Answers
The length of the side BC in the right triangle is 3.76 units.
How to find the length of side BC?We want to find the length of the side BC, for the right triangle ABC.
We know that:
ACB = 90°
ABC = 20°
AC = 4 units.
Now we need to use trigonometric relations to find BC, we can use the relation:
cos(a) = adjacent cathetus/hypotenuse
Replacing what we know we will get:
cos(20°) =BC/4
4*cos(20°) = BC = 3.76
That is the length.
Learn more about right triangles at:
https://brainly.com/question/2217700
#SPJ1

Related Questions
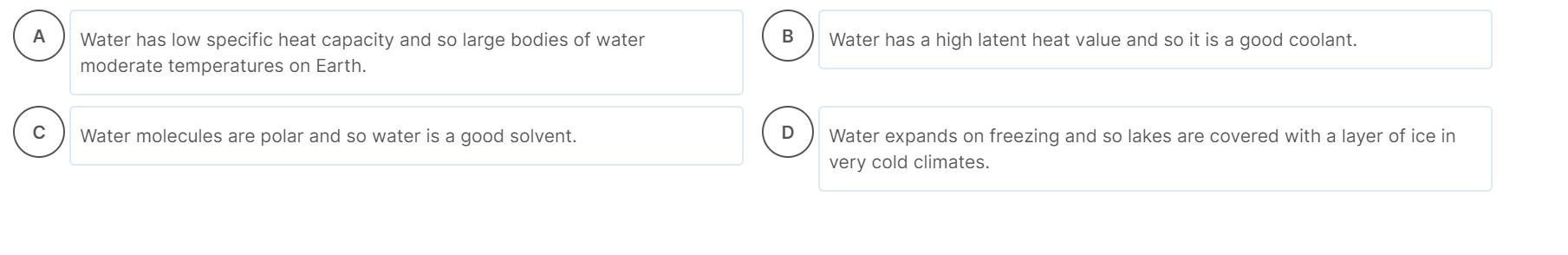
Which of the following statements about water is not true?
Answers
Answer:
Water has a low specific heat capacity and so large bodies of water moderate temperatures on Earth.
Explanation:
Water has a very high specific heat capacity, meaning that it has to absorb a lot of energy to raise the temperature by one degree. Because water has a high specific heat capacity, large bodies of water can moderate the temperature of nearby land.
Hope this helps.
Write the reaction for the formation of the lithium ion, Li+ from an atom of lithium.
Answers
The reaction Li Li+ + e- can be used to symbolise the creation of the lithium ion, Li+, from a lithium atom.
What happens when lithium ions combine chemically?The movement of lithium ions and electrons from the electrode to the cathode is how lithium ion batteries function. Neutral lithium is oxidised and changed into Li+ at the electrode. When the electrons from the anode reaction are absorbed at the cathode, this causes the reduction of Co(IV) to Co(III).
What happens when a lithium particle forms?Only lithium is an alkali element that neither forms the Li anion in solution nor does it do so in solid form. In order to create compounds containing the Li+ cation, lithium can easily lose one of its three electrons due to its high chemical activity.
To know more about lithium ion visit:-
https://brainly.com/question/13073127
#SPJ9
6. Explain why there is no base unit for the dimension of volume.
Answers
What is the number of moles of hydrochloric acid will there be in 10 mL of a given solution with a molarity of 0.5 mol/L?
Answers
Answer:
You are on the right track.
Explanation:
Indeed, your approach here will be to use the molar mass of aluminium hydroxide to convert the mass of the sample to moles and the mole ratio that exists between the two reactants to find the number of moles of hydrochloric acid consumed.
Al
(
OH
)
3
(
s
)
+
3
HCl
(
a
q
)
→
AlCl
3
(
a
q
)
+
3
H
2
O
(
l
)
The balanced chemical equation tells you that the reaction consumes
3
moles of hydrochloric acid and produces
3
moles of water for every
1
mole of aluminium hydroxide that takes part in the reaction.
