Answers
Answer:
all atoms have the same number of protons
Related Questions
a
Jessica has a solution of tea and water. She adds ice to the solution.
Which properties of the solution will change as the ice melts?
a. The mass will decrease.
b. The temperature will decrease.
The volume will increase.
C. The volume will increase
Answers
21(3d − 4) + 100 = 58 State the solution. (If all real numbers
are solutions, enter REALS. If there is no solution, enter NO
SOLUTION.)
Answers
The solution to the equation and value of variable 21(3d - 4) + 100 = 58 is d = 2/3.
Solve the equation 21(3d - 4) + 100 = 58, we can begin by simplifying and isolating the variable:
21(3d - 4) + 100 = 58
Distribute 21 to the terms inside the parentheses:
63d - 84 + 100 = 58
Combine like terms:
63d + 16 = 58
Subtract 16 from both sides:
63d = 42
Divide both sides by 63:
d = 42/63
Simplifying the fraction gives:
d = 2/3
The solution to the equation is d = 2/3.
The solution to the equation 21(3d - 4) + 100 = 58 is d = 2/3. By simplifying the equation, we find that dividing both sides by 63 results in the solution of d = 2/3, which satisfies the original equation.
To know more about variable refer here
https://brainly.com/question/15078630#
#SPJ11
the molecular formula for vitamin c is c6h8o6. what is the empirical formula? question 2 options: cho ch2o c3h4o3 c2h4o2
Answers
c3h4c3
its the smallest number of atoms , so divide all values by 2 as that is the largest common factor of 6 and 8.
how many possible molecular motions can unrestrained gas molecules have?
Answers
Three different molecular movements, including translational, rotational, and vibrational motions, are feasible for unrestrained gas molecules.
Unrestrained gas molecules have three separate types of motion: vibration, rotation, and translation. Translation describes a full molecule travelling linearly in space. A molecule in rotational motion revolves around the core of its mass. Vibrational motion is created by the atoms' internal oscillations within the molecule.
According to the principles of thermodynamics and statistical mechanics, these three motions work together to form the overall behaviour and properties of gas molecules, such as temperature, pressure, and heat capacity.
Learn more about molecular movements:
https://brainly.com/question/31448972
#SPJ4
A flask contains three gases, Nitrogen, Oxygen, and ammonia. The nitrogen has a partial pressure of 9.84 atm, the oxygen has a partial pressure of 643 torr, and the ammonia has a partial pressure of 2865 kPa. What it the total pressure in the flask expressed in atm? (Make sure and do all of your conversions correctly).
Answers
To calculate the total pressure in the flask, we need to convert all the partial pressures to the same unit and then add them together. We can convert the partial pressures of oxygen and ammonia to atm using the following conversions:
1 atm = 760 torr
1 atm = 101.325 kPa
Partial pressure of oxygen in atm = 643 torr / 760 torr/atm = 0.846 atm
Partial pressure of ammonia in atm = 2865 kPa / 101.325 kPa/atm = 28.27 atm
Now we can add up all the partial pressures in atm:
Total pressure in atm = Nitrogen partial pressure in atm + Oxygen partial pressure in atm + Ammonia partial pressure in atm
Total pressure in atm = 9.84 atm + 0.846 atm + 28.27 atm
Total pressure in atm = 38.956 atm
Therefore, the total pressure in the flask is 38.956 atm (rounded to three decimal places).
Explain.Car side mirrors do not work when they are convered with frost. What does frost do no the surface of a mirror to cause a diffuse reflection?
Answers
The side mirrors play an important role in providing the safety of a car and also help the drivers to see the vehicles behind the car. Most of the side mirrors have a small heating element in them which helps to defrost or deice.
When ice, snow or even just condensation builds up on your mirror, it can severely limit your visibility. The frost makes the smooth surface of the mirror rough, so that the parallel rays are reflected in different directions.
There are extensive and multiple refractions which cause the distortion of light and so that the frost looks white, whereas ice is clear also called black ice due to the colour of road surface.
