Answers
Answer:
ANSWER OS HERE
Explanation:
THE O2- has two more extra electrons due to presence of 2 negative charges so a total of 10 electrons and the element that has 10 electrons in the periodic table in its neutral state is Neon (Ne) ( atomic number (10) = no.of protons = no. of electrons) so neutral element with same electron configuration as O2- is Ne. Log in or register to post comments.
The electron configuration of the neutral atom is an useful place to start when trying to determine the electron configuration of an ion.
What is the idea behind this?The neutral atom in your situation is sulfur (S), which is found in group 16 of period 3 of the periodic chart. A neutral sulfur atom has a total of 16 electrons surrounding its nucleus since sulfur has an atomic number of 16. Thus, a neutral sulfur atom will have the following electron configuration. Now, a neutral sulfur atom receives two more electrons to create the sulfide anion, or S2. These two electrons will be added to the 3p-orbitals, which can hold a maximum of two protons, as you can see in the configuration of the neutral atom. between them, a maximum of six electrons. Thus, the sulfide anion's electron configuration will be the electron configuration of neon, the noble gas that appears before sulfur in the periodic table, will be used in the shorthand notation for noble gases for the sulfide anion.
To know more about Electronic configuration, visit-https://brainly.com/question/13497372
#SPJ2
Related Questions
PLS HELP!!!!!
Convert the following measurements. Show all work, including units that cancel.
8.5x10^24 molecules NO2 -> mol
Answers
Answer:
Explanation:
8.5x10^24molecules x\(\frac{1 mol}{6.022x10^2^3 molecules}\) = 14.1 moles NO2
Original unit of molecules cancels with the conversion unit of molecules = moles and you are left with moles as your final unit.
At a manufacturing plant, Xanti adds
205. moles of NO2 gas to a storage
tank as a component to generate nitric
acid. How many liters of NO2 gas did
he add, assuming the system is
maintained at STP?
Answers
The volume (in liters) of NO₂ added to the storage tank at STP is 4592 L
Volume of gas at STPAt standard temperature and pressure (STP),
1 mole of a gas = 22.4 L
With above, we can determine the volume of the NO₂ added. Details below:
How to determine the volume of NO₂ gas addedFrom the question given above, the following data were obtained:
Number of mole of NO₂ added = 205 molesVolume of NO₂ added =?The volume of NO₂ added can be obtained as follow:
1 mole of NO₂ is equivalent to 22.4 L at STP.
Therefore,
205 moles will be equivalent to = 205 × 22.4 = 4592 L at STP
Thus, the volume of NO₂ added to the tank is 4592 L
Learn more about volume at STP:
https://brainly.com/question/15980650
#SPJ1
Can H2 be broken down? (Not H)
Answers
Hello, this is Bing. I can help you with your question. Based on the information I found on the web, **H2** can be broken down into its two atoms of hydrogen (H) by supplying enough energy to overcome the bond that holds them together⁴. This process is called **dissociation** and requires an energy equal to or greater than the **dissociation energy** of H2, which is about 436 kJ/mol⁴.
One way to break down H2 is by using **electricity** to split water (H2O) into hydrogen (H2) and oxygen (O2) through a process called **electrolysis**¹. In this process, water is decomposed into its elements by passing an electric current through it. The electric current is provided by a battery or another source of electricity and the water needs to have an **electrolyte**, such as salt or acid, added to it to make it conductive¹. Two electrodes, usually made of metal or other conductive material, are inserted into the water and connected to the battery. The electrode connected to the positive terminal of the battery is called the **anode** and the one connected to the negative terminal is called the **cathode**¹. When the electric current flows through the water, hydrogen gas bubbles form at the cathode and oxygen gas bubbles form at the anode¹. The overall chemical reaction for electrolysis of water is:
2 H2O → 2 H2 + O2
Another way to break down H2 is by using **heat** to cause a reaction between hydrogen and oxygen that produces water and releases a large amount of energy. This reaction is called **combustion** or **oxidation** and can be ignited by a spark or a flame³. The reaction is very fast and explosive and can be dangerous if not controlled. The overall chemical reaction for combustion of hydrogen is:
2 H2 + O2 → 2 H2O
I hope this helps you understand how H2 can be broken down and what methods are used to do so.