So you can say that you have
0.75
g
⋅
the molar mass of Al
(
OH
)
3
1 mole Al
(
OH
)
3
78
g
⋅
the 1:3 mole ratio
3 moles HCl
1
mole Al
(
OH
)
3
=
0.029 moles HCl
−−−−−−−−−−−−−−
Since you know that the reaction produces the same number of moles of water as the number of moles of hydrochloric acid it consumes--the two chemical species have a
3
:
3
mole ratio in the balanced chemical equation--you can use the molar mass of water to say that the reaction will produce
0.029
moles H
2
O
⋅
18.015 g
1
mole H
2
O
=
0.52 g
−−−−−
The answers are rounded to two sig figs, the number of sig figs you have for the mass of aluminium hydroxide.
Explanation:
#ClaratheBrainlyQueen
Answer: 0.005 mol
Explanation:
Given information
Volume = 10 mL
Molarity = 0.5 mol / L
Given formula
Molarity = Mole / Volume
Convert volume unit to liters
1 L = 1000 mL
10 mL = 10 / 1000 = 0.01 L
Substitute values into the formula
Molarity = Mole / Volume
Mole = Molarity × Volume
Mole = (0.5) × (0.01)
Simplify by multiplication
\(\Large\boxed{Mole~=~0.005~mol}\)
Hope this helps!! :)
Please let me know if you have any questions
Why do we use commas after transition words?
Commas are extra punctuation.
Commas show readers when something should be shouted.
Commas indicate a natural pause in the sentence.
Commas make the sentence look nicer.
Answers
Answer:
C - commas indicate a natural pause in the sentence
Calculate the change in pH when 1.0 L of solid NaOH was added to 1.0 L of buffer solution in the previous case. Compare this change in pH with the change in pH that would occur if 1.0 L of water were added to the same amount of solid NaOH.
Answers
Answer:
When a strong base (OH-) is added to a buffer solution, the hydroxide ions are consumed by the weak acid forming water and the weaker conjugate base of the acid. The amount of the weak acid decreases while the amount of the conjugate base increases.
List the three subatomic particles. Give the relative masses and charges of each (sorry for putting it in college TvT)
Answers
Answer:
Explanation:
The electron is subatomic particle that revolve around outside the nucleus and has negligible mass. It has a negative charge.
relative charge = -1
Relative mass = 1.1836
It was discovered by j. j. Thomson in 1897 during the study of cathode ray properties.
He constructed the glass tube and create vacuum in it. He applied electric current between electrodes. He noticed that a ray of particles coming from cathode to wards positively charged anode. This ray was cathode ray.
Properties of cathode ray:
The ray is travel in straight line.
The cathode ray is independent of composition of cathode.
When electric field is applied cathode ray is deflected towards the positively charged plate.
Hence it was consist of negatively charged particles.
While neutron and proton are present inside the nucleus. Proton has positive charge while neutron is electrically neutral. Proton is discovered by Rutherford while neutron is discovered by James Chadwick in 1932.
Relative mass of proton= 1
Relative mass of neutron = 1
Relative mass of proton = +1
Relative charge of neutron = 0
The number of electron or number of protons are called atomic number while mass number of an atom is sum of protons and neutrons. The umber of protons and electrons are always equal to make the atom electrically neutral.
What is the molar mass of Al(BrO2)
Answers
Answer:
The molar mass is 138.8843 g/mol
PLEASE HELP
We wish to determine the moles of solid AgCl formed when 50.0 ml of 0.250 M AgNO3 reacts with excess MgCl2 according to the equation below.
2AgNO3(aq) + MgCl2(aq) 2Ag Cl(s) + Mg (NO3)2(aq)
In the previous step you determined 0.0125 mol AgNO3 react. How many moles of AgCl form during the reaction?
Answers
The number of moles of AgCl formed during the reaction is 0.0125 mol.
