Select the reagents you would use to synthesize the compounds below from benzene. Use the minimum number of steps. No more than three steps are required in any synthesis.
a. Br2, FeBr3
b. CH3COCi. AICl3
c. SO3, H2SO4
d. Cl2, FeCl3
e. KMnO4, H2O
f. HNO3, H2SO4
g. Fe, H3O then OH
h. CH3Cl, AICl3
m-chlorobenzenesulfonic acid:
2,4,6-tribromoaniline
Answers
Answer:
f and a. That is reagents f[HNO3, H2SO4] is used first then followed by reagent a[Br2, FeBr3]
Explanation:
So, in the production of m-bromonitrobenzene[3-Nitro-1-bromobenzene/1-Bromo-3-nitrobenzene] from benzene from the reagents provided by using the fastest reagents that is to say the reagent with the minimum number of steps, we are going to make use of reagents in option 'f' followed by reagents in option 'a'.
STEP ONE: The first step in the production of m-bromonitrobenzene[3-Nitro-1-bromobenzene/1-Bromo-3-nitrobenzene] from benzene to give the minimum number of steps from the reagents given is nitration by using the reagents in option 'f'' that is HNO3, H2SO4 to give nitrobenzene. The equation for the chemical reaction is given below as:
C₆H₆ + HNO₃, H₂SO₄ -------------------------------------------------------> C6H5NO2.
It is the N⁺O₂ that is been used in the reaction mechanism [from the reaction: HNO₃ + H₂SO₄ < -----------------> N⁺O₂ + HSO₄⁻ + H₂O].
STEP TWO: The next thing to do is to react the nitrobenzene got from the nitration of benzene in step one above with the reagents in option 'a'[Br2, FeBr3]. This step is known as the bromination of nitrobenzene.
C₆H₅NO₂ + Br₂, FeBr3 ------------------------------------------------> C₆H₄BrNO₂.
Related Questions
Which change of state involves an absorption of energy?
Answers
If you found the mass of the Styrofoam packing peanuts and bricks and divided their mass by their
volumes, which of these would you find to have a higher density?
Answers
By dividing the found mass by volume, the density of the object will be obtained. The density of Styrofoam packing peanuts and bricks are almost similar.
Mass of a substance is the quantity of matter present in the substance. The unit of mass is kg.
A substance's volume is how much room it occupies. The unit of volume is \(m^3\).
Density of a substance is the mass of the substance present in the given volume.
Density = Mass / Volume
The unit of density is \(kg/m^3\).
Objects made from the same substance have the same density.
The density of Styrofoam packing peanuts is 2.3-2.4 pounds per cubic foot and Styrofoam bricks is 2-3 pounds per cubic foot and hence are similar with a slight difference.
Therefore, the density of Styrofoam packing peanuts and bricks is almost same.
Learn more about mass, volume and density here:
https://brainly.com/question/13799762
#SPJ9
Which answer is correct?

Answers
Answer:
Titanium (ll) phosphide
Explanation:
Changing from solid to a liquid at or above melting point
is
Answers
Answer:
liquefaction
liquefaction is a process when a something turns into a liquid.
Explanation:
How many molecules are in 24 grams of ozone (03)
Answers
Answer:48
Explanation:
Answer: 3. 0.125 X 10”23 molecules
Explanation:
What is the change in enthalpy for a reaction if the reactants have 270 kJ of energy and the products have 150 kJ of energy
Answers
Answer-150 kj
Explanation:
follow me on insta bbyspot
A covalent bond forms when two atoms share unpaired electrons in their outer shell. Covalent bonds between carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and phosphorus (P) are particularly important and numerous in biological organisms. Rank the following covalent bonds in terms of which molecules would take the least amount of energy to break apart.
Question List (6 items)
(Drag and drop into the appropriate area)
C—H
C—C
C—O
N—H
C—N
O—H
Covalent Bonds
Least Energy to Break
1
2
3
4
5
6
Greatest Energy to Break
Answers
To determine which of the following covalent bonds would require the least energy to break apart in a given molecule: Energy Required to Break: The greatest energy to break is C—H, N—H, O—H, C—N, C—O, and C—C.
