Sasha pulls a block across the floor at a constant speed. Why doesn't the motion of the block change even though Sasha is pulling on it?
Answers
Sasha pulls a block across the floor at a constant speed. The motion of the block does not change even though Sasha is pulling on it because of inertia. Inertia is the property of an object to resist changes in its motion.
Once the block is set in motion, it will continue to move at a constant speed in a straight line unless acted upon by an unbalanced force. Sasha’s pulling on the block provides the unbalanced force necessary to overcome the force of friction between the block and the floor. Once the block is moving at a constant speed, the force of friction is balanced by the force applied by Sasha. Therefore, the block continues to move at a constant speed.
The block would only change its motion if an unbalanced force acts on it. An unbalanced force is a force that is not opposed by another force of equal magnitude and in the opposite direction. For example, if Sasha were to push the block harder or in a different direction, the block’s motion would change.
Similarly, if the force of friction between the block and the floor were to increase, the block’s motion would change. In summary, Sasha’s pulling on the block at a constant speed does not change the block’s motion because of the property of inertia.
To learn more about speed visit;
https://brainly.com/question/17661499
#SPJ11
Related Questions
2H2+O2 - 2H2
What will be the volume of water vapor produced when 8 grams of H2 reacts at STP
Answers
2H₂ + O₂ ⇒ 2H₂ for this reaction the volume of water vapor produced when 8 grams of H₂ reacts at STP is 179.2 Litre.
What do you mean by STP ?The standard temperature and pressure (STP) adverts to the nominal conditions in the atmosphere at sea level. These conditions are 0 degrees Celsius and 1 atmosphere (atm) of pressure.
The formula for STP can be represented as, VP = nRT.
"P" is pressure, "V" is volume, n is the number of moles of a gas, "R" is the molar gas constant and "T" is temperature.
Given:
Number of mole = 8 grams
8 g of hydrogen = 8 × 1
= 8 mol.
At STP 1 mole H₂ occupies 22.4 L volume.
Therefore, 8 moles of hydrogen occupy = 8 × 22.4
= 179.2 L.
Thus, the volume of water vapor produced when 8 grams of H₂ reacts at STP is 179.2 Litre.
To learn more about the STP, follow the link;
https://brainly.com/question/26107741
#SPJ2
What is the molarity of a solution prepared by dissolving 20.0 grams of naoh in sufficient water to make a solution with a total volume of 2.40 liters? 0.120 m naoh 0.208 m naoh 0.416 m naoh 0.833 m naoh
Answers
The molarity of the solution is 0.833 M NaOH.
To calculate the molarity (M) of a solution, you need to determine the number of moles of solute (in this case, NaOH) and divide it by the volume of the solution in liters.
First, convert the given mass of NaOH to moles using its molar mass. The molar mass of NaOH is 22.99 g/mol for Na, 16.00 g/mol for O, and 1.01 g/mol for H. So, the molar mass of NaOH is 22.99 + 16.00 + 1.01 = 40.00 g/mol.
Number of moles of NaOH = 20.0 g / 40.00 g/mol = 0.500 mol.
Next, divide the number of moles by the volume of the solution in liters:
Molarity (M) = 0.500 mol / 2.40 L = 0.208 M.
Therefore, the molarity of the solution prepared is 0.208 M NaOH.
To know more about "Molarity" refer here:
https://brainly.com/question/12123167#
#SPJ11
For the following question, choose TWO answers. Which question should be asked to determine if the reaction supports the Brønsted- Lowry model of acids and bases?
Answers
According to the Brønsted-Lowry model, an acid and its conjugate base differ by a single proton, while a base and its conjugate acid differ by the addition of a single proton. If a reaction produces conjugate acid-base pairs, it supports this model.
To determine if the reaction supports the Brønsted-Lowry model of acids and bases, there are several questions that could be asked. Two possible questions are:
1. Does the reaction involve the transfer of protons (H+ ions) from one molecule to another.This is a key concept in the Brønsted-Lowry model, which defines acids as proton donors and bases as proton acceptors. If a reaction involves the transfer of protons, it supports this model.