To know more about refraction, visit;
https://brainly.com/question/20341265
#SPJ1
ibuprofen, C13H18O2, is the active ingredient in many non-prescription pain relievers.
a) If the tables in a bottle contain a total of 33 g of ibuprofen, how many moles of ibuprofen are in the bottle?
b) How many molecules of ibuprofen are in the bottle?
Answers
Mole measure the number of elementary entities of a given substance that are present in a given sample. Therefore, the moles and number of molecules of ibuprofen is 0.15moles and 9.03×10²²molecules respectively.
What is mole?The SI unit of amount of substance in chemistry is mole. The mole is used to measure the quantity or amount of substance. We know one mole of any element contains 6.022×10²³ atoms which is also called Avogadro number.
Mathematically,
mole =given mass ÷ molar mass
given mass of ibuprofen= 33 g
Molar mass of ibuprofen= 206.29 g/mol
mole of ibuprofen=33 g ÷ 206.29 g/mol
mole of ibuprofen=0.15moles
number of atoms/molecules=number of moles × 6.022×10²³(Avogadro number)
number of atoms/molecules of ibuprofen= 0.15× 6.022×10²³
=9.03×10²²molecules
Therefore, the moles and number of molecules of ibuprofen is 0.15moles and 9.03×10²²molecules respectively.
To know more about mole, here:
https://brainly.com/question/15209553
#SPJ1
What is the molarity of 25.00 grams of powdered (solid) AgNO3 added to 500.00mL of pure water. The molar mass of AgNO3 is 169.874g/mole or also written as 1mole/169.874g
1 Calculate the moles of AgNO3 added.
2 Divide by total volume. M= n/v
3 Ensure your units are correct (that means use your dimensional analysis)
Here are your hints.
The moles of AgNO3 is between 0.100 and 0.500
The numerical value of your concentration should be between 0.100 and 0.500
Answers
The molarity of 25.00 grams of powdered AgNO3 added to 500.00mL of pure water is 0.29 mol/L
StoichiometryFrom the question, we are to determine the molarity of the solution prepared.
First, we will determine the number of moles of AgNO₃ present.
Using the formula,
\(Number\ of\ moles = \frac{Mass}{Molar\ mass}\)
From the given information,
Mass = 25.00 g
Molar mass = 169.874 g/mole
Number of moles = \(\frac{25.00}{169.874}\)
Number of moles of AgNO₃ present = 0.147168 mole
Now, for the molarity of the solution
\(Molarity = \frac{Number\ of\ moles}{Volume}\)
From the given information,
Volume = 500.00 mL = 0.5 L
Molarity = \(\frac{0.147168}{0.5}\)
Molarity = 0.294336
Molarity ≅ 0.29 mol/L
Hence, the molarity of 25.00 grams of powdered AgNO3 added to 500.00mL of pure water is 0.29 mol/L
Learn more on Stoichiometry here: https://brainly.com/question/14805986
In the outer layer of the sun, energy is transported outward by the rising of hot plasma and sinking of cooler plasma. This transfer is known as __________.
a.Electromagnetic radiation
B.Convection
C. Solar flares
D. Conduction
Answers
Answer:
solar flares
Explanation:
We have that The outward transportation of sun rays by the rising of hot plasma and sinking of cooler plasma is Known as
Solar flaresOption D
From the question we are told
In the outer layer of the sun, energy is transported outward by the rising of hot plasma and sinking of cooler plasma. This transfer is known as __
Generally
When the sun give off Heat energy it does this by the rising of hot plasma and sinking of cooler plasma
This Process is defined by scientist around the world as Solar flares and this
is the reason why the rays if the sun reaches earth.
Therefore
The outward transportation of sun rays by the rising of hot plasma and sinking of cooler plasma is Known as
Solar flares
Option D
For more information on this visit
https://brainly.com/question/17756498
Explain why butter and graphite have different melting points?
