Scientists have been collecting atmospheric co2 data for many years to monitor changes over time. Which of the following best describes why an island location, such as the mauna loa observatory in hawaii, is an ideal location to measure global co2 concentrations?.
Answers
Mauna Loa Observatory in Hawaii is an ideal location to measure global atmospheric CO2 concentration. This is because the observatory is situated far away from the main continental sources of atmospheric pollution. This allows for direct measurement of the undisturbed background concentration of CO2 in the atmosphere.
The monitoring of atmospheric CO2 data has been going on for several years to watch over the changes that happen over time. The Global Atmosphere Watch (GAW) is a program established by the World Meteorological Organization to measure and monitor various atmospheric trace gases and their isotopes and other related parameters.The observatory is situated at an elevation of 3,397 meters, which is high enough to make measurements above the "boundary layer," the lowest part of the atmosphere, which is influenced by nearby sources. Mauna Loa is in a remote location, far from urban and industrial pollution sources. Mauna Loa is situated in the middle of the Pacific Ocean, thousands of miles from any large continental sources of atmospheric pollution, which makes it a suitable place for accurate measurements of undisturbed background atmospheric CO2 concentrations.
To Know more about Global Atmosphere Watch visit:
brainly.com/question/29641493
#SPJ11
Related Questions
50 POINTS PLEASE ANSWER CORRECTLY
Two balls collide on a pool table. Before the collision, ball 1 is traveling with a speed of 4 m/s, and ball 2 is at rest. After the collision both balls are in motion.
What has happened in this collision?
A. There was no change in ball 2's velocity, therefore momentum was not conserved.
B. Ball 2's velocity decreased, and it gained some of ball 1's momentum.
C. Ball 1's velocity decreased, and it gained momentum.
D. Ball 2's velocity increased, and it gained some of ball 1's momentum.
Answers
Answer:
D
Explanation:
With the collision, obviously Ball 2's velocity increased, while Ball 1 slowed down a little bit due to the impact. This decrease in velocity caused a decrease in Ball 1's Momentum. Satisfying both conditions, option D is right.
Ammonia (NH3) reacts with oxygen to form nitrogen monoxide and water. All the materials involved in this reaction are gasses. 0.100 moles of each of the reactants are initially introduced to a 5.0-liter reaction vessel. a. What would be the quantity of each gas in the container upon completion of the reaction? b.What would be the partial pressure of each gas on the reaction vessel upon reaction completion if the temperature of the system is 105 degrees C? c. What is the total pressure of all the gases on the reaction vessel at 105 degrees C?
Answers
Answer:
a. 0.02 moles of NH₃, 0 moles of O₂, 0.08 moles of NO, 0.12 moles of H₂O
b. \(P_{NH_3}\) = 12,576.5 Pa, \(P_{NO}\) = 50,306.05 Pa, \(P_{H_2O}\) = 74,459.1 Pa
c. The total pressure is 138,341.64 Pa
Explanation:
a. NH₃ + O₂ → NO + H₂O
The balanced chemical equation is first found to be
4NH₃ + 5O₂ → 4NO + 6H₂O
Therefore, we have;
4 moles of NH₃ reacts with 5 moles of O₂ to form 4 moles of NO and 6 moles H₂O
Dividing by the reactant with the highest number of moles which is 5 moles of oxygen gives;
4/5 moles of NH₃ reacts with 5/5 moles of O₂ to form 4/5 moles of NO and 6/5 moles H₂O