2. Do the reactants and products contain conjugate acid-base pairs.
for more such questions on Brønsted-Lowry model :
https://brainly.com/question/20308475
#SPJ11
Answer:
1. Did an acid donate a hydrogen ion to become a conjugate base?
2. Did a base accept a hydrogen ion to become a conjugate acid?
what is the oxidation state of an individual carbon atom in caco3 ? express the oxidation state numerically (e.g., 1).
Answers
The oxidation state of an individual carbon atom in CaCO3 is +4. The oxidation state of an individual carbon atom in compounds is often determined by analyzing the compound's structure. In the case of CaCO3, the compound is composed of a carbon atom, two oxygen atoms, and a calcium atom. The oxygen atoms are electronegative and will pull electrons away from the carbon atom. As a result, the carbon atom will be oxidized.
In this compound, there are three oxygen atoms (-2 each) and one calcium atom (+2). To calculate the oxidation state of the carbon atom, we can use the following formula:
Oxidation state of C + 3 × oxidation state of O + oxidation state of Ca = 0
Let's plug in the values we know:
Oxidation state of C + 3 × (-2) + (+2) = 0
Simplifying this equation gives us:
Oxidation state of C = +4
Therefore, the oxidation state of the individual carbon atom in CaCO3 is +4.
To know more about oxidation visit :
https://brainly.com/question/13182308
#SPJ11
For each of the following pairs of compounds, choose which will elute faster in a TLC experiment (i. E. , which compound will have a larger Rf value). Explain what factors led to your choice. 6 pt a. Naphthalene or 1-Bromonaphthalene Choice Explanation: 1-Bromonaphthalene is more polar than Naphthalene. If polarity is higher, its Rf value will be less which means that molecule will travel less distance (lower Rf value) during a TLC experiment
Answers
In a TLC experiment, the compound with the larger Rf value will elute faster. In the case of naphthalene and 1-bromonaphthalene, the 1-bromonaphthalene will elute faster and have a larger Rf value.
This is because 1-bromonaphthalene is more polar than naphthalene. Polar compounds have a stronger attraction to the polar stationary phase (such as the silica gel in TLC plates) and will interact more with it, resulting in a lower Rf value.
Naphthalene, on the other hand, is less polar and will have a weaker interaction with the stationary phase, allowing it to travel further and have a higher Rf value.
The polarity of a compound is determined by the presence of functional groups or atoms that create an uneven distribution of charge or electronegativity. In this case, the bromine atom in 1-bromonaphthalene increases its polarity compared to naphthalene, leading to a stronger interaction with the stationary phase.
In summary, the 1-bromonaphthalene will elute faster in a TLC experiment and have a larger Rf value compared to naphthalene due to its higher polarity resulting from the presence of a bromine atom.
To know more about electronegativity refer to this:
https://brainly.com/question/3393418
#SPJ11
List these compounds in order of increasing moles of molecules 2. 0 g of CH4O; 2. 0 g of H2O; and 2. 0 g of CHO molecules
Answers
Using the specified mass and each compound's respective molecular weights, we can compare the number of moles of each compound:
CH4O: Methyl alcohol has a molecular weight of about 32 g/mol.The formula for calculating the number of moles of CH4O is (mass of CH4O) / (molecular weight of CH4O): 2.0 g / 32 g/mol = 0.0625 moles.
H2O: H2O (water) has a molecular weight of about 18 g/mol.H2O's mass divided by its molecular weight yields the number of moles: 2.0 g divided by 18 g/mol, or 0.111 moles.
CHO: CHO (formaldehyde) has a molecular weight of about 30 g/mol.The formula for calculating the number of moles of CHO is (mass of CHO) / (molecular weight of CHO): 2.0 g / 30 g/mol = 0.067 moles.Consequently, the chemicals are CH4O CHO H2O in ascending molecular weight order.
learn more about compound here:
https://brainly.com/question/23654396
#SPJ11
carbon skeletons for amino acid biosynthesis are supplied by intermediates of the citric acid cycle (see the figure). which intermediate would you predict may directly supply the carbon skeleton for synthesis of a five-carbon amino acid?