Answers
Answer:
Differences in melting points between graphite and butter is how their atoms are fixed structurally. Graphite consists of carbon atoms that have covalent bonding with a compact hexagonal arrangement that gives a lot of stability to the compound and makes its melting point elevated to approximately 3500oC.
The allotropes of carbon are diamond and graphite. The allotropes can have same or different physical or chemical properties. Therefore, differences in melting points between graphite and butter is due difference in structure.
What are allotropy?Allotropy is a property by which an element exist in more than one form. The different forms of element are called allotropes.
The features of graphite are :
1.Graphite form strong covalent bonds.
2. Graphite has delocalized electrons but they don't have metallic bonding.
3. There are total four electrons per carbon but only three electrons are available for covalent bond. One electron per carbon is responsible for delocalization.
4. Graphite forms a giant structures.
Graphite consists of carbon atoms that have covalent bonding with a compact hexagonal arrangement that gives a lot of stability to the compound and makes its melting point elevated.
Therefore, differences in melting points between graphite and butter is due difference in structure.
To know more about allotropy, here:
https://brainly.com/question/16914774
#SPJ2
one way to determine if a molecule is polar is to place it between two oppositely charged metal plates. what happens when polar molecu;es are placed between these
Answers
Polar molecules' poles point in the direction of the oppositely charged metal plate when they are sandwiched between the plates.
Positive and negative poles are found in opposite poles in polar molecules. For instance, water molecules will have their negative pole facing the positive end and vice versa when they are placed between two metal plates that have opposing charges.
The charges on the metal plate will force them to align themselves since opposing charges repel one another and like charges attract.
Therefore, when polar molecules are sandwiched between two metal plates with opposing charges, the poles will point in the direction of the plate with the opposite charge.
To learn more about metal plates, refer:-
https://brainly.com/question/20078989
#SPJ4
a. He is as wise as his sister. (change into Negative) 6. He put the cup on the shelf. (change into interrogative) c. He said to her. Will you play tomorrow? (change into indirect) d. Doctors cure patients. (Change into passive) e. She (not eat) vet. (Rewrite it using correct tense) t Everybody has eaten..............? (use correct question tag)
Answers
Answer:
a. He is as wise as his sister. (change into Negative)
⇒Neither he is wise nor his sister.
b. He put the cup on the shelf. (change into interrogative)
⇒Did he put the cup on the shelf?
c. He said to her. Will you play tomorrow? (change into indirect)
⇒He asked me that whether I play the next day.
d. Doctors cure patients. (Change into passive)
⇒Patients were cured by doctors.
e. She (not eat) yet. (Rewrite it using correct tense)
⇒she hadn't eaten yet.
f. Everybody has eaten..............? (use correct question tag)
⇒Everybody has eaten, haven't they?
Question 2 of 30
A television commercial shows happy people while describing some medical
symptoms. These symptoms include feeling tired and sad. The medication
being advertised by the commercial was approved by the FDA to treat a
disease that causes these symptoms. The narrator says that it is available by
prescription only and contains 1% of the active ingredient. What can you infer
about this medication?
OA. The people in the commercial are happy because they were
treated by the medication.
B. The medication would be more effective if it contained 10% of the
active ingredient.
C. Anyone who has the symptoms should request a prescription
from his or her doctor.
D. The medication can treat people who have the disease described.
Answers
The medication can treat people who have the disease described. According to the commercial, the drug has FDA approval to treat a condition whose symptoms are listed.
Additionally, the narrator notes that the medication only comes with a prescription and has 1% of the active substance. We can deduce from this information that the drug can effectively treat persons who have the condition generating these symptoms, but obtaining it requires a prescription. The commercial provides no support for the other possibilities.
Therefore, the correct option is D.