The energy needed to break a covalent link between two atoms is known as covalent bond energy. Atoms share electrons in their outer shell to create covalent bonds, which can result in a stable and energetically advantageous arrangement of electrons. The atoms involved, their electronegativities, and the length of the connection all affect how strong a covalent bond is. Covalent bonds often have higher electronegativities and shorter bond lengths. Covalent bonds between the atoms of carbon, hydrogen, oxygen, nitrogen, and phosphorus play a crucial role in the synthesis of complex macromolecules like proteins, nucleic acids, and carbohydrates in living things. Understanding covalent bond energy is crucial for formulating medications that can target specific biological molecules and for predicting the reactivity of compounds.
Learn more about covalent bonds here:
https://brainly.com/question/2326897
#SPJ4
What is the molality of a solid solution containing 0.125 g of chromium and 81.3 g of iron?
A: 2.96E-5 m
B: 11.6 m
C: 11.6E3 m
D: 0.0296 m
E: none of these
Answers
Around 0.0296 molarity is the molality of the solid solution. D: 0.0296 m is the closest possible response.
How come we compute molality?When examining a solution's vapour pressure and temperature-related characteristics, concentrations expressed in molality are utilised. Molality is utilised since its value is unaffected by temperature fluctuations. On the other hand, a solution's volume is marginally temperature-dependent.
Finding the moles of the solute (chromium) and the mass of the solvent (iron) in kilogrammes is the first step in calculating the molality of the mixture:
moles of Cr = 0.125 g / 52 g/mol = 0.00240 mol
mass of Fe = 81.3 g
mass of solvent = mass of Fe - mass of Cr = 81.3 g - 0.125 g = 81.175 g = 0.081175 kg
The quantity of moles of solute per kilogramme of solvent is the definition of molality, which we can utilise now:
molality = moles of solute / mass of solvent in kg
molality = 0.00240 mol / 0.081175 kg = 0.0295 mol/kg
To know more about molarity visit:-
https://brainly.com/question/8732513
#SPJ1
how many grams of oxygen can be prepared by the decomposition of 12 grams of mercury oxide
Answers
Taking into account the reaction stoichiometry, 0.886 grams of O₂ can be prepared by the decomposition of 12 grams of mercury oxide.
Reaction stoichiometryIn first place, the balanced reaction of the decomposition of mercury oxide is:
2 HgO → 2 Hg + O₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
HgO: 2 moles Hg: 2 moles O₂: 1 moleThe molar mass of the compounds is:
HgO: 216.59 g/moleHg: 200.59 g/moleO₂: 32 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
HgO: 2 moles ×216.59 g/mole= 433.18 grams
Hg: 2 moles ×200.59 g/mole= 401.18 grams
O₂: 1 mole ×32 g/mole= 32 grams
Mass of oxygen formedThe following rule of three can be applied: if by reaction stoichiometry 433.18 grams of HgO form 32 grams of O₂, 12 grams of HgO form how much mass of O₂?
\(mass of O_{2} =\frac{12 grams of HgOx 32 grams of O_{2}}{433.18 grams of HgO}\)
mass of O₂= 0.886 grams
Then, 0.886 grams of O₂ can be prepared by the decomposition of 12 grams of mercury oxide.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
Describe echolocation. Give an example of an animal that uses echolocation.
Answers
dolphin and whales use echolocation
What are 5 things that change so slow they are almost unnoticeable
Answers
Answer:
people ,shape ,behavior, grades ,friends
Which seasons in Atlanta GA have worst AQI
Answers
In Atlanta, GA, certain seasons are associated with poorer air quality due to various factors such as weather conditions, human activities, and geographical location.
Typically, the seasons with the worst AQI in Atlanta, GA, are summer and early fall. This is primarily due to the combination of high temperatures, stagnant air masses, and increased pollution from various sources.
During the summer months, Atlanta experiences hot and humid weather, which can contribute to the formation of ground-level ozone. Ozone is a harmful pollutant that is created when pollutants from vehicles, power plants, and industrial activities react with sunlight and heat. High levels of ozone can cause respiratory issues and other health problems.