Which is the same as 4/5 moles of NH₃ reacts with 1 mole of O₂ to form 4/5 moles of NO and 6/5 moles H₂O
Multiplying by 0.100 gives;
0.1×4/5 moles of NH₃ reacts with 0.1 mole of O₂ to form 0.1×4/5 moles of NO and 0.1×6/5 moles H₂O
The quantity of each gas in the container upon completion of the reaction is therefore;
(0.1 - 0.1×4/5) = 0.02 moles of NH₃
0 moles of O₂
0.08 moles of NO
0.12 moles H₂O
b. Given that the temperature = 105°C, we have;
PV = nRT
P = nRT/V
Where:
n = Total number of moles = 0.02 + 0.08 + 0.12 = 0.22 moles
R = Universal gas constant = 8.3145 J/(mol·K)
T = Temperature = 105°C = 378.15 K
V = Volume = 5 litre = 0.005 m³
P = 0.22×8.3145×378.15/0.005 = 138,341.64 Pa
From Dalton's law of partial pressure, we have;
Partial pressure Pₓ = Xₓ × P
Where:
Xₓ = Mole fraction
Which gives for ammonia NH₃ with 0.02 moles;
Mole fraction = 0.02/0.22 = 1/11
\(P_{NH_3}\) = 1/11 × 138,341.64 = 12,576.5 Pa
For the 0.08 moles of NO, we have
Mole fraction = 0.08/0.22 = 4/11
\(P_{NO}\) = 4/11 × 138,341.64 = 50,306.05 Pa
For the 0.12 moles H₂O
P = 0.12×8.3145×378.15/0.005 = 74,459.1 Pa
Mole fraction = 0.12/0.22 = 6/11
\(P_{H_2O}\) = 6/11 × 138,341.64 = 74,459.1 Pa
c. The total pressure = 12,576.5 Pa + 50,306.05 Pa + 74,459.1 Pa = 138,341.64 Pa.
What is the pH of a solution whose H + concentration is 4.0 10 –9?
Answers
Answer:
he pH of a solution is defined as the negative log10 [H+] ... 1 x 10-11. 11. Acidic Solution. 1 x 10-4. 4. 1 x 10-10. 10. 1 x 10-5. 5. 1 x 10-9.
Explanation:
m
A 35.0mL sample of 0.150 M acetic is titrated with 0.150 M NaOH solution. Calculate the pH after the following volumes of base have been added?
a) 0 mL b) 17.5 mLc) d) 35.0 mL e) 35.5 mL
Answers
The pH of the acetic acid solution after the addition of different volumes of NaOH solution are as follows:
a) pH = 2.87. Before any NaOH is added, the solution consists of 0.150 M acetic acid, which is a weak acid with a pKa of 4.76. At equilibrium, the concentrations of acetic acid and acetate ions are equal, and the pH can be calculated using the Henderson-Hasselbalch equation: pH = pKa + log([acetate]/[acetic acid]). Since no NaOH has been added yet, the concentrations of acetate and acetic acid are both equal to 0.150 M, so pH = 4.76 + log(0.150/0.150) = 2.87.
b) pH = 3.53. After adding 17.5 mL of NaOH solution, the concentration of acetic acid has decreased to 0.075 M, while the concentration of acetate ions has increased to 0.075 M. Using the Henderson-Hasselbalch equation with these new concentrations gives: pH = 4.76 + log(0.075/0.075) = 3.53.
c) pH = 9.09. After adding 35.0 mL of NaOH solution, all of the acetic acid has been converted to acetate ions. At this point, the solution consists of a 0.150 M acetate ion solution, which is the conjugate base of acetic acid. The pH of this solution can be calculated using the equation: pH = pKa + log([base]/[acid]). Since the pKa of acetic acid is 4.76, the pH of the solution is: pH = 4.76 + log(0.150/0) = 9.09.
d) pH = 9.28. After adding 35.0 mL of NaOH solution, there is still an excess of base in the solution. The pH can be calculated using the same equation as in part (c), but with the new concentration of acetate ions: pH = 4.76 + log(0.300/0) = 9.28.
e) pH = 9.35. After adding 35.5 mL of NaOH solution, the concentration of base is now greater than the concentration of acetate ions, resulting in a basic solution. The pH can be calculated using the equation: pH = 14.00 - pOH = 14.00 - (-log[OH-]) = 9.35.