Learn more about Medication, here:
https://brainly.com/question/11098559
#SPJ1
Which of the following is the correct sequence of events in cholesterol synthesis? (Note: not all events in the sequence are included)
HMG-CoA > isopentyl pyrophosphate > farnesyl pyrophosphate > geranyl pyrophosphate
isopentyl pyrophosphate > squalene > oxidosqualene > geranyl pyrophosphate
dimethylallyl pyrophosphate > geranyl pyrophosphate > farnesyl pyrophosphate > squalene
HMG –CoA > phosphomevalonate > squalene >farnesyl pyrophosphate
mevalonate > dimethylallyl
pyrophosphate > farnesyl pyrophosphate > gernayl pyrophosphate
Answers
The correct sequence of events in cholesterol synthesis is HMG –CoA > phosphomevalonate > isopentenyl pyrophosphate > dimethylallyl pyrophosphate > geranyl pyrophosphate > farnesyl pyrophosphate > squalene > lanosterol > cholesterol.
HMG-CoA: The precursor of cholesterol is HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A). The synthesis of HMG-CoA occurs in the cytoplasm of cells.
Isopentenyl pyrophosphate: HMG-CoA undergoes a series of reactions to form mevalonate. Isopentenyl pyrophosphate is formed from mevalonate by the conversion of mevalonate to mevalonate-5-phosphate.
Dimethylallyl pyrophosphate: Isopentenyl pyrophosphate condenses with dimethylallyl pyrophosphate to produce geranyl pyrophosphate.
Geranyl pyrophosphate: Geranyl pyrophosphate is transformed into farnesyl pyrophosphate by the addition of another molecule of isopentenyl pyrophosphate.
Farnesyl pyrophosphate: Farnesyl pyrophosphate is transformed into squalene by the removal of two phosphate groups.
Squalene: Two squalene molecules join together to form the first cyclic intermediate, lanosterol.
Lanosterol: Lanosterol is then transformed into cholesterol through a series of reactions.
Therefore, the correct option among the given options in the question is HMG –CoA > phosphomevalonate > squalene >farnesyl pyrophosphate.
You can learn more about cholesterol at: brainly.com/question/29661052
#SPJ11
answer questions 3 and 4 for a brainly!

Answers
Answer:
The correct answer is -
3. Butyne
4. Pentene
Explanation:
The names of such organic compounds are classified on the number of the carbon atoms and based on a number these prefixes are attached -
Meth - one carbon
Eth - two carbons
Prop - three carbons
But - four carbons
Pent - five carbons, and so on
The bonds can be identified by names of an organic compound by their suffixes -
- ane = single bond
- ene = double bond
-yne = triple bond
Thus, for question 3, carbon numbers are 4 and triple bond so Butyne, and similarly for question 4, 5 carbon atoms and double bond so Pentene.
Why are computer models useful in studying phenomena in the universe?
Answers
Answer:
Computer Models are used to study the complex phenomena in the universe.
Explanation:
The term 'models' can be defined as a representation of something that is real. A model is used to learn the phenomena of the natural world for easy understanding of it.
The computer models are used to study the large and complex data in the universe. These models are used to perform various calculations that, in real, would take years to do. It helps in studying the changes in variables, which in turn helps the scientist to know of any climatic change, or other changes in the nature.
Balance the following equation by oxidation number method:
Zn + HNO3-----> Zn(NO3)2 + NO + H2O
Answers
By using the oxidation number approach, find the solution to the equation Zn + HNO3 4Zn(NO3)2 Plus N2O + H2O.
Zn HNO3 undergoes what kind of reaction?Chemical Reaction Type: We only have one displacement reaction for this reaction. Balanced Approaches: By employing diluted HNO3, the reaction between HNO3 with Zn will result in Zn(NO3) + H2.
Is the displacement reaction in Zn H2O?Balanced Approaches: You have a displacement reaction reaction in this zinc and water reaction. Cold water does not cause the response to occur. Water must be scorching hot or at least very hot. The number the hydrogen atoms just on output side of the formula must be correctly counted in order to balance the equation.