Given the reaction:2AgNO3(aq) + MgCl2(aq) → 2Ag Cl(s) + Mg (NO3)2(aq)We are supposed to determine the moles of solid AgCl formed when 50.0 ml of 0.250 M AgNO3 reacts with excess MgCl2 and in the previous step, we found that 0.0125 mol of AgNO3 reacts.
We can use the stoichiometry method to find the moles of AgCl formed.
To do so, we will have to balance the given chemical equation and find out the number of moles of AgCl formed from the given reactants.
The balanced chemical equation is:2AgNO3(aq) + MgCl2(aq) → 2Ag Cl(s) + Mg (NO3)2(aq)From the equation, we can say that 2 moles of AgCl form from 2 moles of AgNO3 reacted.
In the previous step, we have found the number of moles of AgNO3 reacted, which is 0.0125 mol.
As per the balanced chemical equation, 2 moles of AgCl form from 2 moles of AgNO3 reacted.
Therefore, the number of moles of AgCl formed = (0.0125 mol AgNO3 reacted × 2 moles AgCl / 2 moles AgNO3) = 0.0125 mol AgCl.
The number of moles of AgCl formed during the reaction is 0.0125 mol.
For more questions on AgCl
https://brainly.com/question/15393967
#SPJ8
11. A 4.175 gram sample of a certain hydrate of copper (II) sulfate, CuSO,• xH,O, is heated until all
the water is driven off. The resulting anhydrous compound weighs 3.120 grams. What is the
formula of the hydrate?
Answers
The formula of the hydrate = CuSO₄• 3H₂O
Further explanationGiven
4.175 grams sample CuSO₄• xH₂O
3.120 grams anhydrous compound CuSO₄
Required
The formula
Solution
mass of H₂O driven off :
= 4.175 - 3.12
= 1.055 g
MW CuSO₄ = 159.5 g/mol
MW H₂O = 18 g/mol
mol ratio of CuSO₄ : H₂O :
= 3.12/159.5 : 1.055/18
= 0.01956 : 0.05861
= 1 : 3
Based on the data provided, the formula of the hydrated salt is CuSO4.3H20
What is the formula of the hydrate?The formula of the hydrate is determined from the mole ratio of the anhydrous saltand water.
Mass of water = 4.175 - 3.120
mass of water = 1.055 g
Mole ratio= mass/molar mass
molar mass of CuSO4 = 160 g/mol
molar mass of H2O = 18 g/mol
CuSO4 = 3.120/160 = 0.0195
H2O = 1.055/18 = 0.0586
CuSO4 = 0.0195/0.0195 = 1
H2O = 0.0586/0.0195 = 3
Therefore, the formula of the hydrated salt is CuSO4.3H20
Learn more about hydrated salts at: https://brainly.com/question/24920157
The percent ionization of a 0.350 M HC,H,O2 solution. Ka(HC2H302)
Answers
Answer:
The answer is 56
Explanation:
i need points
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
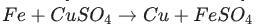
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
How does heat affect water and its state of matter
Answers
Answer:If a liquid is heated the particles are given more energy and move faster and faster expanding the liquid. The most energetic particles at the surface escape from the surface of the liquid as a vapour as it gets warmer. Liquids evaporate faster as they heat up and more particles have enough energy to break away.
Explanation:
When the volume of a gas is changed from 11.5 cm3 to __ cm3, the temperature will change from 415.0 K to 200.0 K

Answers
Answer:
Explanation:
Charles Law: The volume of and ideal gas is directly proportional to the temperature when the pressure is kept constant
V₁ - initial Volume = 11.5 cm³ ; T₁ = Initial temperature = 415 K ;
V₂ - final volume ;T₂ =final temperature = 200 K
\(\dfrac{V_{1}}{T_{1}}=\dfrac{V_{2}}{T_{2}}\\\\\\\dfrac{11.5}{415}=\dfrac{V_{2}}{200}\\\\\\\dfrac{11.5}{415}*200=V_{2}\\\\\\V_{2}=5.5 \ cm^{3}\)
Which of the following properties allows water molecules to stick to othe types of molecules?