Answers
The predicted intermediate that can directly supply the carbon skeleton for the synthesis of a five-carbon amino acid, is a-ketoglutarate. Correct answer: letter D.
This is because a-ketoglutarate is an intermediate of the Krebs cycle, and as such can directly supply the carbon skeleton for the synthesis of a five-carbon amino acid.
What is a-ketoglutarate?Five-carbon amino acids are essential for the synthesis of proteins in the body. a-Ketoglutarate is a key intermediate in the Krebs cycle that can directly supply the carbon skeleton for the synthesis of a five-carbon amino acid.
This makes a-ketoglutarate an important molecule for the synthesis of proteins in the body.
Which intermediate would you predict may directly supply the carbon skeleton for synthesis of a five-carbon amino acid?
Group of answer choices:
A) succinate
B) malate
C) cotrate
D) a-ketoglutarate
E) isocitrate
Learn more about amino acids:
https://brainly.com/question/2526971
#SPJ4
Which combination of elements would form an ionic bond?
Oxygen and sulfur
fluorine and iron
calcium and sodium
carbon and bromine
Answers
true or false; some organisms are not affected by any abiotic factors
Answers
Answer:
True
Explanation:
Answer:
False
Explanation:
pls help with give brainiest Which statement best explains why the sun's

Answers
What volume would the 1.75 moles of CO₂ occupy at STP?
Answers
Answer:
39.2
Explanation:
1.75 moles × 22.4 liters = 39.2
How have humans influenced the Antarctic tundra ecosystem?
Humans have moved into the area and cleared out several plants and trees.
Humans have increased the greenhouse gasses found in the atmosphere.
Humans have adapted to live in colder areas and moved into the Antarctic tundra.
Humans have hunted several species that are only found in the Antarctic tund.
Answers
Answer: b
Explanation:
if the co2 level in air rises to 0.700% (by volume), what is its partial pressure if the air pressure is 760 mmhg?
Answers
The mole fraction of carbon dioxide in the mixture is
P(CO₂) = 0.00004 × 76mmHg
= 0.304mmHg
The key to this problem is the fact that each component of a gas mixture contributes to the total pressure exerted by the mixture in proportion to the number of molecules in the mixture.
Partial pressures of gases are most often expressed as mole fractions.
P(gas) = χ(gas) × P(mixture)
This is the proportional to the number of molecules.
As you know, 1 mole of a substance is exactly 6.022⋅10²³
molecules of that substance. This is known as the Avogadro number Nₐ.
This means that the number of molecules, say x and Avogadro's number
No. can be used to express the number of moles of a gas.
From a mole = Number of Molecules × Nₐ
Now, the percentage composition of a gas mixture is the number of molecules each gas contributes to 100 molecules of the mixture.
In this case, the air must contain 0.04% carbon dioxide. This means that there are 0.04 CO₂ molecules in 100 air molecules.
For example, the number of moles of carbon dioxide in 100 air molecules is ⁿCO² = 0.04 molecules × Nₐ
= 0.04 Nₐ
This air sample is
n (total) = 100 molecules × Nₐ
= 100⋅Nₐ
This means that the mole fraction of carbon dioxide in the mixture is
P(CO₂) = 0.00004 × 76mmHg
= 0.304mmHg
Learn more about Carbon Dioxide:
https://brainly.com/question/3049557
#SPJ4
How many moles are there in 12.5 g of methanol, CH3OH?
A) 0.0326 mol
B) 0.39 mol
C) 69.0 mol
D) 30.7 mol
Answers
Answer:
B (0.39 mol).
Explanation:
Hope this helped!