To know more about oxidation visit:
https://brainly.com/question/376562
#SPJ1
stoichometry in chemistry!! please someone help i'm begging you, if i fail this then i fail chemistry

Answers
Answer:
You can find in the given attachemnet



Choose the correct products for the double replacement reaction below. Click here to access the solubility rules to determine which product, if any, forms a solid precipitate in the reaction. AgNO3 KCl Upper K l Upper Pb (Upper N Upper O Subscript 3) Subscript 2 Baseline right arrow. ? K O2 NCl KNO3 AgCl AgK ClNO3 K2NO3 AgCl2.
Answers
In both cases, the precipitates were AgCl and PbI2 respectively.
What is a precipitate?A precipitate is a solid product obtained from the reaction of two aqueous phase species. Let us now consider the two reactions listed in the question;
AgNO3(aq) + KCl(aq) ----> AgCl(s) + KNO3(aq)2KI(aq) + Pb(NO3)2 (aq) ----->PbI2(s) + 2KNO3(aq)We can see that in both cases, the precipitates were AgCl and PbI2 respectively.
Learn more about precipitates: https://brainly.com/question/24846690
Answer:
B and B
Explanation:
edge 2022
Energy appears in many forms what form of energy is lightning
Answers
Answer:
thermal and light energy
in a molecule of ch4 the hydrogen atoms are spatially? (a) tetrahedron.
(b) pyramid.
(c) rectangle.
(d) square.
Answers
In a molecule of CH₄, the hydrogen atoms are spatially tetrahedron-shaped. The correct answer is option (a).
In the methane (CH₄) molecule, the carbon atom is in the center, and the four hydrogen atoms are positioned around it. Because of the four electron pairs surrounding the carbon atom, the CH₄ molecule is tetrahedral. The carbon atom has four valence electrons that share with hydrogen to form four single covalent bonds, resulting in a complete outer electron shell for the carbon atom and a complete outer electron shell for the hydrogen atoms.
The electron-pair geometry is tetrahedral, while the molecular geometry is also tetrahedral. The methane molecule's tetrahedral geometry is determined by the arrangement of the carbon and hydrogen atoms.
Thus, in a molecule of CH₄, the hydrogen atoms are spatially tetrahedron-shaped. The correct answer is option (a).
Learn more about covalent bonds here:
https://brainly.com/question/33425898
#SPJ11
Sulfuric acid decomposes into sulfur trioxide gas and water. What would be the respective coefficients for balancing?.
Answers
Chemical equations must be balanced, which requires that the quantity and type of atoms on both sides of the reaction arrow be equal.
What are the balanced reaction's coefficients?As a result, chemical equations must be balanced, which requires that the quantity and type of atoms on both sides of the reaction arrow be equal. Coefficients are the values added in front of formulas to balance equations; they multiply each atom in a formula.The number in front of the variable phrases is the coefficient, just like in algebra. The number in front of the formula is the coefficient in chemistry.
Sulphur trioxide = SO_3
Sulfuric acid = H_2SO_4
SO 3(g) + H2O (l) ---> H2SO4 (aq)
To learn more about balanced reaction's refer to:
https://brainly.com/question/26694427
#SPJ4
Ethanol (1) has a specific heat of 2.443/9-°C and mercury's is 0.14 J/g. C. Which substance is the easier one to warm to a higher temperature? Why?
Answers
Answer:
\( \small \sf Answer \rightarrow Mercury \: is \: easier \: to \: warm. \)
Explanation:
Specific Heat:
The specific Heat of any material is the heat absorbed or evolved by the material to raise or fall it's temperature by 1°C per unit mass of the material.
The heat absorbed or evolved (Q) is directly proportional to the mass of substance(m) and rise or fall of temp(∆T)
The general formula for specific heat is,
\( \sf \: \: \: \: \: s = \frac{Q}{\Delta T} \)
Where s is specific heat,Q is the Heat absorbed or evolved & ∆T is change is temp.Now coming to your question,
The specific heat of ethanol is 2.443 J/g °C
& specific heat of mercury is 0.14 J/g ° C
The definition states that, if the specific heat would be more, more amount of heat will be required for per unit change in temperature and the corresponding substance will be difficult to warm. Similarly, substance with high specific heat won't warm as fast as substance with less specific heat.