To know more about acetic acid click here:
https://brainly.com/question/15202177
#SPJ11
Using the reaction of Calcium Chloride and Sodium Phosphate, what would be the limiting reactant if 15mL of 0.2SOM Calcium Chloride was combined with 15mL of 0.325M Sodium Phosphate? Show your calculations. Include the Balanced Chemical Equation, with phases. 2). The solubility of Barium Hydroxide is listed in the solubility table as 5.60g of Barium Hydroxide Octahydrate at 15°C in 100mL of Water. Show your work for the following calculations. Give all values to three significant figures. How many moles of Barium Hydroxide is this? How many grams of Barium Hydroxide can dissolve in 100mL of water at 15°C? What is the molarity of Barium lons? What is the molarity of Hydroxide ions?
Answers
The limiting reactant is Calcium Chloride, and Barium Hydroxide's molarity is 0.11M with a solubility of 5.60g in 100mL of water at 15°C.
1. The limiting reactant in the reaction between Calcium Chloride and Sodium Phosphate would be Sodium Phosphate, as it has a lower number of moles.
The balanced chemical equation for the reaction is: \(CaCl_2\)(aq) + \(Na_3PO_4\)(aq) → \(Ca_3(PO_4)_2\)(s) + 6NaCl(aq).
The calculation for the limiting reactant can be shown as follows:
Moles of \(CaCl_2\) = (0.2 mol/L) x (0.015 L) = 0.003 mol
Moles of \(Na_3PO_4\) = (0.325 mol/L) x (0.015 L) = 0.004875 mol
Therefore, \(Na_3PO_4\) is the limiting reactant.
2. The molar mass of Barium Hydroxide Octahydrate is 315.46 g/mol.
Moles of Barium Hydroxide Octahydrate that can dissolve in 100mL of water at 15°C = (5.60 g) / (315.46 g/mol) = 0.0178 mol
Grams of Barium Hydroxide that can dissolve in 100mL of water at 15°C = 5.60 g
Molarity of Barium ions = (0.0178 mol) / (0.1 L) = 0.178 M
Molarity of Hydroxide ions = 2 x (0.0178 mol) / (0.1 L) = 0.356 M.
Learn more about the limiting reactant at
brainly.com/question/1366311
#SPJ4
which point marks the highest temperature and pressure at which it's possible to determine whether a sample of pure x is a liquid or a gas?
Answers
The point that marks the highest temperature and pressure at which it's possible to determine whether a sample of pure x is a liquid or a gas is called the critical point.
At the critical point, the substance can no longer be compressed into a liquid and expanding it does not result in vaporization. At this point, the temperature and pressure are at their highest and are called the critical temperature and critical pressure, respectively.
The critical point marks the boundary between the liquid and gas phases, and any further increase in pressure or temperature will result in a single homogeneous phase. The critical temperature and pressure are unique properties of each substance and can be used to identify and differentiate between different materials.
Learn more about critical point here: https://brainly.com/question/7805334
#SPJ4
a 1.5 m solution of nacl has a volume of 0.534 l. if this is diluted to 0.80 m, what will be the final volume?
Answers
Dilution formula is: M conc . Vol conc = M diluted . Vol diluted
1.5 M . Vol conc = 0.80 M . 0.10L
Vol conc = 0.80 M . 0.10L / 1.5M = 0.053L
Does NaCl produce a solution?When water molecules push the ions apart, the ionic bond holding sodium and chloride ions together is destroyed. The water molecules surround the sodium and chloride atoms in this image after the salt compounds have been separated. The salt then starts to dissolve and turns into a homogeneous solution.
0.9% sodium chloride Injection, USP is a sterile, nonpyrogenic, isotonic sodium chloride and water solution for injection. Each mL of solution contains 9 mg of sodium chloride. It is only offered in single-dose vials and doesn't contain any bacteriostats, antibacterial agents, or extra buffers. Drugs for injection are diluted or dissolved using it.
learn more about water molecules
https://brainly.com/question/1313076
#SPJ1
Help plz (will mark brainleist)

Answers
Answer:
energy disturbance easy
PLEASE HELP!!!!! NEED HELP ASAP!!!!!