On the basis of strength of intermolecular forces, which of the following elements would be expected to have the highest melting point?
Answers
Answer:
I think its Kr
Explanation:
sorry if I'm wrong
Bromine (Br₂) has the highest melting point among the given elements. Therefore, option (A) is correct.
What are intermolecular forces?Intermolecular forces can be described as the attractive and repulsive forces that develop between the molecules of a substance. These forces are responsible for most of the chemical and physical properties of matter. The intermolecular forces between the molecules are Vander Waals forces.
Forces that exist between the molecules themselves are known as intermolecular forces. The particles of solids and liquids are held together by intermolecular forces.
The intermolecular forces are Dipole-Dipole Interactions, Ion-Dipole Interactions, Ion-Induced Dipole Interactions, Dipole-Induced Dipole Interaction, and Dispersion Forces.
As the size of the atoms increases the strength of the intermolecular force also increases and a large amount of energy will be required to break bonds. Since Bromine has the largest size among the all other elements. Therefore, Bromine will have the highest melting point.
Learn more about intermolecular forces, here:
https://brainly.com/question/9007693
#SPJ6
Your question was incomplete, but the complete question was,
On the basis of the strength of intermolecular forces, which of the following elements would be expected to have the highest melting point?
A) Br₂
B) Cl₂
C) F₂
D) N₂
The electron affinity of Te is 190 kJ/mol. Write the equation for which this is the energy change.
Answers
The equation for the electron affinity of Te (tellurium) can be written as:
Te(g) + e⁻ → Te⁻(g)
Electron affinity explained.
Electron affinity (EA) is a physical property of an atom that refers to the energy change that occurs when a neutral atom in the gas phase gains an electron to form a negatively charged ion. The electron affinity of an atom is typically measured in units of energy per mole (kJ/mol or eV/atom).
The electron affinity (EA) is defined as the energy change that occurs when a neutral atom in the gas phase gains an electron to form a negatively charged ion. The equation for the electron affinity of Te (tellurium) can be written as:
Te(g) + e⁻ → Te⁻(g)
The electron affinity of Te, which is given as 190 kJ/mol, represents the energy released when an isolated Te atom in the gas phase gains an electron to form a Te⁻ ion, also in the gas phase.
Learn more about electron affinity below.
https://brainly.com/question/977718
#SPJ1
How are electricity and magnetism related?
Moving magnets can make electrical currents.
Electricity can create a magnetic field.
Electricity and magnetism are not related.
Protons and electrons are repelled by magnets.
Need help now this work is due by 5:00
Answers
Answer: Electricity and magnetism are essentially two aspects of the same thing, because a changing electric field creates a magnetic field, and a changing magnetic field creates an electric field.
Answer:How are electricity and magnetism related?
Moving magnets can make electrical currents.
Electricity and magnetism are not related.
Protons and electrons are repelled by magnets.How are electricity and magnetism related?
Moving magnets can make electrical currents.
Electricity and magnetism are not related.
Protons and electrons are repelled by magnets.How are electricity and magnetism related?
Moving magnets can make electrical currents.
Electricity and magnetism are not related.
Protons and electrons are repelled by magnets.
Explanation:
The average weather conditions over a long period of time is known as
Answers
Answer: it’s climate
Explanation:
The rate constant for the first-order decomposition at 45 degree C of dinitrogen pentoxide, N2O5, dissolved in chloroform, CHCI3, is 6.2*10^-4 min^-1. What is the rate of the reaction when [N2O5]=0.40 M? 2N2O5 -> 4NO2 + O2?
Answers
Answer:
The rate law for a first-order reaction is:
rate = k[N2O5]
where k is the rate constant and [N2O5] is the concentration of N2O5.
Plugging in the values given:
rate = (6.2*10^-4 min^-1) * (0.40 M) = 2.48*10^-4 M/min
Therefore, the rate of the reaction when [N2O5]=0.40 M is 2.48*10^-4 M/min.
