Answers
The number of moles of \(MnO_2\) required is 3.345 moles.
In the given reaction, the balanced equation shows that for every 4 moles of \(MnO_2\), 4 moles of \(KMnO_4\) are produced. Therefore, we can use the stoichiometry of the reaction to calculate the moles of \(MnO_2\) and the moles of \(KMnO_4\)
Given:
Mass of \(MnO_2\) = 291 g
Molar mass of\(MnO_2\) = 87 g/mol
To find the moles of \(MnO_2\), we use the formula:
Moles = Mass / Molar mass
Moles of \(MnO_2\) = 291 g / 87 g/mol = 3.345 mol
Now, since the stoichiometry of the reaction tells us that the ratio of \(MnO_2\)to \(KMnO_4\) is 4:4, we can conclude that 3.345 moles of \(MnO_2\)will produce an equal number of moles of \(KMnO_4\)
Therefore, the moles of \(KMnO_4\) produced will also be 3.345 mol.
However, the question asks for the moles of NaOH, which is not directly related to the given reaction. We cannot determine the moles of NaOH based on the information provided.
To find the moles of NaOH, we would need additional information or another relevant equation that includes NaOH.
Know more about moles here:
https://brainly.com/question/29367909
#SPJ8
Related Questions
What happens to the amount of solution when we add food colour to it?
Answers
Answer:
We need more? What else is in the question? This is unanswerable.
Explanation:
Scientists digging in a cave found an unknown substance. The scientists found that the substance’s molecules were moving around each other. The scientists increased the speed of the substance’s molecules and caused a phase change. How did the scientists do this, and how did this affect the substance?
The scientists transferred energy . . .
Answers
Answer:
By adding heat energy It became a gas Explanation:The molecules moving about another is a clear indicator that the substance is a liquid. In a liquid, the molecules stick together and move about one another. When the scientist increase the speed of the of substance, the kinetic energy increases. if this increase causes a phase change, then the substance becomes a gas. So, by adding heat energy a substance becomes gaseous if it is a liquid already.
Explanation:
Decide which element probably forms a compound with chlorine that has a chemical formula most and least similar to the chemical formula of the compound formed by chlorine and strontium.

Answers
Answer:
a. Beryllium,
b. Nitrogen
Explanation:
Most similar to strontium is beryllium, they both are in the 2d group.
Least similar to strontium is nitrogen. Strontium is a metal in the 2d group, Nitrogen is non-metal in the 15th group
What type of reaction is shown below:
Al₂O3 (s) → Al(s) + +O₂(g)
O Acid/Base Neutralization
O Synthesis
O Double Replacement
O Decomposition
O Single Replacement
O Combustion
Answers
Answer: Decomposition
Explanation:
The compound, (Aluminum Oxide), decomposes to form Aluminum and Oxygen.
To determine the mass of CO2 that forms from a given mass of CaCO3, which of the following must be known? Check all that apply.
Answers
To determine the mass of CO₂, the following must be known :
the molar mass of CaCO₃ the mole ratio of CaCO₃ to CO₂ the molar mass of CO₂Further explanationReaction
Decomposition of CaCO₃
CaCO₃ ⇒ CaO + CO₂
Given the mass of CaCO₃, so to determine the mass of CO₂ :
1. Find the mol of CaCO₃ from the molar mass of CaCO₃
\(\tt n_{CaCO_3}=\dfrac{mass~CaCO_3}{MW~CaCO_3}\)
2. Find the mole ratio of CaCO₃ : CO₂(from equation = 1 : 1)
\(\tt n_{CaCO_3}\div n_{CO_2}=1\div 1\)
3. Find the mass of CO₂ from the molar mass of CO₂
\(\tt mass_{CO_2}=n_{CO_2}\times MW_{CO_2}\)
Answer:
1 3 and 5
Explanation:
What would be the volume in liters of an 25.15 liter sample of gas at 201 °C and 2.31 atm if conditions were changed to STP?
Answers
The volume of the gas at STP would be 23.93 liters.