2. In the production of ammonia, what mass of ammonia can be produced if 1 mole of hydrogen gas is used?
3. If 80g of sodium hydroxide react with aluminum chloride, how many moles of sodium chloride will be produced?
Answers
In recent years, the output of ammonia has remained mostly steady. Ammonia output was projected to reach 150 million metric tonnes worldwide in 2022. With over 64.6 million metric tonnes produced, East Asia has the largest ammonia output.
How does NaOH produce sodium?Sodium metal is produced via the Castner method, which involves electrolyzing molten sodium hydroxide at a temperature of around 330 °C. At that temperature, the molten sodium would begin to dissolve in the melt; below that temperature, the liquid would harden.
One mole (or one gramme) of sodium chloride (NaCl) has a molecular weight of 58.44, making 58.44g the molecular weight of one gramme. You may create 1M NaCl by dissolving 58.44g of sodium chloride in a final amount of 1 litre.
learn more about ammonia
https://brainly.com/question/14854495
#SPJ1
How can you tell the difference between two clear liquids
Answers
Answer:
To identify a pure liquid substance using the physical properties of solubility, density, and boiling point. The physical properties of a pure substance can be measured without changing the composition of the substance.
Explanation:
What form of chemical weathering is responsible for breaking the serpentinite down on Ruby Jones Hall
Answers
The form of chemical weathering that is responsible for breaking the serpentinite down on Ruby Jones Hall is hydrolysis.
Hydrolysis is a type of chemical weathering that occurs when minerals in rocks react with water and create new compounds as a result. It is particularly important in the weathering of silicate minerals, including the serpentinite found on Ruby Jones Hall. During hydrolysis, water molecules split into hydrogen and hydroxide ions and then react with the minerals. This reaction alters the minerals and creates new ones, often resulting in a softer, weaker rock that is more easily eroded. The process of hydrolysis breaks down the serpentinite on Ruby Jones Hall. Serpentinite is a rock made primarily of the mineral serpentine.
Serpentine is a magnesium-rich mineral that is susceptible to hydrolysis because it reacts readily with water to form other minerals. When water reacts with serpentine, it breaks down the mineral and produces new minerals, including clay minerals like kaolinite and smectite. These new minerals are much softer and more easily eroded than the original serpentine, which is why serpentinite is often found in areas with high rates of weathering and erosion.
To know more about hydrolysis refer to:
https://brainly.com/question/30718479
#SPJ11
A new chemistry teacher arrives in her new room and discovers that the room was left in total chaos by her predecessor. While organizing the room, she finds a box on a shelf in the prep room labeled "Electrochemistry demonstration". The box contains some metal strips, and a solution in a bottle. The metal is labeled "Metal E" and the solution is labeled "1 M nitrate salt of E". In an attempt to organize the activity, she looks throughout the room for similar samples. She manages to find samples of metals and their nitrate solutions labeled A, B, C and D in addition to the original sample. In a file cabinet, she finds a paper labeled "Electrochemistry Activity" which lists five metals: zinc, aluminum, magnesium, silver and tin She is not able to find anything that identifies which metals correspond to which letters. She makes some quick observations, summarized below.
Answers
Answer:
D in addition to the original sample. In a file cabinet, she finds a paper labeled "Electrochemistry Activity" which lists five metals: zinc, aluminum, magnesium, silver and tin She is not able to find anything that identifies which metals correspond to which letters. She makes some quick observations, summarized below.
Explanation:
84.9 g of solid iron reacts with oxygen gas forming iron(III) oxide. How many moles of oxygen will react
Answers
Answer:
1.14 moles of oxygen will react
Explanation:
The balanced equation of reaction between iron and oxygen is
4 Fe + 3 O₂ → 2 Fe₂O₃
By stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of each compound participate in the reaction:
Fe: 4 molesO₂: 3 molesFe₂O₃: 2 molesThe molar mass of iron being Fe 55.85 g/mole, then the following amount of mass reacts by stoichiometry: 4 moles* 55.85 g/mole=223.4 g
You can apply the following rule of three: if by stoichiometry 223.4 grams of Fe react with 3 moles of O₂, 84.9 grams of Fe react with how many moles of O₂?
