Answers
Answer:
101.50 g H₂O
Explanation:
The mole ratio of HNO₃ and H₂O is 6 : 2
Hence, 16.9 moles of HNO₃ will produce = 2/6×16.9 = 5.63 moles of H₂O
Also,
Mass = Moles × M.Mass
Mass = 5.63 mol × 18.02 g/mol
Mass = 101.50 g H₂O
Related Questions
Determine the volume of sulfuric acid solution needed to prepare 37.4 g of aluminum sulfate,
Al2(SO4)3by the reaction
2Al(s) + 3H2SO4. (aq) Al (SO,) (ag) + 3H2 (g)
The sulfuric acid solution, whose density is 1.104 g/mL, contains 15.0% H›SO; by mass.
Answers
The volume of the acid is seen as 0.22 L.
What is the volume of the acid that you need?We know that the concentration of the raw acid can be given as;
Co = 10pd/M
We have;
P = percentage
d = density
M = molar mass
Co = 10 * 15 * 1.104/98
Co = 1.69 M
We know that in this as what we have is the raw acid and we have to find the molarity of the raw acid and that we have to use it to obatin the volume of the acid that we need.
We know that;
Number of moles = Concentration * volume
37.4 g/98 g/mol = 1.69 M * V
V = 0.38/1.69
V = 0.22 L
Learn more about acid:https://brainly.com/question/16751313
#SPJ1
Experiment 4: A chemist mixes aqueous solutions of sodium hydroxide and aluminum chloride in a double-displacement reaction, which forms a white solid precipitate and a clear solution. Write the complete, balanced molecular equation for the reaction. Include physical states.
balanced equation:
Answers
The balanced molecular equation for the reaction between sodium hydroxide (NaOH) and aluminum chloride (\(AlCl_3\)) in aqueous solution can be written as follows: 2NaOH(aq) + 3\(AlCl_3\)(aq) → 3NaCl(aq) + \(Al(OH)_3\)(s)
In this reaction, sodium hydroxide (NaOH) reacts with aluminum chloride (\(AlCl_3\)) to form sodium chloride (NaCl) and aluminum hydroxide (\(Al(OH)_3\)). The coefficients in the balanced equation indicate the stoichiometric ratio between the reactants and products.
The physical states of the substances are indicated by the symbols (aq) for aqueous solutions and (s) for the solid precipitate.
The reaction is a double-displacement reaction, also known as a precipitation reaction. Double-displacement reactions involve the exchange of ions between two compounds, resulting in the formation of a precipitate.
In this case, sodium hydroxide and aluminum chloride react to form sodium chloride and aluminum hydroxide, with aluminum hydroxide being the white solid precipitate.
It's worth noting that the actual reaction might involve hydrated forms of the compounds, such as NaOH·x\(H_2O\) and \(AlCl_3\)·y\(H_2O\). However, for simplicity, these hydrated forms are not included in the balanced equation.
Overall, the balanced equation represents the chemical reaction between sodium hydroxide and aluminum chloride, showing the reactants, products, and their stoichiometric ratios.
For more such question on balanced molecular equation visit:
https://brainly.com/question/11904811
#SPJ8
48g of 02 produce how many grams of Al2O3
Answers
Taking into account the reaction stoichiometry, 102 grams of Al₂O₃ are formed when 48 grams of O₂ react.
Reaction stoichiometryIn first place, the balanced reaction is:
4 Al + 3 O₂ → 2 Al₂O₃
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Al: 4 moles O₂: 3 molesAl₂O₃: 2 molesThe molar mass of the compounds is:
Al: 27 g/moleO₂: 32 g/moleAl₂O₃: 102 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Al: 4 moles ×27 g/mole= 108 gramsO₂: 3 moles ×32 g/mole= 96 gramsAl₂O₃: 2 moles ×102 g/mole= 204 gramsMass of Al₂O₃ formedThe following rule of three can be applied: if by reaction stoichiometry 96 grams of O₂ form 204 grams of Al₂O₃, 48 grams of O₂ form how much mass of Al₂O₃?