In addition to ozone, Atlanta also experiences increased levels of particulate matter (PM) during the summer and early fall. PM refers to tiny particles suspended in the air, which can come from sources such as vehicle exhaust, industrial emissions, and wildfires.
These particles can be inhaled into the lungs and can have detrimental effects on respiratory health.
It's important to note that air quality can vary from year to year and is influenced by various factors. Local regulations, weather patterns, and changes in pollutant emissions can all impact the AQI during different seasons.
Monitoring air quality reports and taking necessary precautions such as reducing outdoor activities during times of poor air quality can help individuals stay informed and protect their health.
For more such question on air quality visit:
https://brainly.com/question/21173066
#SPJ8
What is the correct way to represent the ionic compound sodium fluoride
Answers
Answer:
NaF
may be this helpful!

The correct way to represent the ionic compound sodium fluoride is NaF (Na+ and F- ions).
The correct way to represent the ionic compound sodium fluoride is NaF. In this compound, sodium (Na) and fluoride (F) form an ionic bond. Sodium is a metal from Group 1 of the periodic table, while fluoride is a non-metal from Group 17.
Ionic compounds consist of positively charged ions (cations) and negatively charged ions (anions) held together by electrostatic attractions. In NaF, sodium loses one electron to become a sodium cation (Na+), while fluoride gains one electron to become a fluoride anion (F-). The resulting compound, NaF, consists of Na+ and F- ions arranged in a crystal lattice structure.
The chemical formula NaF represents the stoichiometric ratio of sodium to fluoride in the compound. It indicates that for every sodium cation, there is one fluoride anion present.
NaF is commonly used as a source of fluoride in various applications, including water fluoridation, toothpaste, and as a component in certain industrial processes.
Learn more about ionic compound from the given link:
https://brainly.com/question/2687188
#SPJ11
Good Morning I have a question I need help and can not find the answer o it maybe someone help me? The question is _______ Most Of the Energy that drives water cycle comes from__________? (this is from Science)
Answers
Answer:
hydro, water
Explanation:
Name four types of salts
Answers
Answer:
Any ionic molecule formed of a base and an acid, which dissolves in water to produce ions is known as a salt. The four common types of salts are:
1. NaCl or sodium chloride is the most common kind of salt known. It is also known as table salt.
2. K2Cr2O7 or potassium dichromate refers to an orange-colored salt formed of chromium, potassium, and oxygen. It is toxic to humans and is also an oxidizer, which is a fire hazard.
3. CaCl2 or calcium chloride looks like table salt due to its white color. It is broadly used to withdraw ice from roads. It is hygroscopic.
4. NaHSO4 or sodium bisulfate produces from hydrogen, sodium, oxygen, and sulfur. It is also known as dry acid. It has commercial applications like reducing the pH of swimming pools and spas and others.
13. An aerosol spray can of deodorant with a volume of 0.410 L contains 3.0 g of propane gas (CH3) as propellant. What is the pressure in the can at 20°C? VRT 3. og festa
Answers
Answer: The pressure in the can is 4.0 atm
Explanation:
According to ideal gas equation:
\(PV=nRT\)
P = pressure of gas = ?
V = Volume of gas = 0.410 L
n = number of moles = \(\frac{\text {given mass}}{\text {Molar mass}}=\frac{3.0g}{44.1g/mol}=0.068mol\)
R = gas constant =\(0.0821Latm/Kmol\)
T =temperature =\(20^0C=(20+273)K=293K\)
\(P=\frac{nRT}{V}\)
\(P=\frac0.068mol\times 0.0820 L atm/K mol\times 293K}{0.410L}=4.0atm\)
Thus the pressure in the can is 4.0 atm
due to the change in the number of electrons, the metal and nonmetal atoms both become _____
Answers
How many milliliters of gasoline have a mass of 2.4 kg ( D=0.74g/mL )?
Answers

Answer:
3243.2ml
Explanation:
Converting 2.4kg to g = 2.4X1000
=2400g
Since Density is mass/volume,
then volume is = mass/density
volume = 2400/0.74
=3243.2ml
How should the solute and solvent be mixed in the container?.
Answers
Fill to the 250-mL mark with water after mixing a tiny amount of water with the solute to dissolve it.