\(moles of O_{2} =\frac{84.9 grams of Fe*3 moles ofO_{2} }{223.4 grams of Fe}\)
moles of O₂= 1.14 moles
1.14 moles of oxygen will react
Which type of reaction is
NaOH + KNO3 NaNO3 + KOH
-->>
Answers
Answer:
Explanation:
NaOH + KNO3 --> NaNO3 + KOH. combustion. CH4 + 2 O2 --> CO2 + 2 H2O. single displacement. 2 Fe + 6 NaBr --> 2 FeBr3 + 6 Na. double displacement.
PLEASE SHOW WORK PLEASE !!!! need help
Question 7 Calculate the pH of 0.81 M Mg(OH)₂. Show your work to earn points. Use the editor to format your answer Question 8 Calculate the pH of 0.27 M solution of the pyridine (CsHsN; K=1.7 x 10%)
Answers
7. the pH of 0.81 M Mg(OH)₂ solution is 9.19.
8. the pH of 0.27 M pyridine solution is 9.11.
Mg(OH)₂ is a base which dissociates to produce two OH⁻ ions.
Mg(OH)₂ → Mg²⁺ + 2 OH⁻
Let the concentration of OH⁻ ions produced be x.
Therefore, the concentration of Mg²⁺ is 0.81-x
Mg(OH)₂ → Mg²⁺ + 2 OH⁻
Initial concentration (M) 0 0
Change (M) -x +2x
Equilibrium Concentration 0.81-x x x
Using Kb for Mg(OH)₂,Kb = Kw/Ka
Kw = 1.0 × 10⁻¹⁴ at 25 °C.
For Mg(OH)₂,Kb = [Mg²⁺][OH⁻]²/Kw= (x)²/0.81 - x
Kb = 4.5 × 10⁻¹² = x²/0.81 - x
On solving the equation,x = 7.7 × 10⁻⁶M
Therefore, the concentration of OH⁻ ions = 2 × 7.7 × 10⁻⁶ = 1.54 × 10⁻⁵ M
To calculate the pH of the solution, use the formula:
pOH = - log [OH⁻]= - log 1.54 × 10⁻⁵pOH = 4.81pH = 14 - 4.81 = 9.19
Thus, the pH of 0.81 M Mg(OH)₂ solution is 9.19.
Let the concentration of OH⁻ ions produced be x.
Therefore, the concentration of C₅H₅NH⁺ is 0.27 - x.
C₅H₅N + H₂O ⇌ C₅H₅NH⁺ + OH⁻
Initial concentration (M) 0.27 0
Change (M) -x +x
Equilibrium Concentration 0.27-x x
Using Kb for C₅H₅N,Kb = Kw/Ka
Kw = 1.0 × 10⁻¹⁴ at 25 °C.
For C₅H₅N,
Kb = [C₅H₅NH⁺][OH⁻]/[C₅H₅N]= (x) (x)/(0.27-x)Kb = 1.7 × 10⁻⁹
= x²/(0.27-x)
On solving the equation,
x = 1.3 × 10⁻⁵ M
Therefore, the concentration of OH⁻ ions = 1.3 × 10⁻⁵ M
To calculate the pH of the solution, use the formula:
pOH = - log [OH⁻]= - log 1.3 × 10⁻⁵pOH
= 4.89pH = 14 - 4.89 = 9.11
Thus, the pH of 0.27 M pyridine solution is 9.11.
learn more about pH here
https://brainly.com/question/12609985
#SPJ11
a 1) How would you make 1 liter of a 10% NaCl solution from a solid stock? Provide details of what kind of containers you would use.
Answers
To make 1 liter of a 10% NaCl solution from a solid stock, you will require the following materials and containers.MaterialsSolid NaClDistilled water1-Liter volumetric flask250-mL volumetric flask 2-beakersProcedureTo prepare 1 liter of a 10% NaCl solution, the following procedure should be followed:Measure out 100g of NaCl using a balance.