\(mass of Al_{2} O_{3} =\frac{48 grams of O_{2} x204 grams of Al_{2} O_{3}}{96 grams of O_{2}}\)
mass of Al₂O₃= 102 grams
Finally, 102 grams of Al₂O₃ are formed when 48 grams of O₂ react.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
#SPJ1
Graphite, an allotrope of carbon, is converted into cubic diamond through a process that may take a billion years or longer.
a. True
b. False
Answers
Answer:
The given statement is true.
Explanation:
The given statement is true.
As per an estimate, to convert graphite into diamond nearly 1 billion to 3.3 billion years of time is required.
Graphite converts into diamond when high-pressure is applied deep in the earth core.
How many of the three types of subatomic particles are present in a neutral atom of 15N?

Answers
Explanation:
if we have a neutral 15N is means that the atomic mass of it is 15.
So it has:
7 protons
7 electrons
and 15-7 = 8 neutrons
Answer: 7 protons, 7 electrons and 8 neutrons.
Which of the following is not the name of an amino acid?
A. Lysine
B. Valine
C. Methane
D. Phenylalanine
(Edge)
Answers
Answer:
C. Methane
Explanation:
Hopefully this isnt too late!
Answer:
C. Methane
(Photo for proof at the bottom.)
Explanation:
All amino acids contain an amino group, a carboxyl group, and an R group. An amino group consists of NH2. A carboxyl group consists of COOH. And an R group usually consists of a hydrogen or carbon atom bonded to the amino acid. Methane only consists of CH4, which clearly does not meet the criteria of an amino acid.
Here's a photo of Edge incase you're doubtful.
Please click the heart if this helped.

Find the wavelength of the electromagnetic radiation with a frequency of 3x10^-18Hz PLEASE ANSWER
Answers
Answer:
λ = 1×10²⁶m
Explanation:
Given data:
Wavelength of radiation = ?
Frequency of radiation = 3×10⁻¹⁸Hz
Solution:
Formula:
c = f × λ
c = speed of wave = 3×10⁸ m/s
by putting values,
3×10⁸ m/s = 3×10⁻¹⁸Hz × λ
λ = 3×10⁸ m/s / 3×10⁻¹⁸s⁻¹
λ = 1×10²⁶m
Please how to do question 4.
Answers
Answer:
all you have to so answer it all done
Is [HN.CHR-CO]n a homo polymer or a copolymer
Answers
Answer:(−NH−CHR−CO−)n is a homopolymer. The reason is that it has a single monomer (NH2−CHR−COOH), α−amino acid.
If a polymer consists of only one kind of monomer then it is called a homopolymer, while a polymer that consists of more than one kind of monomer is called a copolymer
reaction will be spontaneous at all temperatures if _____
Answers
If a reaction has a negative ΔG and a positive ΔS, the reaction will be spontaneous at all temperatures.
If a reaction is spontaneous at all temperatures, it implies that the reaction will occur without the need for any external intervention, such as the addition of energy. For a reaction to be spontaneous, it must satisfy the criteria of thermodynamic favorability, which is determined by the change in Gibbs free energy (ΔG) associated with the reaction.
The relationship between ΔG, temperature (T), and the equilibrium constant (K) of a reaction is described by the equation ΔG = ΔH - TΔS, where ΔH is the change in enthalpy and ΔS is the change in entropy.
To ensure spontaneity at all temperatures, two conditions must be met:
ΔG must be negative: A negative ΔG indicates a thermodynamically favorable reaction, meaning the products have a lower Gibbs free energy than the reactants. If ΔG is negative, the reaction will proceed spontaneously in the forward direction.
ΔS must be positive: A positive ΔS signifies an increase in the overall entropy of the system. Higher entropy means more disorder, and spontaneous reactions often involve an increase in randomness. When ΔS is positive, it can compensate for the enthalpic term, ΔH, allowing the reaction to proceed spontaneously.
For more such questions on spontaneous visit:
https://brainly.com/question/30127476
#SPJ8
Why is scientific notation used?
to round numbers to the nearest whole number
to promote reproducibility of data
to increase the validity of data
to express very large or very small numbers
Answers
The correct option is (d) To express very large or very small number.
What is the Scientific Notation?