Which complex ion can exhibit geometric isomerism? Assume that M is the metal ion, A and B are ligands, and the geometry is octahedral. a) [MA.12+ b) [MAB]2+ c) [MA4B2]2+ d) [MA3B312+
Answers
Answer:
[MA4B2]2+
Explanation:
Octahedral species are species that has a coordination number of of six. This means that six ligands are attached to the central metal atom/ion.
For any octahedral specie to exhibit geometrical or cis-trans isomerism, it must have the formula EX2Y4 as in [MA4B2]2+, hence the answer above.
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
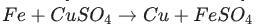
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
What will most likely cause a sudden wind to start blowing? Help ;-;
A. Clouds
B. Humidity
C. Precipitation
D. Air pressure
Answers
it is D. Air pressure!!! :)
Draw 4 decyne structure
Answers
The structure of the alkyne 4 - decyne is shown in the image attached.
How do you draw 4-decyne?Alkyne functional group compounds with a triple bond made of carbon and carbon contain 4-decyne (C). The triple bond is found at the fourth carbon position in the ten-carbon chain (decyne) of 4-decyne.
Nine hydrogen atoms (H) are joined to the first nine carbon atoms (C), starting from the left side.
In conclusion, 4-decyne is made up of a chain of ten carbon atoms, with the fourth carbon atom serving as the center of a carbon-carbon triple bond (C). Except for the carbon involved in the triple bond, all carbon atoms are connected to hydrogen atoms.
Learn more about chemical structure:https://brainly.com/question/32300619
#SPJ1

1. If you place 30.0 L of ethyl acetate (C4H8O2) in a sealed room that is 7.25 m long, 2.75 m wide, and 2.75 m high, will all the ethyl acetate evaporate? If some liquid remains, how much will there be? The vapor pressure of ethyl acetate is 94.9 torr at 25 °C, and the density of the liquid at this temperature is 0.901 g/mL. Treat the room dimensions as exact numbers.
Answers
There will be 0.4589 mL of ethyl acetate left in the space after evaporation.
What is evaporation?The conversion of a liquid substance into a gas is known as evaporation. As a result of the liquid absorbing energy from its surroundings, molecules begin to travel faster and faster until they finally become a vapour and escape into the environment. Usually, the energy is absorbed as heat, but it can also be in the form of light or electricity.
No, the ethyl acetate won't all evaporate. The amount of ethyl acetate that will stay in the space after evaporation can be determined using the ideal gas law. As per the ideal gas law, PV = nRT
P is the overall system pressure, V is the room's volume, n is the amount of ethyl acetate in moles, R is the ideal gas constant, and T is the temperature.
To solve for n, the quantity of moles of ethyl acetate, we can rearrange the equation as follows: n = PV/RT
When the values are plugged in, we get:
n = (94.9 torr)(7.25 m x 2.75 m x 2.75 m)/(8.314 J/K mol)(298 K)
\(n = 4.666 \times 10^{-3} mol\)
The molar mass of ethyl acetate (88.11 g/mol) can then be used to compute the mass of ethyl acetate:
Mass = \(n \times M = (4.666 x 10^{-3} mol)(88.11 g/mol)\) = 0.4125 g
Using the density of ethyl acetate (0.901 g/mL), it is possible to determine the volume of the liquid that is still present:
Volume = mass/density = (0.4125 g)/(0.901 g/mL) = 0.4589 mL
As a result, there will be 0.4589 mL of ethyl acetate left in the space after evaporation.
To learn more about evaporation, visit:
brainly.com/question/24258
#SPJ1
How many liters will 2.76 mol CO2 occupy?
2.76 mol
II
Answers
This problem is providing information about the moles of carbon dioxide, 2.76 mol, and asks for the volume this amount takes up, turning out to be 61.8 L according to the Avogadro's law:
Avogadro's law:In chemistry, gas laws are used to relate the behavior of gases by virtue of the their pressure, volume, temperature and moles; thus several gas laws exist for us to do so, however, we here focus on the Avogadro's law which relates the volume and moles when both temperature and pressure are held constant.