Write the chemical formula for the covalent compounds below
EXAMPLE- dinitrogen trioxide= N2O3
nitrogen monoxide =
dihydrogen oxide =
diphosphorus pentoxide =
sulfur trioxide =
Answers
Answer:
down below
Explanation:
NO
H20
P4O10
SO3
1. NO
2. H2O
3. P4O10
4. SO3
How many moles of potassium chloride (KCl) can be produced by the decomposition of 4.0 mol of potassium chlorate (KClO3).
Answers
It takes 4 moles of potassium chlorate to make 6 moles of oxygen gas.
How many moles of KCl are generated from the breakdown of one mole of KClO3?Due to the fact that there will only be 1 chemical present, this indicates that approximately 2 moles of KCL03 will disintegrate. It will break down into two moles of KCL and three moles of oxygen.
How many grammes of oxygen gas are created when 5.00 moles of potassium chlorate are broken down?We can observe that three moles of oxygen are created from two moles of potassium chlorate. As a result, we can say that 1.9684 g of oxygen will be created during the entire breakdown of 5 g of $KClO 3$.
To know more about potassium chlorate visit:-
brainly.com/question/488887
#SPJ1
A fundamental equation of thermodynamics, the Gibbs-Helmholtz equation, is a linear equation that relates free energy change, AG, to absolute temperature, T. The equation is AG = AH -TAS, where AH is enthalpy change and AS is entropy change. Using the above equation, find AG at 400 K for a reaction in which AH = 61.0 kcal and AS = 0.020 kcal/K. 7. A cost equation is known to be y = 10x + 250, where x is the number of units produced and y is the cost in $. Find the total cost of producing 5 000 units. Round your answer to four significant digits (SD).
Answers
At 400 K, the free energy change (ΔG) for the reaction is 53.0 kcal. The total cost of producing 5,000 units is $50,250.
To find ΔG at 400 K using the Gibbs-Helmholtz equation, we need to substitute the given values of ΔH and ΔS into the equation.
ΔG = ΔH - TΔS
Given;
ΔH = 61.0 kcal
ΔS = 0.020 kcal/K
T = 400 K
Substituting these values into the equation;
ΔG = 61.0 kcal - (400 K)(0.020 kcal/K)
ΔG = 61.0 kcal - 8.0 kcal
ΔG = 53.0 kcal
Therefore, at 400 K, the free energy change (ΔG) for the reaction is 53.0 kcal.
To find the total cost of producing 5,000 units using the cost equation y = 10x + 250, we need to substitute x = 5,000 into the equation.
y = 10x + 250
Given;
x = 5,000
Substituting x = 5,000 into the equation:
y = 10(5,000) + 250
y = 50,000 + 250
y = 50,250
Therefore, the total cost of producing 5,000 units is $50,250.
To know more about free energy change here
https://brainly.com/question/32317964
#SPJ4
How much heat is released or absorbed in the reaction of 10.0 grams of SiO2 (quartz) with excess hydrofluoric acid?SiO2(s) + 4HF(aq) → SiF4(g) + 2H2O (kJ/mol) −910.9 −320.8 −1615 −285.8a. 1.25 kJ absorbedb. 1.25 kJ releasedc. 11.3 kJ absorbedd. 11.3 kJ releasede. 6.56 kJ released
Answers
The correct answer is d. 11.3 kJ released, which is the value obtained by rounding the actual heat of hydrofluoric acid released to two significant figures.
Based on the given thermochemical equation, the reaction releases 910.9 kJ/mol of SiO2 reacted. To determine the amount of heat released or absorbed in the reaction of 10.0 grams of SiO2 with excess hydrofluoric acid, we need to first convert the mass of SiO2 to moles.