DO IT PLS
6. A helium laser emits light with a wavelength of 363 nm. What is the frequency of the light?
Answers
4.which is a product of the process shown above?
a. carbon dioxide
b. sugar
c. sunlight
d. water
5.where does the energy that drives this process come from?
a. oxygen
b. sugar
c. sunlight
d. water
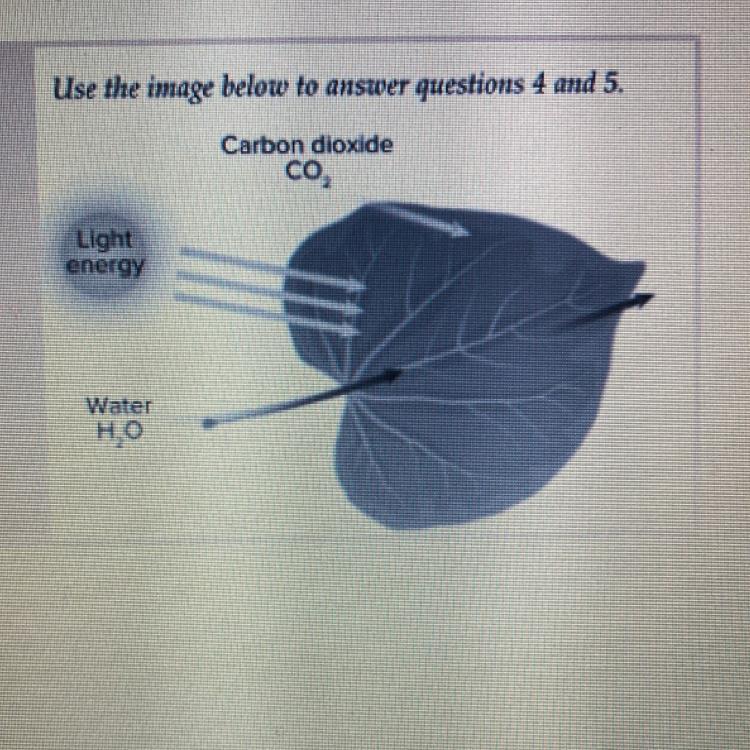
Answers
Answer:
4. b 5.C
Explanation:
Photosynthesis makes sugar for plants and sunlight is the energy used to convert CO2 and water into glucose
The product of the process of photosynthesis is sugar and the energy to dive the process of photosynthesis comes from sunlight.
What is photosynthesis?Photosynthesis is defined as a process by which plants and other photosynthetic organisms convert the light energy in to chemical energy through the process of cellular respiration.
Some of the energy which is converted is stored in molecules of carbohydrates like sugar and starches which are made up of from carbon dioxide and water . Photosynthetic organisms which can perform photosynthesis are algae and cyanobacteria. Photosynthesis is largely responsible for producing and maintaining the content of oxygen in earth's atmosphere.
The process begins with proteins absorbing light energy which are called reaction centers and contain a green pigment which is called chlorophyll . In plants ,these pigments are present inside organelles called chloroplasts while in bacteria they are present in plasma membrane.
Learn more about photosynthesis,here:
https://brainly.com/question/26494694
#SPJ2
You measure the following dimensions of a rectangular metal block of metal A: length =13.0; cm; width = 6.4cm; height = 8.2cm It has a mass of 1287 gWhat is the density of metal in g/cm^ 3 ?

Answers
The density of the rectangular block of metal in g/cm³ is 1.87 g/cm³
How to calculate density?Density is a measure of the mass of matter contained by a unit volume. It can be calculated by dividing the mass of the substance by its volume as follows;
Density = mass ÷ volume
According to this question, the dimensions of a rectangular metal block of metal A are as follows: length = 13.0cm; width = 6.4cm; height = 8.2cm. If it has a mass of 1287g, the density can be calculated as follows;
Volume = 13 × 6.4 × 8.2 = 682.24cm³
Density = 1287g ÷ 682.24cm³
Density = 1.87 g/cm³
Learn more about density at: https://brainly.com/question/28858363
#SPJ1
Amino acid Is a compound that contains at least
Answers
They contain Carbon, Nitrogen, Hydrogen, Oxygen, and Sulfur
7. What is the volume of the
composite
solid?