Measure the weight of an empty 250-mL volumetric flask.Add the NaCl to a 250-mL beaker and add a small amount of distilled water to it to dissolve the NaCl.Carefully pour the dissolved NaCl solution into the 250-mL volumetric flask. Add distilled water to the mark on the flask to make up the volume. Stopper the flask and invert it several times to mix the solution.Measure the weight of the 1-Liter volumetric flask.Add the 250-mL volumetric flask solution to a 1-Liter volumetric flask.Add distilled water to the mark on the flask to make up the volume.
Stopper the flask and invert it several times to mix the solution.The final volume of the solution will be 1 liter of a 10% NaCl solution.PrecautionsEnsure the NaCl has completely dissolved before adding more water to avoid making a less concentrated solution.Measure the weight of the volumetric flask before and after adding the solution to calculate the volume of solution that was added.Use distilled water to prepare the solution.
To know more about volumetric flask visit:-
https://brainly.com/question/28997155
#SPJ11
According to the following reaction, how much energy is evolved during the reaction of2.50 L B2H6 and 5.65 L Cl2 (Both gases are initially at STP)? The molar mass of B2H6 is 27.67 g/mol.B2H6(g) + 6 Cl2(g) → 2 BCl3(g) + 6 HCl(g) ΔH°rxn = -1396 kJ
Answers
The reaction of \(2.50 L B$_2$H$_6$\) and \(5.65 L Cl$2$\) at STP produces \($-145.2\ kJ$\) of energy, based on the balanced equation with \(\Delta H{rxn} = -1396\ kJ$.\)
To calculate the energy that evolved during the reaction of 2.50 L \(B$_2$H$_6$ and 5.65 L Cl$_2$\), we need to first determine the number of moles of each gas.
Using the ideal gas law, we can calculate the number of moles of \(B$_2$H$_6$ and Cl$_2$\)at STP (Standard Temperature and Pressure):
\($PV = nRT$\)
\(V =$ 2.50 L B$_2$H$_6$\) at STP
\(T =$ 273 K\)
\(R =$ 0.0821 L atm/(mol K)\)
\(P =$ 1 atm at STP\)
\($n_{B_2H_6} = \frac{PV}{RT} = \frac{(1 atm)(2.50 L)}{(0.0821 L\ atm\ mol^{-1}\ K^{-1})(273 K)} = 0.104\ mol\ B_2H_6$\)
Similarly, for\(Cl$_2$\), we have:
\(V =$ 5.65 L Cl$_2$\) at STP
\($n_{Cl_2} = \frac{PV}{RT} = \frac{(1 atm)(5.65 L)}{(0.0821 L\ atm\ mol^{-1}\ K^{-1})(273 K)} = 0.252\ mol\ Cl_2$\)
Since the reaction equation shows that 6 moles of \(Cl$_2$\) react with 1 mole of \(B$_2$H$_6$\), we have an excess of \(Cl$_2$\) in this case, and we can assume that all the \(B$_2$H$_6$\) is consumed during the reaction.
Therefore, the amount of energy evolved during the reaction of 0.104 mol\(B$_2$H$_6$\) and 0.252 mol \(Cl$_2$\) is given by:
\($\Delta H_{rxn} = -1396\ kJ/mol$\)
Multiplying this by the number of moles of \(B$_2$H$_6$\) gives:
\($\Delta H = \Delta H_{rxn} \times n_{B_2H_6} = -1396\ kJ/mol \times 0.104\ mol = \boxed{-145.2\ kJ}$\) of energy evolved during the reaction.
The negative sign indicates that the reaction is exothermic, meaning that it releases energy.
Learn more about ideal gas law,
https://brainly.com/question/28257995
#SPJ4
an acid is a molecule/compound (i.e. hcl, ch3cooh), that when added to a solvent (water) will dissociate donating additional h (protons) to the resulting solution. therefore, an acid is a molecule that increases the hydrogen ion concentration of the solution. the molecule is an acid, whereas the solution containing the acid is acidic. some acids are considered strong and some are considered weak. what is the difference between a strong and weak acid? for an acid to be strong, when it is mixed in water the majority of the molecules will dissociate (very little is left intact), therefore it adds a large number of h into the solution, creating a strong acidic solution (ph
Answers
Since it dissociates almost entirely, HCl is a powerful acid.