A) Viscosity
B) Adhesion
C) Surface Tension
D) Density
Answers
Answer:
B.) Adhesion
Explanation:
A.) is incorrect. Viscosity is a measure of internal friction that affects the fluidity of a liquid.
B.) is correct. Adhesion is the ability of water to stick to other substances (which are not water).
C.) is incorrect. Surface tension is the tension created by the bonds of the surface particles.
D.) is incorrect. Density is a ratio that compares mass to volume.
The empirical formula for C6H12 is..? I don’t know what method or formula I need to solve this?
Answers
The empirical formula tells us how many atoms of each element are in a molecule regardless of the structure of the molecule, that is, regardless of which element is bonded to which element or what type of bond there is. It is the simplest way to represent a molecule.
As far as I can see the molecule C6H12 is already represented by its empirical formula, so in this case, you don't have to use any formula. You know that there are 6 atoms of carbon and 12 atoms of hydrogen, that is the information that the empirical formula gives.
So, the empirical formula for C6H12 is C6H12
When an oceanic plate and a continental plate collide, which one will subduct?
1. Oceanic
2.Continental
3.Both
4.Neither one
Answers
Both — rate :) hope this helps
When an oceanic plate and a continental plate collide, both will subduct. Therefore, option 3 is correct.
What is an oceanic plate ?Oceanic plates are plates found beneath the ocean. As previously stated, oceanic plates are denser than continental plates. This is due to the fact that they are made of denser rocks. Basalt is the most common material found in oceanic plates.
When compared to continental crust, oceanic crust formed at spreading ridges is relatively homogeneous in thickness and composition.
Because oceanic crust is naturally denser, when an oceanic plate converges with a continental plate, the oceanic crust will always subduct beneath the continental crust. Convergent boundaries are frequently linked to larger earthquakes and increased volcanic activity.
Thus, option 3 is correct.
To learn more about an oceanic plate, follow the link;
https://brainly.com/question/13569781
#SPJ6
You have a 5M stock solution of NaCl (Formula Weight: 58.44g/mole), a 0.25M stock solution of glucose (Formula Weight; 180.156g/mole), and a bottle of solid Tris base (Formula Weight: 121.1g/mole). How would prepare (be specific) 250mL of a single solution containing 150mM Tris, 25mM glucose, and 150mM NaCl. g
Answers
Answer:
4.54g of Tris base,25mL of the 0.25M stock solution of glucose and 7.5mL of the 5M stock solution of NaCl must be added and complete the volume in a volumetric flask to 250.0mL
Explanation:
To prepare the single solution we need to find the moles of each solute (Tris, glucose and NaCl) from the stock solutions anf the solid:
Moles Tris:
0.250L *(0.150mol / L) = 0.0375moles Tris * (121.1g/mol) = 4.54g of Tris base must be added
Moles glucose:
0.250L * (0.025mol/L) = 6.25x10⁻³mol glucose * (1L / 0.25mol) = 0.025L = 25mL of the 0.25M stock solution of glucose must be added
Moles NaCl:
0.250L * (0.150mol / L) = 0.0375mol NaCl * (1L / 5mol) = 0.0075L =
7.5mL of the 5M stock solution of NaCl
You must add:
4.54g of Tris base,25mL of the 0.25M stock solution of glucose and 7.5mL of the 5M stock solution of NaCl must be added and complete the volume in a volumetric flask to 250.0mL
why is often difficult to identify a highly weathered mineral
Answers
Weathering changes the chemical and physical nature of an element that is why it is often difficult to identify a highly weathered mineral.
The breakdown and alteration of rocks and minerals at or near the Earth's surface as a result of exposure to various weathering agents, such as water, wind and temperature changes is known as weathering.