In such a way, since no information on the constant variables is given, we assume the mentioned carbon dioxide is at STP, (0 °C and 1 atm), which means that we can use the following equivalence statement derived from the ideal gas law (PV=nRT):
22.4 L = 1 mol
Hence, we calculate the required volume:
\(2.76molCO_2*\frac{22.4L\ CO_2}{1molCO_2} =61.8L\ CO_2\)
Learn more about ideal gases: https://brainly.com/question/11676583
The following Lewis diagram represents the valence electron configuration of a main-group element.
This element is in group
.
According to the octet rule, this element would be expected to form an ion with a charge of
.
If is in period 5, the ion formed has the same electron configuration as the noble gas
.
The symbol for the ion is
.
Answers
This element is in group 1.
According to the octet rule, this element would be expected to form an ion with a charge of +1.
If X is in period 5, the ion formed has the same electron configuration as the noble gas Krypton
The symbol for the ion is Rb⁺
What is electronic configuration?Electronic configuration refers to the arrangement of electrons in the orbitals of an atom or molecule, indicating the energy level of the electrons, the number of electrons in each energy level, and the number of electrons in each orbital.
Considering the given element:
It has one valence electron, hence it is in group 1. Group 1 elements form ions with a charge of +1.
Losing one electron will give the ion the same electron configuration as Kyrton since it is the noble gas in Period 4.
The element is rubidium and the ion is Rb⁺.
Learn more about electronic configuration at: https://brainly.com/question/26084288
#SPJ1

Lana is testing her hypothesis that marigolds grow faster in red light than in yellow light. If the plants in yellow light grow faster during her experiment what should Lana do next? A. Assume that she made a mistake during the experiment B. Report that her hypothesis was useless C. Conclude that the experiment did not work D. Repeat the experiment to confirm the result
Answers
If the plants in yellow light grow faster during her experiment, Lana should repeat the experiment to confirm the result. Option D.
Testing hypothesesIn science, hypotheses are tested using experiments. Two hypotheses are usually formed during the course of experimentation:
Null hypothesisAlternate hypothesisThe null hypothesis is set up to either be accepted is found to be true or rejected if found to be false. The alternate hypothesis is just there for guidance.
Hypotheses being found not to be true does not always mean that the reverse will be true.
Thus, if a complete opposite is obtained during testing, the next thing would be to repeat the experiment in order to be double sure there was not a mistake.
More on hypotheses testing can be found here: https://brainly.com/question/8169133
#SPJ1
12. 5.6g of solid copper was heated with 476.2 J at room temperature (25°C). Given that
copper has a C of 0.38 J/g°C, what would its final temperature be?
Answers
Answer:
The final temperature of the copper would be 311.3°C.
I hope this helps you
helppp nowwww plsss

Answers
Explanation:
The River rushing down through the bottom of the canyon would be your answer but if not then you can try The Rocks falling due to the gravity :)
What is the balanced chemical
reaction for the synthesis of
carbon with hydrogen?
Answers
The balanced chemical reaction for the synthesis of carbon with hydrogen is CH₄ = C + 2H₂
Balanced chemical equation explainedBalance chemical equation refers to chemical reactions in which the moles and atoms on both the reactant side and product side are the same.
It is a way of balancing chemical equation in which the atoms of each chemicals on the product and reactant are the same.
The balanced chemical reaction for the synthesis of carbon with hydrogen is CH₄ = C + 2H₂
In this reaction, one molecule of methane is formed by combining one atom of carbon with two molecules of hydrogen gas.
Learn more about balanced chemical equation below.
https://brainly.com/question/11904811
#SPJ1
What is electro-magnetic radiation?
Answers
It is a kind of radiation including visible light ,radio waves, gamma rays, and x-rays in witch electric and magnetic fields vary simultaneously. Hope that helps :)
The following two organic compounds are structural isomers to each other. Carefully identify and justify the structural isomers type (skeletal, functional, or positional) with their common molecular formula

Answers
Structural isomers are molecules with the same molecular formula but with different structural formulae. This means that they have the same number and types of atoms, but they are arranged differently. The following two organic compounds are structural isomers of each other.