Molar mass of SiO2 = 60.08 g/mol
10.0 g SiO2 ÷ 60.08 g/mol = 0.166 mol SiO2
Since the reaction ratio between SiO2 and heat is 1 mol SiO2 : 910.9 kJ, we can calculate the amount of heat released or absorbed by multiplying the number of moles of SiO2 by the heat released per mole:
0.166 mol SiO2 x 910.9 kJ/mol = 151.2 kJ
The heat released in the reaction of 10.0 grams of SiO2 with excess hydrofluoric acid is 151.2 kJ. However, the answer choices do not match this value exactly. The closest answer choice is e. 6.56 kJ released. This answer is incorrect, as it is much lower than the actual heat released in the reaction.
learn more about hydrofluoric acid here:
https://brainly.com/question/24194581
#SPJ11
Option 1: Individual Activity
Clear the coordinates you currently have on the interactive map from Part E by clicking on the red X symbol in the coordinates menu. Directly below it, click on the Add Coordinates button. Enter the coordinates N 35°00’ 00”, latitude and E 33°00’ 00” longitude. Then zoom out so that you can see the entire country where these coordinates are located. What is the name of the country and the body of water that surrounds it?
Option 2: Group Activity
Using the interactive online map from parts D and E, locate a country that you have never been to but would like to visit. Without the other members of your group watching, type in the name of this place in the address box. Then write down only its latitude and longitude coordinates on a piece of paper. Next, trade your paper with a partner who has also looked up and written down the coordinates of a place. Using your partner’s coordinates and the online map, locate the country your partner would like to visit. Do this by clicking the Add Coordinates button below the address and coordinates boxes and then entering your partner’s coordinates.
Check with your partner to make sure you got the location right. After you determine the location your partner would like to visit, write the coordinates and the name of the country in the answer space.
Answers
The country located at coordinates N 35°00' 00", latitude and E 33°00' 00" longitude is Cyprus and is surrounded by the Mediterranean Sea. The country I would like to go to would be the one located at coordinates N 41°89'01" E 12°49'22".
What is a geographic coordinate?Geographic coordinates is a term that refers to a reference system that has the objective of identifying a point located on Earth using a system of numbers, letters or symbols.
The geographical coordinates take as reference points the Greenwich meridian (longitude 0°) and the line of the equator (latitude 0°). On the other hand, longitudes range from 0° to -180° or 180° and latitudes range from 0° to 90° or -90°.
According to the above, it can be inferred that the location of the coordinates N 35°00' 00", latitude and E 33°00' 00" longitude is the country of Cyprus surrounded by the Mediterranean Sea.
Learn more about geographic coordinates in: https://brainly.com/question/10760270
P4(s) + Cl₂(g) → PCI3(l) + energy 4. Calculate the gram-formula mass of the product. Show all work. g/mol
Answers
The gram formula mass of the product, PCl₃, is 137.5 g.
What is the gram formula mass of a compound?The amount of a compound that has the same mass in grams as the formula mass in an atomic mass unit is said to have the compound's gram formula mass. It is also known as the molar mass.
Every element's atom has a characteristic mass, and each compound's molecule has a characteristic mass determined by the compound's formula.
The gram formula mass of the product PCl₃ is calculated below:
The atomic mass of P = 31 g
The atomic mass of Cl = 35.5 g
The gram formula mass of PCl₃ = 31 + 35.5 * 3
The gram formula mass of PCl₃ = 137.5 g
Learn more about gram formula mass at: https://brainly.com/question/490195
#SPJ1
two types of fine chemical
Answers
Answer:
Flavors and fragrances
how much heat is evolved when 27.0 g of glucose is burned according to this equation? c6h12o6 6o2 → 6co2 6h2o; δ h comb. = -2808 kj/mol. a. 421 kJ
b. 241 kJ
c. 280 kJ
d. 136 kJ
Answers
The amount of heat evolved when 27.0 g of glucose is burned according to the equation is (A) 421 kJ.
The balanced chemical equation for the combustion of glucose is
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O.