Minerals can undergo physical changes as a result of weathering, such as being broken up into smaller pieces or having their color and texture altered. Additionally, it may result in chemical alterations such as the removal or addition of specific chemical components.
This may lead to the creation of brand-new minerals or the modification of already existing minerals into new ones. Highly weathered minerals might not still possess the same physical and chemical characteristics as their unweathered counterparts.
Learn more about Weathering at:
brainly.com/question/29057766
#SPJ1
A flashbulb of volume 1.70 mL contains O2(g) at a pressure of 2.30 atm and a temperature of 18.0°C. How many grams of O2(g)
the flashbulb contain
Answers
Answer:
0.0001637 mol
Explanation:
PV = nRT
Very important formula in chem
2.3 atm * 0.0017 L = n * 0.082057 * 291 K
= 0.0001637 mol O2
This concept is very important in chem, practice it.
A large stone weighs 53.0kg. How many pounds does it weigh?
Answers
Answer:
116.6 lbs
Explanation:
There are 2.2 lbs per Kilogram of weight - and likewise 0.454 Kilograms per pound - but instead of dividing by .454 I multiplied the weight by 2.2 to get 116.6 pounds (of course you could round up and get 117 but 116.6 is a little more accurate).
The weight of the stone in pounds will be 116.8 pounds.
We have a large stone of which weighs 53 Kg.
We have to find its weight in pounds.
How many pounds are equivalent to 1 Kg ?In 1 kilogram there are 2.2046 lbs.
According to the question -
Weight of stone in kilograms = 53 Kg
Assume that the weight of stone is equal to A kg. Then -
A = 53 Kg
Now, in order to convert A kg into pounds, we will multiply it by 2.2046.
Therefore, Weight of stone in kilograms is equivalent to = 53 x 2.2046 = 116.8 pounds.
Hence, the weight of the stone in pounds will be 116.8 pounds.
To solve more questions on unit conversions, visit the link below-
https://brainly.com/question/13091877
#SPJ2
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide.
Part A
Enter a balanced chemical equation for this reaction.
Answers
Answer:
Explanation:
The balanced chemical equation for the reaction between solid sodium and liquid water to produce hydrogen gas and aqueous sodium hydroxide is:
2 Na (s) + 2 H2O (l) -> 2 NaOH (aq) + H2 (g)
In this reaction, two atoms of sodium (Na) react with two molecules of water (H2O) to produce two molecules of sodium hydroxide (NaOH) in aqueous form and one molecule of hydrogen gas (H2).
You place a ton of radioactive material into a lead box and close the box.
What radioactive decay particle(s) will escape the box?
Answers
Answer:
Beta particles and neutrons
Explanation:
Lead is not particular good for shielding beta particles and neutrons. Lead shielding is used for shielding x-rays and gamma-rays due to its high density and atomic mass.
Materials made with lead are used by people dealing with all kinds of radioactive particles to prevent radioactive poisonings.
But lead cannot effectively protect against beta particles and neutrons.
What are two different forms of a single gene called?
A.factors
B.alleles
C.traits
D.chromosomes
Answers
Answer:
B. Alleles
Explanation:
calculate the mass of nickel metal which will react with 25mL of 0.15mol/L (
hydrochloric acid to produce nickel (II) chloride. 0.110625g
Answers
Answer:
0.110625 g of Ni
Explanation:
The first step in solving this problem is to put down the accurate chemical reaction equation.
Ni(s) + 2HCl(aq) ---> NiCl2(aq) + H2(g)
Secondly, we obtain the amount of HCl that reacted from the information provided.