Carefully identify and justify the structural isomers type (skeletal, functional, or positional) with their common molecular formula.Common molecular formula: C6H14Structural isomers:(i) Hexane: Hexane is a straight-chain alkane with six carbon atoms and no double bonds or rings. The carbon atoms are linked together in a linear or straight-chain configuration in the skeletal isomer. The skeletal isomer differs in terms of the arrangement of atoms in its molecule. This indicates that it is a skeletal isomer.(ii) 2-methylpentane: It is a branched-chain alkane with six carbon atoms and no double bonds or rings. It differs from the first molecule in terms of the location of a methyl group on the second carbon of the five-carbon chain, rather than a straight six-carbon chain. This difference is due to a change in the positioning of the carbon atoms in the molecule. As a result, it is a positional isomer, as it differs by the position of the functional group or substituent. Therefore, the skeletal and positional isomerism types are present between these two compounds.For such more question on molecular
https://brainly.com/question/24191825
#SPJ8
Mechanical digestion begins in the_____ and involves physical processes, such as chewing.
Answers
Answer:
begins in the mouth
What is the difference between Claire’s test of the collision scene where Vehicle 2 fell off the cliff and the film, Iceworld Revenge, where it did not?*
Which claim is more convincing?
Claim 1: The vehicles in Iceworld Revenge had different masses; in Claire’s test, the vehicles had the same mass.
Claim 2: The friction of the surface that was used in Iceworld Revenge was different from the friction of the surface in Claire’s test.
Did the Science Seminar cause your thinking about the claims to change? Explain.
Answers
Complete Question:
Check the file attached to get the complete question
Answer:
In the film Ice word Revenge, vehicle 2 did not fall of the cliff because, but in Claire's test, vehicle 2 off the cliff because
Explanation:
In Claire's test, the weight of vehicle 1 is either equal to or greater than the weight of vehicle 2, so it was sufficient to push it down the cliff. In the film Ice word revenge, the weight of vehicle 1 is less than the weight of vehicle 2, it is not sufficient to make it fall off the cliff ( Note: Looking exactly the same in the movie, as Claire claimed, does not mean they have the same mass). Therefore if Claire wants a collision that will not make the vehicle 2 fall off the cliff, he should collide it with a vehicle of lesser mass/weight.
Match the object with its characteristic.
Earth
?
Electromagnet
Venus
?
Permanent magnet
Refrigerator
magnet
?
Not magnetic
Answers
Earth is an electromagnet
A refrigerator magnet is a permanent magnet
Venus is not magnetic
Answer:
Earth is electromagnet
Refrigerator is permanent magnet
Venus is not
Explanation:
what is the formula for co3+ and se2-?
Answers
The formula for Co3+ is Co3+ because it represents the ion of cobalt that has lost three electrons, leaving it with a 3+ charge.
What is chemical formula and how they are formed ?
A chemical formula is a symbolic representation of a chemical compound that shows the types of elements present in the compound and the relative number of atoms of each element. For example, the chemical formula for water is H2O, which indicates that it is made up of two hydrogen atoms and one oxygen atom.
Chemical formulas are formed by identifying the elements that make up a compound and determining the relative number of each element in the compound. The number of each element is represented by a subscript following the chemical symbol of the element. For example, the chemical formula for methane is CH4, which indicates that there is one carbon atom and four hydrogen atoms in each molecule of methane.
The formula for Se2- is Se2- because it represents the ion of selenium that has gained two electrons, giving it a 2- charge.
To know more about reaction visit :-
https://brainly.com/question/11231920
#SPJ1
Write the equilibrium expression for the following reaction. CaO(s) + CH₄(g) + 2H₂O(g) —> CaCO₃(s) + 4H₂(g)
Answers
The equilibrium expression can be written as follows:
K = [CaCO₃] × [H₂]⁴
------------------
[CaO] × [CH₄] × [H₂O]²
In this equilibrium expression, the square brackets represent the concentrations of the species involved in the reaction, and the coefficients of the balanced equation indicate the stoichiometric coefficients of the corresponding species.