To determine the amount of heat evolved when 27.0 g of glucose is burned, we need to use the molar mass of glucose and the given heat of combustion (ΔHcomb) in kJ/mol. The molar mass of glucose (C₆H₁₂O₆) can be calculated as follows:
Molar mass of C = 12.011 g/mol
Molar mass of H = 1.008 g/mol
Molar mass of O = 15.999 g/mol
Molar mass of glucose = (6 x 12.011) + (12 x 1.008) + (6 x 15.999) = 180.156 g/mol
So, 27.0 g of glucose is equal to 27.0/180.156 = 0.1499 mol of glucose.
The amount of heat evolved when 1 mol of glucose is burned is ΔHcomb = -2808 kJ/mol.
So, the amount of heat evolved when 0.1499 mol of glucose is burned is:
ΔHcomb x 0.1499 mol = -2808 kJ/mol x 0.1499 mol = -420.8 kJ (rounded to one decimal place)
Therefore, the amount of heat evolved when 27.0 g of glucose is burned is 420.8 kJ. However, since the answer choices are given in kJ and not in kJ/mol, we need to round the answer to the nearest whole number. The correct answer is therefore (a) 421 kJ.
Learn more about heat of combustion here: https://brainly.com/question/14086312
#SPJ11
Is Palladium an elementary substance or a compound?
Answers
Answer:
Elementary substance, since it is in the periodic table of elements.
Hope that helps!
-SCSB
Explanation:
A sample of carbon dioxide gas occuples a volume of 2.5L at standard temperature and pressure (STP). What will be the volume of a sample of argon gas that has the same number of moles and pressure but twice the absolute temperature?
Answers
Answer:
The correct answer is 5.0 L
Explanation:
STP are defined as T=273 K and P= 1 atm
By using the ideal gas equation, we can calculate the number of moles (n) of the gas at a volume V=2.5 L:
PV= nRT
⇒n= (PV)/(RT) =(1 atm x 2,5 L)/(0.082 L.atm/K.mol x 273 K)= 0.112 mol
For a sample of argon gas, with the same number of moles (0.112 mol) but twice the temperature (T = 273 K x 2= 546 K):
V= (nRT)/P = (0.112 mol x 0.082 L.atm/K.mol x 546 K)/1 atm = 5.0 L
That is consistent with the fact that when a gas is heated, it expanses. So, if the temperature increases twice, the volume also increases twice.
If rates of nitrogen fixation increased tenfold in aquatic ecosystems, would you expect a tenfold increase in primary productivity? Yes or No
Answers
The given statement " If rates of nitrogen fixation increased tenfold in aquatic ecosystems, would you expect a tenfold increase in primary productivity" is true because because nitrogen is a limiting nutrient in terrestrial ecosystems.
In the terrestrial ecosystem the nitrogen is the most important limiting nutrient. The Nitrogen gas is the one of the major abundant gas in the atmosphere that occupying 78 % by the volume. Nitrogen plays the important role in the controlling the aquatic and the terrestrial ecosystem. The nitrogen and the phosphorus are the most common limiting nutrient in the aquatic ecosystem.
Therefore, the presence of the high amount of the nitrogen in the atmosphere will leads to the release of the large amount of pollutants.
To learn more about ecosystem here
https://brainly.com/question/13550229
#SPJ4
When refering to a gas, the higher the temperature, the faster the particles move:
True
False
Answers
Answer:
TrueExplanation:
In Gas,The movement of particles depends upon their temperature. When, the temperature is high the movement of the particles is faster.When, the temperature is low the movement of the particles is slower down and move close together.Hence,
The above statement is true for, the gas particles moves faster in high temperature.
What is the molar mass of AI(CIO4)3?
Answers
Answer:
Molar mass is 325.33
Explanation:
Molar mass is 325.33

If 6.73 g of Na2CO3 is dissolved in enough water to make 250.0 mL of solution, what is the molarity of the sodium carbonate solution
Answers
If 6.73 g of Na2CO3 is dissolved in enough water to make 250.0 mL of solution. The molar concentration of sodium carbonate will be 0.254 M.