Scientific notation is a way to present numbers that are too large or too small to be easily written in decimal form. The three components of scientific notation are coefficient, base and exponent. The proper format to write a scientific notation is a x 10^b, where a is a number or decimal number and b is the power of 10 to make scientific notation equivalent to original number. When a number between 1 and 10 is multiplied by a power of 10 then the number is expressed in scientific notation. For example, 10000000 can be written as 10⁷, which is the scientific notation and the exponent is positive here. Similarly, for the negative exponent 0.000001 can be can be represented as 10-⁷. Hence, the scientific notation is used to express very large or very small numbers.
Learn more about Scientific Notation here: https://brainly.com/question/15361382
#SPJ1
Which one is a decomposition reaction?
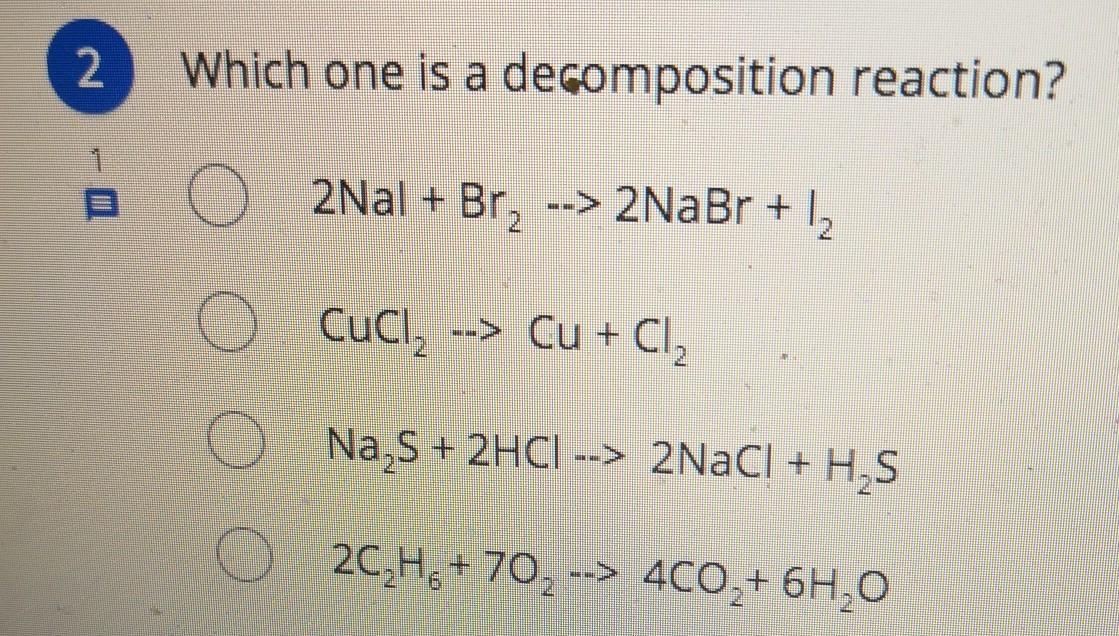
Answers
Answer:
b no
Explanation:
because it is decomposing into two elements
Starting with appropriate unlabeled organic compounds, show syntheses of each of the following:
Draw the reagents needed to produce C6H5—C≡C—T.
Answers
In first reaction alkyne is treated with base to remove proton later alkyne anion react with T20 to form tritium isotope labelled product.
What is reagent?
Reagent is a substance used to bring about a chemical reaction, or added to test if a reaction occurs. In a chemical reaction, one or more reactants are combined to form one or more products. A reagent is typically used to start, speed up, or determine the progress of a reaction. Common reagents include acids, bases, oxidizers, reducing agents, and salts. Reagents can also be used in qualitative or quantitative analysis to detect, measure, or separate chemicals in a sample.
For structure refer attached file
For more information about reagent please visit:
https://brainly.com/question/30024442
#SPJ4

After being treated with a base in the initial reaction to remove the proton, the alkyne anion then reacts with T20 to produce a product labeled with the tritium isotope.
A reagent in a chemical process is what?