Volume of HCl (V)= 25 ml
Concentration of HCl (C)= 0.15 mol/L
Then, to find the number of moles of HCl (n);
n= CV
Substitution values
n= 25/1000 × 0.15
n= 3.75 ×10^-3 moles
Mass of 3.75 ×10^-3 moles of HCl = number of moles × molar mass
Molar mass of HCl= 36.5 gmol-1
Therefore;
Mass of HCl = 3.75 ×10^-3 moles × 36.5 gmol-1
Mass of HCl= 0.136875 g of HCl
Thirdly we determine the mass of Ni reacted;
If 1 mole of Ni reacted with 2 moles of HCl according to the reaction equation
Then x moles of Ni reacts with 3.75 ×10^-3 moles of HCl
x= 1 × 3.75 ×10^-3 moles/ 2
x= 1.875 × 10^-3 moles of Ni
Mass of Ni= 1.875 × 10^-3 moles of Ni × 59 gmol-1
Mass of Ni= 0.110625 g of Ni
Which of these waves has the greatest wavelength? (3 points) Wave shown with 2 wavelengths. Wave shown with 3 wavelengths. Wave shown with 1 wavelength stretch over a short distance. Wavelength shown with 1 wavelength stretched over a long distance.
Answers
The waves that has the greatest wavelength is Wavelength shown with 1 wavelength stretched over a long distance.
Waves explained.A wave could be a disturbance or variety that voyages through a medium or space, carrying vitality without transporting matter. Waves can take different shapes and happen totally different sorts of waves, counting mechanical waves and electromagnetic waves.
Mechanical waves require a medium to propagate, meaning they require a substance like water, discuss, or a strong fabric to transmit the wave. Illustrations of mechanical waves incorporate water waves, sound waves, and seismic waves. In these waves, particles of the medium sway or vibrate in a design, exchanging energy from one molecule to another.
Electromagnetic waves, on the other hand, don't require a medium and can travel through vacuum, such as in space. Electromagnetic waves comprise of electric and attractive areas swaying opposite to each other and to the heading of wave engendering. Illustrations of electromagnetic waves incorporate obvious light, radio waves, microwaves, infrared waves, bright waves, X-rays, and gamma beams.
Learn more about waves below.
https://brainly.com/question/26116832
#SPJ1
Draw 4 decyne structure
Answers
The structure of the alkyne 4 - decyne is shown in the image attached.
How do you draw 4-decyne?Alkyne functional group compounds with a triple bond made of carbon and carbon contain 4-decyne (C). The triple bond is found at the fourth carbon position in the ten-carbon chain (decyne) of 4-decyne.
Nine hydrogen atoms (H) are joined to the first nine carbon atoms (C), starting from the left side.
In conclusion, 4-decyne is made up of a chain of ten carbon atoms, with the fourth carbon atom serving as the center of a carbon-carbon triple bond (C). Except for the carbon involved in the triple bond, all carbon atoms are connected to hydrogen atoms.
Learn more about chemical structure:https://brainly.com/question/32300619
#SPJ1

What are some factors that influence the effectiveness of a chemical sanitizer?
Answers
Chlorine is the most commonly used chemical sanitizer agent and there are so many factors that influences the effectiveness of these chemical sanitizers like- Temperature, Concentration, Contact time, Water Hardness, pH but bacterial cell history does not affect the efficiency of sanitizers.
Factors influencing the effectiveness of chemical sanitizer:
Temperature: Temperature for the sanitizer should lie between 75°F and 120°F to work properly. At the higher temperatures, chlorine compounds may cause corrosion to some metal items. Concentration: If concentration of sanitizing agent is too high, it will be toxic and lower concentration result in an inadequate reduction of microorganismsContact time: If the contact time of sanitizer is too long, it evaporates before achieving the desired disinfection.Water hardness: Hard Water reduces the effectiveness of sanitizer.pH: With raise in pH, chlorine becomes less effective as a sanitizer.To know more about Sanitizers:
brainly.com/question/9635668
#SPJ4
What happens when a pan full of water is heated on a stove?

Answers
• As the liquid is heated, the particles in liquids gain kinetic energy when they are heated and start to move faster.
,• So, option B is correct, ,kinetic energy of water moleculs increases.
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8