Molarity is defined as the concentration of solute or a substance in given volume of a solution. The data guven in the question is as follows,
mass of sodium carbonate = 6.73 g
volume of solution =250 mL
Molar mass of sodium carbonate = 105.99 g/mol
So the molarity can be calculated as:
M =\(\frac{n}{V}\)
=\(\frac{6.73}{250}\) × \(\frac{1}{105.99}\) ×\(\frac{1000ml}{1L}\)
=0.254M Na₂CO₃
Thus, molarity of sodium carbonate is 0.254 M.
The dissociation of sodium carbonate in the ions, we get:
Na₂CO₃→ 2Na⁺ + CO₃⁻²
From the above equation, it can be interpreted as there is 1:2 mol ratio between sodium carbonate and sodium ion. The molarity of sodium will be:
Na⁺ =2×0.245=0.508 M
Also, it is seen that there is 1:1 mol ratio between sodium carbonate and carbonate ion, therefore, its molarity will be:
CO₃⁻² = 0.245M
Thus, the molarity of the sodium carbonate will be 0.254 M.
To look more about molarity click here
brainly.com/question/8732513
#SPJ4
a flask of an unknown gas with a pressure of 759 torr was attached to an open-end manometer. the mercury level was 2.4 cm higher at the open end than at the flask end. the atmospheric pressure when the gas pressure was measured was atm. report your answer to the hundredths place.
Answers
The atmospheric pressure when the gas pressure was measured is approximately 0.99 atm.
To determine the gas pressure inside the flask, we need to consider the pressure difference between the gas and the atmospheric pressure. The pressure difference can be determined by measuring the height difference of the mercury levels in the open-end manometer.
Pressure inside the flask (P_gas) = 759 torr
Height difference in the manometer (h) = 2.4 cm
The pressure difference between the gas and the atmospheric pressure can be calculated using the equation:
P_gas - P_atm = ρgh
Where:
P_atm is the atmospheric pressure
ρ is the density of mercury (13.6 g/cm³)
g is the acceleration due to gravity (9.8 m/s²)
h is the height difference in meters
First, we need to convert the height difference from centimeters to meters:
h = 2.4 cm = 0.024 m
Substituting the given values into the equation, we have:
759 torr - P_atm = (13.6 g/cm³ * 0.024 m * 9.8 m/s²)
Simplifying the equation, we can convert grams to kilograms and cancel out the units:
759 torr - P_atm = (0.3264 kg/m² * 9.8 m/s²)
To convert torr to atm, we divide by 760:
0.998 - P_atm = 0.3264 * 9.8 / 760
0.998 - P_atm = 0.0042
P_atm = 0.998 - 0.0042
P_atm = 0.9938 atm
Therefore, the atmospheric pressure when the gas pressure was measured is approximately 0.99 atm.
To know more about gas visit:
https://brainly.com/question/24719118
#SPJ11
PLEASE HEP QUICK!! I'M BEING TIMED!!
1. _____ - the phase change in which a substance changes from a liquid into a gas.
25 points
Vaporization
Condensation
Heat of vaporization
Evaporation
2. _____ - the process that changes a substance from a liquid to a gas at temperatures below the substances boiling point.
25 points
Heat of vaporization
Vaporization
Evaporation
Condensation
3. _____ - the energy a substance must absorb in order to change from a liquid to a gas.
25 points
Vaporization
Evaporation
Heat of vaporization
Condensation
4. The arrangement of molecules in water becomes less orderly as water melts and _____ orderly as water freezes.
25 points
more
less
Other:
Answers
Answer:
Evaporation, conensation, Heat of vaporization, more
Explanation:
im pretty sure this is correct
Answer: create a question.
Explanation:
what is the balance of S8+Br2=S3Br7
Answers
Answer:
3S₈ + 28Br₂ => 8S₃Br₇
Explanation:
Start with either sulfur (S) or bromine (Br) and balance ...
3S₈ + Br₂ => 8S₃Br₇ or S₈ + 7/2Br₂ => S₃Br₇
Balance the remaining reactant ...