In the field of chemical research, a "substance or compound that is given to a system in order to bring about a chemical reaction or is added to check whether a reaction is occurring or not" is referred to as a reagent. A similar response is utilized to validate the discovery of the presence of another drug.
A catalyst can speed up a certain chemical reaction, whereas a reagent is a material or mixture used in chemical analysis or other reactions.
See attached file for response.
To learn more about reagent use link below:
https://brainly.com/question/26905271
#SPJ4

u make 1.000 L of an aqueous solution that contains 35.0 g of sucrose (C12H22O11).Part AWhat is the molarity of sucrose in this solution?Express your answer to three significant figures.M
Answers
Answer:
0.102 M
Explanation:
Given data
Mass of sucrose (solute): 35.0 gVolume of solution: 1.000 LStep 1: Calculate the moles of solute
The molar mass of sucrose is 342.3 g/mol. The moles corresponding to 35.0 g of sucrose are:
\(35.0g \times \frac{1mol}{342.3g} =0.102mol\)
Step 2: Calculate the molarity of sucrose in the solution
The molarity is equal to the moles of solute divided by the liters of solution.
\(M=\frac{0.102mol}{1.000L} =0.102 M\)
Which change to the ecosystem had the largest effect on the the population of trout in Wisconsin?
Answers
The largest effect on the population of trout in Wisconsin was caused by the introduction of non-native species into the ecosystem.
The introduction of non-native species into an ecosystem can cause a disruption in the food chain, leading to a decrease in the population of native species. In Wisconsin, the introduction of non-native species such as the brown trout and rainbow trout has had a significant impact on the population of native brook trout.
The non-native species compete with the native brook trout for food and habitat, which has led to a decrease in the brook trout population. This highlights the importance of preserving the natural balance of ecosystems and avoiding the introduction of non-native species.
To know more about ecosystem, click here.
https://brainly.com/question/13979184
#SPJ1
What sample at STP has the same number of molecules as 5 L of NO2
Answers
Answer:
5l NO
2
at STP
No. of molecules=
22.4
5
mol=
22.4
5
×N
A
molecules
A) 5ℊ of H
2
(g)
No. of moles=
2
5
mol=
2
5
×N
A
molecules
B) 5l of CH
4
(g)
No. of moles of CH
4
=
22.4
5
mol=
22.4
5
N
A
molecules
C) 5 mol of O
2
=5N
A
O
2
molecules
D) 5×10
23
molecules of CO
2
(g)
Molecules of 5l NO
2
(g) at STP=5l of CH
4
(g) molecules at STP
Therefore, option B is correct.
Was this answer helpful?
Which of the following processes release energy? a. ball rolling down a hill b. formation of copper (II) oxide from copper and oxygen c. formation of ice from liquid water d. condensation of water on a wind shield of a car
Answers
Answer:
d. condensation of water on a wind shield of a car
Explanation:
Condensation involves the conversion of moist air into liquid.
Gas has a higher energy compared to liquid. This is why Gas particles move at random motion and faster in relation to solid and liquid particles due to the high energy content.
The conversion of the gas to liquid means that there was loss or release of energy which validates the answer.
pls help 40 points!! Augie created this chart about the two kinds of waves.
A 2-column table with 3 rows titled 2 Kinds of Waves. The first column labeled Title 1 has entries the transfer energy is perpendicular to the direction of the wave motion, particles move up and down, particles move only slightly. The second column labeled Title 2 has entries the transfer direction is parallel to the direction of wave motion, particles move side to side, particles move only slightly.
Which best labels the chart?
Title 1 is “Longitudinal Waves,” and Title 2 is “Transverse Waves.”
Title 1 is “Transverse Waves,” and T
Answers
its b
i did the quiz and got it right it is b
Answer:
b
Explanation:
took test on edge
which of the following is a true statement when the moon is closest to the sun in its orbit around earth
Answers
where are the answers choices?
A student decreases the temperature of a 547 cm3 balloon from 590 K to 210 K.
Assuming constant pressure, what should the new volume of the balloon be?
Round your answer to one decimal place.