Hence mercury will be easier to warm up compared to ethanol, and this could a reason to why Mercury is the only metal liquid at room temperature, because it have less specific heat!
\( \small \sf Answer \rightarrow Mercury \: is \: easier \: to \: warm \: to \: a \: high \: temperature.\)
Thanks for joining brainly community!
Boreal forests are characterized by _______ trees. A. Creosote b. Eucalyptus c. Coniferous d. Deciduous Please select the best answer from the choices provided A B C
Answers
Answer:
c
Explanation:
coniferous trees, Tianga also called Boreal forest biome (major life zone).
hope it's perfect.
Answer: C is correct.
A second concern was discovered when a chemical dosing machine in the bottling line had a minor release during a change out of the chemical dimethyl dicarbonate (DMDC). The employees were evacuated without injury.
These two incidents led the winery to determine the level of risk and potential solutions. A risk assessment team was formed, and an assessment was performed. The team determined that the SO2 and DMDC exposure risks both presented multiple fatality – level risk and required immediate risk treatment.
Management sets the expectations, context, and objectives of the assessment. The risk assessment team was established that included the consultant as facilitator, the winemaker, assistant winemaker, cellar manager, operations manager, bottling
department manager, maintenance manager, and Health and Safety Executive (HSE) manager.
Data were collected regarding the SO2 and DMDC operations, equipment and instruments used, instructions, chemicals, and their SDSs, operator training, procedures, and available incident information. A search for similar events involving SO2 and DMDC were also conducted. Employees were interviewed to learn from their experiences, concerns, and suggestions.
The two procedures were observed to document and understand the sequence of tasks and potential risks associated with tasks. Photographs, tank quantities, room dimensions and configurations, distances to exits, means of egress, and other physical attributes were collected.
After reviewing the information, the potential concerns of fatalities or serious incidents were discussed. Workplace exposures such as pure SO2 releases and DMDC releases which present a potential for fatalities or serious incidents must be given the highest priority and controlled to an acceptable level. As a side note, the consultant explained that unlike like less-serious workplace incident rates, fatality, and serious incident/injury rates have not declined and do require serious attention. FSI exposures that can result in environmental releases, explosions, and disasters have been found to involve some of the following factors (14):
• Unusual and nonroutine work
• Nonproduction tasks
• Facility modification or construction activities
• Shutdowns and startups for repair and maintenance tasks
• Exposure to high-energy sources (e.g. electrical, steam, pneumatic, chemical) • Upsets (situations going from normal to abnormal).
Answers
The risk assessment team, consisting of various members from different departments within the winery, conducted a comprehensive assessment of the potential risks associated with sulfur dioxide (SO2) and dimethyl dicarbonate (DMDC) operations.
Data was collected regarding the equipment, chemicals, procedures, training, incident history, and physical attributes of the workplace. The team also interviewed employees to gather their experiences, concerns, and suggestions.
After reviewing the collected information, the team identified the potential risks of fatalities or serious incidents related to workplace exposures of SO2 and DMDC. These risks were considered of utmost priority and required immediate control to ensure an acceptable level of safety. The consultant highlighted the importance of addressing these serious risks, as fatality and serious incident rates have not shown a decline and demand serious attention.
Factors contributing to the potential for environmental releases, explosions, and disasters were identified, including unusual and nonroutine work, nonproduction tasks, facility modification or construction activities, shutdowns and startups for repair and maintenance tasks, exposure to high-energy sources (such as electrical, steam, pneumatic, chemical), and situations transitioning from normal to abnormal (upsets).
Based on the assessment and the identified risks, it is crucial for the winery to implement effective control measures to minimize the potential for fatalities, serious incidents, and environmental disasters. These measures may include improving procedures, enhancing operator training, implementing stricter safety protocols, and ensuring proper handling and storage of chemicals.
To know more about sulfur dioxide (SO2)
https://brainly.com/question/24925697
#SPJ11
Convert a density of 55.3 lbs/ft3 into g/mL.