The concentration of a pure solid (CaCO₃ in this case) is not included in the equilibrium expression, as it remains constant throughout the reaction.
The equilibrium constant (K) represents the ratio of the product concentrations to the reactant concentrations at equilibrium, each raised to the power of their respective stoichiometric coefficients. The specific values of these concentrations depend on the initial conditions, and K remains constant as long as the temperature is unchanged.
It is important to note that the equilibrium constant expression is written based on the balanced chemical equation. The stoichiometric coefficients determine the relationship between the concentrations of reactants and products, allowing us to express the equilibrium state quantitatively using the equilibrium expression.
For more such question on equilibrium. visit :
https://brainly.com/question/30843966
#SPJ8
what types of energy are involved in a chemical reaction
- activation energy
- chemical energy
- released energy
is this energy required for a chemical reaction to take place, and
- absorbed energy
- chemical energy
- released energy
is the energy associated with every substance
Answers
chemical energy
i thnk this ithe answer
What is the molarity when 1.25 mol is dissolved in 3.50 L of solution?
Answers
Answer:
0.36 mol/L
Explanation:
The formula for molarity M=m/L
m stands for moles
L stands for liters
Plug it into the equation...
M=1.25 mol/3.5 L
M=0.35714285714
The mass number of an atom is 18 and the atom has 8 protons. What is the number of neutrons?
Answers
Answer:
10 neutrons
Explanation:
The mass number is equivalent to the protons + neutrons so...
18 - 8 = 10 neutrons
calculate the pH of the solution obtained if 40cm^3 of 0.2M HCl was added to 30cm^3 of 0.1M NaOH
Answers
To calculate the pH of the solution obtained by mixing HCl and NaOH, we need to consider the neutralization reaction between the two compounds. The reaction between HCl (hydrochloric acid) and NaOH (sodium hydroxide) produces water (H₂O) and forms a salt (NaCl).
Given:
Volume of HCl solution (V₁) = 40 cm³
Concentration of HCl solution (C₁) = 0.2 M
Volume of NaOH solution (V₂) = 30 cm³
Concentration of NaOH solution (C₂) = 0.1 M
1. Determine the moles of HCl and NaOH used:
Moles of HCl = Concentration (C₁) × Volume (V₁)
Moles of HCl = 0.2 M × 0.04 L (converting cm³ to L)
Moles of HCl = 0.008 mol
Moles of NaOH = Concentration (C₂) × Volume (V₂)
Moles of NaOH = 0.1 M × 0.03 L (converting cm³ to L)
Moles of NaOH = 0.003 mol
2. Determine the limiting reagent:
The stoichiometry of the reaction between HCl and NaOH is 1:1, meaning that they react in a 1:1 ratio. Whichever reactant is present in a smaller amount will be the limiting reagent.
In this case, NaOH is present in a smaller amount (0.003 mol), which means it will be fully consumed during the reaction.
3. Determine the excess reagent and its remaining moles:
Since NaOH is the limiting reagent, we need to find the remaining moles of HCl.
Moles of HCl remaining = Moles of HCl initially - Moles of NaOH reacted
Moles of HCl remaining = 0.008 mol - 0.003 mol
Moles of HCl remaining = 0.005 mol
4. Calculate the concentration of HCl in the resulting solution:
Volume of resulting solution = Volume of HCl solution + Volume of NaOH solution
Volume of resulting solution = 0.04 L + 0.03 L
Volume of resulting solution = 0.07 L
Concentration of HCl in the resulting solution = Moles of HCl remaining / Volume of resulting solution
Concentration of HCl in the resulting solution = 0.005 mol / 0.07 L
Concentration of HCl in the resulting solution ≈ 0.071 M
5. Calculate the pH of the resulting solution:
pH = -log[H⁺]
pH = -log(0.071)
Using logarithm properties, we can determine the pH value:
pH ≈ -log(0.071)
pH ≈ -(-1.147)
pH ≈ 1.147
Therefore, the pH of the solution obtained by mixing 40 cm³ of 0.2 M HCl and 30 cm³ of 0.1 M NaOH is approximately 1.147.