Please help
Answers
Answer:
≈194.7
Explanation:
(547cm^3/590K) / (V2/210K)
V2 = 194.69 or 194.7
:D
What is the name of Si3N4
A) silicon nitride
B) trisilicon tetranitride
Answers
What is the name of Si3N4
A) silicon nitride .
Answer:
\(\textrm {A) silicon nitride}\)
Explanation:
\(\textrm {The title of the compound}\) \(\mathrm {Si_{3}N_{4} }\) \(\textrm {is silcon nitride.}\)
\(\textrm {It cannot be called trisilicon tetranitride because a nitride group}\\\textrm {has 3 nitrogen atoms, and tetranitride would mean it has 4 times}\\\textrm {the number of atoms.}\)
\(\textrm {Furthermore, tri- and tetra- are prefixes used for substituent}\\\textrm {groups present in compounds. Here, since only both of them}\\\textrm {are present, it cannot be called so.}\)
If you do not answer this you will feel bad for not answering it. Then hopefully you will come back and answer it.
N2SO4 + 2NaOH --> H2O + Na2SO4
How many molecules of water are produced if 2.0g of sodium sulfate are produced in the reaction above?
Question 3 options:
8.5 x 1021 molecules
8.5 x 1023 molecules
6.77 x 1022 molecules
2.0 x 1023 molecules
Answers
The balanced chemical equation for the reaction is:
N2SO4 + 2NaOH → Na2SO4 + 2H2O
From the equation, we can see that for every 1 molecule of sodium sulfate (Na2SO4), 2 molecules of water (H2O) are produced. Therefore, to find the number of molecules of water produced, we need to determine the number of molecules of sodium sulfate produced in the reaction.
To do this, we need to use the molar mass of sodium sulfate and Avogadro's number. The molar mass of sodium sulfate is 142.04 g/mol, and Avogadro's number is 6.022 x 10^23.
The number of molecules of sodium sulfate produced in the reaction can be calculated as follows:
(2.0 g) / (142.04 g/mol) = 0.01405 mol
And the number of molecules of water produced can be calculated as follows:
0.01405 mol x 2 molecules/mol = 0.0281 x 6.022 x 10^23 molecules/mol = 1.69 x 10^23 molecules
Round 1.69 to 2.0 and your answer is 2.0 x 1023 molecules
A student wants to do a scientific research on how a nuclear fusion can be used as a power source on earth. What type of scientist does the student want to become?
Answers
A nuclear scientist because they are more focused on extracting the nuclear energy of atoms.
what mass of glucose c6h12o6 would be required to prepare 5000 mL of a 0.215 M solution
Answers
Approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M.
To determine the mass of glucose (C6H12O6) required to prepare a 0.215 M solution in 5000 mL, we need to use the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
First, let's convert the volume of the solution from milliliters (mL) to liters (L):
5000 mL = 5000/1000 = 5 L
Now, we can rearrange the formula to solve for moles of solute:
moles of solute = Molarity (M) x volume of solution (L)
moles of solute = 0.215 M x 5 Lmoles of solute = 1.075 mol
Since glucose (C6H12O6) has a molar mass of approximately 180.16 g/mol, we can calculate the mass of glucose using the equation:
mass of solute = moles of solute x molar mass of solute
mass of glucose = 1.075 mol x 180.16 g/mol
mass of glucose = 194.0 g (rounded to three significant figures)
Therefore, approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M. It's important to note that the molar mass of glucose used in this calculation may vary slightly depending on the level of precision required.
For more such questions on glucose visit:
https://brainly.com/question/397060
#SPJ8
The sum of three numbers is 68. The first number is 7 less than the second. The third number is 3 times the second. What are the numbers ?
Answers
Answer:
8,15 and 45 respectively
Explanation:
let x be the second number
x-7 = the first number
3x= the third number
x-7+x+3x=68
5x-7=68+7
5x=68+7
5x=75
divide both side by 5
x=15

What is the calibration of this graduated cylinder? calibration
A. 5 mL
B. 2 mL
C. 1 mL
D. 10 mL

Answers
The answer is 1ml. The answer is 1ml because of calibration of this graduated cylinder
Answer:
1 mL
Explanation:
According to your definition, it is the difference between marked spaces divided by the # of spaces between marked values.