Answers
The density of 55.3 lbs/ft³ is 0.884 g/mL.
To convert density from pounds per cubic foot (lbs/ft³) to grams per milliliter (g/mL), we can use the following conversion factors:
1 pound = 453.59237 grams
1 foot = 30.48 centimeters (cm)
1 inch = 2.54 centimeters (cm)
1 milliliter (mL) = 1 cubic centimeter (cm³)
First, we convert pounds to grams:
55.3 lbs = 55.3 lbs * 453.59237 g/lb = 25050.364 grams
Next, we convert cubic feet to milliliters:
1 ft³ = (30.48 cm)³ = 28316.8466 cm³
1 cm³ = 1 mL
Finally, we calculate the density in g/mL:
Density = (25050.364 g) / (28316.8466 mL) ≈ 0.884 g/mL
Therefore, the density of 55.3 lbs/ft³ is approximately 0.884 g/mL.
Learn more about density from the given link
https://brainly.com/question/1354972
#SPJ11
Which of the following statements is wrong about cathode rays?A.They travel in straight lines towards cathodeB.They produce heating effectC.They carry negative chargeD.They produce X-rays when strike with material having high atomic masses
Answers
The statements wrong about cathode rays is none of the given option. All the given option is true about the cathode rays.
The statements for the cathode rays is given as :
A. They travel in straight lines towards cathode. This statement is true about the cathode rays.
B. They produce heating effect. This statement is true about the cathode rays.
C. They carry negative charge. This statement is true about the cathode rays.
D. They produce X-rays when strike with material having high atomic masses. This statement is true about the cathode rays.
Thus, none of the option is incorrect about the cathode rays.
To learn more about cathode rays here
https://brainly.com/question/26500956
#SPJ4
Carbon cycle – What are the main reservoirs
of the carbon cycle? Where do the inorganic and organic carbon
cycles interact? What are the major differences and similarities
between the inorganic and organic carbon?
Answers
The main reservoirs of the carbon cycle are the atmosphere, oceans, land (including vegetation and soils), and fossil fuels. In these reservoirs, carbon exists in both inorganic and organic forms.
The inorganic carbon cycle involves the exchange of carbon dioxide (CO2) between the atmosphere and oceans through processes like photosynthesis and respiration.
Organic carbon, on the other hand, is found in living organisms, dead organic matter, and soil organic matter. It is cycled through processes such as decomposition and consumption by organisms. The interactions between the inorganic and organic carbon cycles occur primarily in the biosphere, where photosynthesis converts inorganic carbon into organic carbon compounds. While inorganic carbon is primarily in the form of CO2, organic carbon is present in complex organic molecules. Both forms of carbon play crucial roles in energy transfer, nutrient cycling, and climate regulation.
Learn more about Carbon Cycle
brainly.com/question/13729951
#SPJ11
Can someone please help with this chemistry question
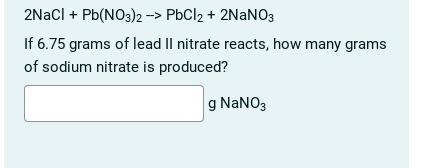
Answers
The mole ratio of NaNO₃ to Pb(NO₃)2 is 2:1. Therefore, 6.75 g of Pb(NO₃)₂ will produce 2 x 6.75 g = 13.5 g of NaNO₃.
What is mole?Mole is a unit of measurement used in chemistry to measure the amount of a substance, usually expressed in terms of the number of atoms, molecules, or other units in a given mass of that substance. The mole, also known as Avogadro's number, is a very large number equal to 6.022 x 10^23. This means that a mole of a substance contains 6.022 x 10^23 atoms or molecules, depending on the substance. The mole is used to measure the amount of a substance in a given sample, and is essential to many chemical calculations.
To learn more about mole
https://brainly.com/question/24191825
#SPJ1
A solution that has a high amount of hydrogen ions is called.
Answers
Answer:
a solution with high amount of hydrogen ions is called an acidic solution