4 in.
3 in.
3 in.

Answers
Answer:
The volume of Component 1 is 36 cubic inches.
Explanation:
To calculate the volume of a composite solid, we need to determine the individual volumes of the different components and then add them together.
In this case, the composite solid consists of multiple components with the following dimensions:
Component 1:
Length: 4 inches
Width: 3 inches
Height: 3 inches
To find the volume of Component 1, we multiply the length, width, and height together:
Volume of Component 1 = Length x Width x Height = 4 in x 3 in x 3 in = 36 cubic inches
Therefore, the volume of Component 1 is 36 cubic inches.
Please provide the dimensions of the remaining components of the composite solid, and I will calculate the total volume by summing up the individual volumes.
What type of words should you include when you write your hypothesis
Answers
Answer:
An if/then statement. If ____happens, then _____ happens.
Explanation:
100 points
Can someone please help me with this puzzle?
I'm giving 100 points.
please don't give me random answers I will report.
Answers
Answer:
Where is the puzzle!??
I surely can help you
Answer:
I don't see the puzzle and this is not a random answer I literally don't see the puzzle so I can't help fix it plz
A force of 6 N is applied to a ball for 2 s. What is the impulse?
Answers
Answer:
The answer is 12 NsExplanation:
The impulse of an object can be found by using the formula
impulse = force × timeFrom the question
force = 6 N
time = 2 s
We have
impulse = 6 × 2
We have the final answer as
12 NsHope this helps you
The atomic mass is...
A. Equal to the number of protons
B. equal to the number of protons + neutrons
C. Equal to the number of protons-electrons
D.Equal to the number of electrons
E. none of the above
Answers
Why does the solubility of many substances increase with temperature? (Remember what an increase in temperature means on a microscopic scale.)
Answers
The solubility of many substances increases with temperature, there are exceptions. Some substances exhibit a decrease in solubility with temperature due to specific interactions or changes in solute-solvent interactions at higher temperatures.
The increase in solubility of many substances with temperature can be attributed to the effect of temperature on the kinetic energy and intermolecular interactions of molecules.
On a microscopic scale, an increase in temperature corresponds to an increase in the kinetic energy of molecules. As the kinetic energy increases, the molecules move more rapidly and collide with each other and with the solvent molecules more frequently and with greater force.
These increased collisions and kinetic energy result in enhanced molecular interactions and overcome the forces holding the solute particles together. This increased energy disrupts the intermolecular forces within the solute, allowing the solvent molecules to surround and interact more effectively with the solute particles, leading to greater solubility.
Additionally, an increase in temperature can cause solvent molecules to move more freely, reducing their cohesion and allowing them to interact more readily with solute particles.
for more questions on solubility
https://brainly.com/question/24057916
#SPJ8
In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor. hydrogen+oxygen⟶water
If you burn 46.2g of hydrogen and produce 413g of water, how much oxygen reacted?
mass of oxygen:
Answers
Answer:
ok, here is your answer
Explanation:
AI-generated answer
To find the mass of oxygen that reacted, we need to use the Law of Conservation of Mass, which states that in a chemical reaction, the mass of the reactants equals the mass of the products.
First, we need to find the number of moles of hydrogen that reacted:
Molar mass of hydrogen (H₂) = 2.016 g/mol
Number of moles of H₂ = mass/molar mass = 46.2 g/2.016 g/mol = 22.92 mol
Next, we need to use the balanced chemical equation to find the number of moles of water produced:
hydrogen + oxygen → water
2H₂ + O₂ → 2H₂O
From the equation, we can see that for every 2 moles of H₂, 1 mole of O₂ is required to produce 2 moles of H₂O. Therefore, the number of moles of O₂ required to produce 22.92 moles of H₂O is:
Number of moles of O₂ = 1/2 x 22.92 mol = 11.46 mol
Finally, we can find the mass of oxygen that reacted by using its molar mass:
Molar mass of oxygen (O₂) = 32.00 g/mol
Mass of oxygen = number of moles x molar mass = 11.46 mol x 32.00 g/mol = 366.72 g
Therefore, the mass of oxygen that reacted is 366.72 g.
mark me as brainliest