In contrary, a weak acid like acetic acid (CH3COOH) does not dissociate well in water because it retains a large number of bound-up H+ ions.
Acetic acid splits into CH3COO, and H+.
In conclusion, the amount of free H+ ions discharged into solution increases with acid strength. The pH value for that acid is inversely correlated with the amount of free H+.
Because they allow other molecules to bind to H+ ions in solution, acids are frequently referred to as H+ ion donors.
Any acid that is entirely ionized in an aqueous solution is considered to be strong. When present in water, hydrogen chloride (HCl) totally ionizes to produce hydrogen and chloride ions.
Weak acid is one that ionizes very little in an aqueous solution. A very well-liked weak acid that is present in vinegar is acetic acid.
To know more about strong and weak acid, click on the link below:
https://brainly.com/question/12811944
#SPJ9
31.1 grams of O2 and 84.3 grams of F2 are placed in a container with a volume of94.9 L. Find the total pressure if the gasses are at a temperature of 55.77 ° c
Answers
In this question, we have:
31.1 grams of O2
84.3 grams of F2
94.9 L of total volume
55.77°C of temperature which is equal to 328.92 K
Now, to find the pressure of this container, we can find the number of moles of each gas, and add both values together making it one value of moles and then we will use the Ideal gas law to find the pressure, so let's start with O2
The molar mass of O2 is 32g/mol and we have 31.1 grams
32g = 1 mol
31.1g = x moles
x = 0.972 moles of O2
Now for F2, the molar mass is 38g/mol, and we have 84.3 grams
38g = 1 mol
84.3g = x moles
x = 2.22 moles of F2
Now we add these values, 0.972 + 2.22 = 3.192 moles
And now we can use the ideal gas law formula:
PV = nRT
Remember that R is the gas constant, 0.082
P * 94.9 L = 3.192 * 0.082 * 328.92
94.9P = 86.1
P = 0.91 atm
Staye two variables the students definitely controlled during the investigation
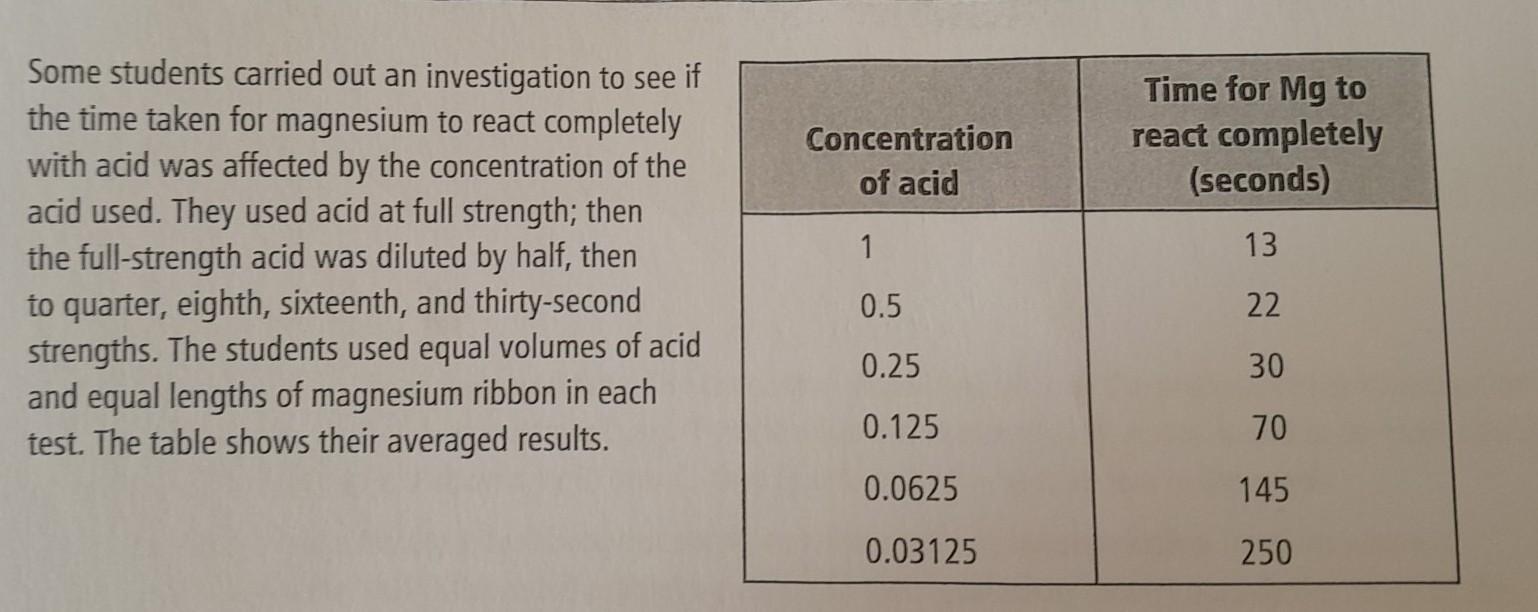
Answers
The two variables that the students definitely controlled during the investigation are:
The volume of acid usedThe length of magnesium ribbon usedWhy are these variables used?The volume of acid used: The students used equal volumes of acid in each test. Controlling the volume of acid ensures that the amount of acid is the same in each test, which helps to keep this variable constant.