3S₈ + 56/2Br₂ => 8S₃Br₇
Remove fractions by multiplying by the fraction's denominator
2(3S₈ + 56/2Br₂ => 8S₃Br₇) => 6S₈ + 56Br₂ => 16S₃Br₇
Reduce to smallest whole number ratio => standard equation at STP ...
3S₈ + 28Br₂ => 8S₃Br₇
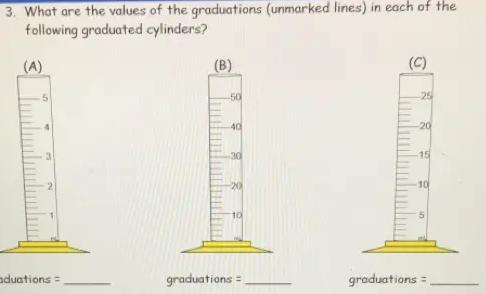
3. What are the values of the graduations (unmarked lines) in each of the
following graduated cylinders?
Q
(A)
50
3
graduations =
(B)
50
-40
-30
-20
10
graduations =
(C)
25
-20
-15
10
5
graduations =
Answers
The values of the Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
Graduated Cylinder - A popular piece of scientific equipment used to measure the volume of a liquid is a graduated cylinder, sometimes referred to as a measuring cylinder or mixing cylinder. Its form is slender and cylindrical. Each marked line on the graduated cylinder indicates the volume of liquid that has been measured.
The clear graduated cylinder is widely used for volume measurements and is regarded to be more accurate than a beaker since it contains permanently marked incremental graduations.
To calculate the value of graduations-
1. Subtract the two values to get the value between the labeled graduations.
2. Determine how many spaces there are between the two graduations. Keep in mind that volume equals space.
3. Divide the number of gaps by the value between the graduations.
For cylinder A.
2 ml- 1ml = 1ml
Number of spaces = 5
∴1/ 5 = 0.2
Graduation = 0.2ml
For cylinder B -
20-10 = 10
number of spaces = 5
∴10/ 5 =2
Graduation = 2ml
For cylinder C-
10-5 = 5
Number of spaces= 5
∴5/5 = 1
Graduation = 1ml
Hence , Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
To learn more about graduated cylinders refer-https://brainly.com/question/26216613
#SPJ9
Your question is incomplete. Please find the missing image below.
True or False? You can observe physical properties of matter without changing the identity of the substance
Answers
true
Germanium has ______ valence electrons and chlorine has ____ valence electrons
Answers
Answer:
Germanium has 4 valence electrons and chlorine has 7 valence electrons.
Explanation:
last shell of chlorine atom has 7 electrons in it. Therefore, there are 7 valence electrons present in an chlorine atom.
Solid state electronics arises from the unique properties of silicon and germanium, each of which has four valence electrons and which form crystal lattices in which substituted atoms (dopants) can dramatically change the electrical properties.
If the half-life of a radioactive substances is 590 million years and you have 40 atoms of it, how many half-lives will have passed when 5 atoms remain
Answers
Answer:
3
Explanation:
Applying,
\(2^{n'}\) = R/R'............... Equation 1
Where n' = number of halflives that have passed, R = Original atom of the substance, R' = atom of the substance left after decay.
From the question,
Given: R = 40 atoms, R' = 5 atoms
Substitute these values into equation 1
\(2^{n'}\) = 40/5
\(2^{n'}\) = 8
\(2^{n'}\) = 2³
Equation the base,
n' = 3
1. A student in lab measures 4.6 grams of copper for an experiment. Upon further analysis he determines that he should have measured out 4.7 grams of copper. What is his percent error?
Answers
Answer:
\(2.13\%\)
Explanation:
Quantity of copper measured by a student = 4.6 grams
Original quantity of copper = 4.7 grams
Error in measurement = Original quantity of copper - Quantity of copper measured by a student \(=4.7-4.6=0.1\) grams
To find the percent error, apply the following formula:
Percent error = (Error in measurement / Original quantity of copper) × 100
\(=\frac{0.1}{4.7}(100)=2.13\%\)