Difference between 2 marked values: 5 mL
# Of Spaces between marked values: 5
Calibration: 5 mL / 5 mL = 1 mL
5 moles of Fe(OH)3 react with sulfuric acid to produce Fe2(SO4), and water.
A) How many moles of sulfuric are required?
B) How many moles of each product are produced?
C) How many grams of each product is produced?
Answers
For 5 moles of Fe(OH)3, 5 moles of sulfuric acid are needed.
What is moles?Moles are small, burrowing mammals found in many parts of the world. They are members of the family Talpidae, and range in size from about two inches to about eight inches in length, depending on the species. Moles have cylindrical bodies with short, velvety fur and enlarged front feet adapted for digging. They feed primarily on small insects and earthworms, and their long claws make them excellent tunnelers. Moles live in underground burrows which they dig out with their front feet and claws. They are solitary creatures, and they are active day or night. Moles are rarely seen above ground, and they are generally considered to be harmless animals.
A) In order to calculate the number of moles of sulfuric acid needed to react with 5 moles of Fe(OH)3, we can use the balanced chemical equation for this reaction: 3Fe(OH)3 + 3H2SO4 → Fe2(SO4)3 + 6H2O. From this equation, we can see that for every 3 moles of Fe(OH)3, 3 moles of sulfuric acid are required. Therefore,
for 5 moles of Fe(OH)3, 5 moles of sulfuric acid are needed.
B) From the balanced chemical equation for this reaction, we can see that for every 3 moles of Fe(OH)3, 3 moles of Fe2(SO4)3 are produced. Therefore, for 5 moles of Fe(OH)3, 5 moles of Fe2(SO4)3 are produced. Additionally, for every 3 moles of Fe(OH)3, 6 moles of water are produced. Thus, for 5 moles of Fe(OH)3, 10 moles of water are produced.
C) The mass of each product produced can be calculated by using the molar mass of the corresponding compound. For example, the molar mass of Fe2(SO4)3 is 392.16 g/mol. Therefore, for 5 moles of Fe2(SO4)3, the mass of the product produced is 1960.8 g. Similarly, the molar mass of water is 18.015 g/mol. Therefore, for 10 moles of water, the mass of the product produced is 180.15 g.
To know more about moles click-
https://brainly.com/question/15356425
#SPJ1
Please please help asap!!!

Answers
Answer:
5
Explanation:
identify the condensed structure that corresponds to the following lewis structure. the lewis structure has a main backbone, which is four carbons long. bonded to the first carbon are 3 hydrogen atoms. bonded to the second carbon are 2 hydrogen atoms. bonded to the third carbon are 2 chlorine atoms. finally, bonded to the fourth carbon are 3 hydrogen atoms.
Answers
The condensed structure that corresponds to the the lewis structure has a main backbone is 2,2 dichloro butane.
A Lewis structure, also known as a Lewis point formula, Lewis point structure, electron point structure, or Lewis electron point structure, is a diagram showing the bonds between the atoms of a molecule and the lone pairs of electrons present in the molecule.
The Lewis structure is a very simplified representation of the valence shell electrons in a molecule. Used to describe how electrons are arranged around individual atoms in a molecule. Electrons are represented as "dots" or as lines between two atoms to join electrons.
The Lewis structure is based on the concept of the octet rule in which atoms share electrons, so each atom has eight electrons in its outer shell. For example, the oxygen atom has six electrons in its outer shell.
Learn more about Lewis stricture here:-https://brainly.com/question/20300458
#SPJ4

Select the structure that corresponds
to the name:
3,5,6-trichloro-2-heptanol

Answers
Answer:C both
Explanation:
The Structure that correspondsto the name: 3,5,6-trichloro-2-heptanol is C both.
How nomenclature is done in organic compound ? First name the principal carbon chain and also check type of bonds single or double present on the carbon chain. Name the functional group according to Seniority table. Name the substituent alphabetically. Both are isomers∵ all are substituent and OH alcohol is added on the principal carbon chain both names are suggested.
Learn more about nomenclature of organic compound here: http://brainly.brainly.com/question/1594044
#SPJ2