The length of magnesium ribbon used: The students used equal lengths of magnesium ribbon in each test. Controlling the length of magnesium ribbon ensures that the amount of magnesium is the same in each test, which helps to keep this variable constant.
Find out more on control variables here: https://brainly.com/question/25801453
#SPJ1
An acid is added to water, and a new equilibrium is established. What is the system after the acid is added? A. pH w = 1 x 10-14 B. pH w -14 C. pH > pOH and Kw = 1 x 10-14 D. pH > pOH and Kw > 1 x 10-14
Answers
Answer:
C. pH > pOH; Kw = 1.0 * 10^-14
Explanation:
The ion product of water, Kw = [H+]*[OH-] = 1.0 * 10^-14. It is a constant.
When an acid or base is added to water, its ion product does not change as it a constant. However, the relative concentrations of H+ ions and OH- ions will change depending on whether an acid or base is added to water.
When an acid is added to water, the concentration of H+ ions increases while that of OH- ions decreases, and vice versa.
Therefore, in the above situation where an acid is added to water, pH > pOH; Kw = 1.0 * 10^-14
Which statements about the polypeptide Gly-Tyr-Gly-Phe-Met-Ser are CORRECT? Select all that apply. Glycine is the N-terminal residue. Glycine is the C-terminal residue. Serine is the C-terminal residue. Serine is the N-terminal residue. Methionine is the N-terminal residue.
Answers
Glycine is the N-terminal residue and Serine is the C-terminal residue.
From the given polypeptide Gly-Tyr-Gly-Phe-Met-Ser, the correct statements are:
Glycine is the N-terminal residue: This is correct because glycine is the first amino acid in the sequence, making it the N-terminal residue.
Serine is the C-terminal residue: This is correct because serine is the last amino acid in the sequence, making it the C-terminal residue.
Methionine is the N-terminal residue: This statement is incorrect. Although methionine is present in the sequence,
it is not the first amino acid. Glycine is the first amino acid, so it is the N-terminal residue.
Therefore, the correct statements are:
Glycine is the N-terminal residue.
Serine is the C-terminal residue.
Learn more about Glycine from the given link
https://brainly.com/question/170426
#SPJ11
A conclusion drawn through reasoning is called an
evaluation
inference
oversight
understanding
Answers
2. What percentage of the offspring
will have the red flowers?
Answers
Answer:
1/4 of the offspeing will have the red flowers