The volume of gas at STP (Standard Temperature and Pressure), we need to use the Ideal Gas Law, which states that PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the gas constant, and T is temperature. First, we need to calculate the number of moles of gas in the initial sample. We can use the formula n = PV/RT, where P is the initial pressure, V is the initial volume, R is the gas constant, and T is the initial temperature.
n = (2.31 atm) x (25.15 L) / [(0.0821 L atm/mol K) x (201 + 273.15 K)]
n = 1.067 moles
Now, we can use the molar volume of gas at STP, which is 22.4 L/mol, to calculate the volume of gas at STP.
V = n x 22.4 L/mol
V = 1.067 moles x 22.4 L/mol
V = 23.93 L
Therefore, the volume of the gas at STP would be 23.93 liters.
For more such questions on gas
https://brainly.com/question/25736513
#SPJ11
PLEASE ANSWER QUICKLY!!
100 NaNO3
90
Solute per 100 g of H₂O (g)
0,80
NH,CI
70 KNO3
60
50
40
30
20
10
0
0 10 20 30 40 50 60 70 80 90 100
Temperature (°C)
KCIO3
60 g KNO3 has
been added to
100 g H₂O at
30 °C. What
type of solution
is this?
A. unsaturated
B. saturated
C. supersaturated

Answers
If 60 grams of the substance are added to 100 g of water, the solution can be categorized as supersaturated.
How saturated is this solution?The graph shows the number of grams that can be dissolved in 100 grams of water at different temperatures. In general, solubility increases with temperature.
According to the graph, at a temperature of 30°C, it is possible to dissolve a total of 48 to 49 grams of \(KNO_{3}\). This information implies that if we add 60 grams at this temperature not all the substance would be dissolved, and therefore the solution would be supersaturated.
Learn more about solubility in https://brainly.com/question/31493083
#SPJ1
Did the entropy of the ice cubes increase or decrease over time?
Answers
The distances in light years between both the Sun and the Solar System's planets are listed below. Mercury: 3.33 light minutes 6 light minutes for Venus Planet Earth: 8.3 light minutes Martian: 12,7 light minutes Jupiter: 43 light-minutes
In particular, the distance travelled by electromagnetic waves (with the term light referring to the spectral fraction visible from the human eye) in the vacuum during a sidereal year is described as the length of a light year (365 Days, 6 hours, 9 minutes, and 10 seconds).
In the vacuum, light travels at a speed of 299 792,458 km/s (c). Simply multiply that amount by the time period under consideration to get the distance in miles. 9461 billion kilometres, roughly (or 63 241 times the distance between Earth and the Sun, also called astronomical units, is 149 597 870,700 km).
3,3 light minutes for Mercury
Venus: six minutes of light
8,3 light minutes on Earth
12,7 light minutes for Mars
: 43 light minutes for Jupiter
1,3 light hours for Saturn
to know more about light, visit this: -
https://brainly.com/question/15200315
#SPJ1
Most jet fuel has an average molecular formula of C13H28 and a density of 804 g/L. Calculate the moles of carbon that are released from the fuel when a jet takes from Charlotte, NC to San Francisco, CA, a distance of 3680 km. Assume that the jet consumes 3.48 L of fuel per 100. km.
Answers
Answer:
Number of moles = 558.46 mol
Explanation:
Density = 804 g/L
Molecular formula = C13H28
Molar mass = 184.37 g/mol
Distance = 3680 km
Rate = 3.48 L of fuel per 100. km
Volume is calculated as follow;
3.48 = 100
x = 3680
x = 3680 * 3.48 / 100
x = 128.064 L
Number of moles = Mass / Molar mass
Mass = Density * Volume
Mass = 804 * 128.064 = 102963.456 g
Number of moles = 102963.456 / 184.37
Number of moles = 558.46 mol
The high concentration of salt in the medullary fluid is believed to be achieved in the loop by a process known as countercurrent exchange multiplication. The principle of this process is analogous to the physical principle applied in the conduction of hot exhaust gases past cold incoming gas so as to warm it and conserve heat.
Answers
The counter-contemporary mechanism Increasing salt consumption expanded sodium excretion, however additionally suddenly precipitated the kidney to preserve water.
Excess sodium become for this reason launched in focused urine. This technique of protective the body's water become so green that the guys without a doubt drank much less whilst their salt consumption become highest.
The counter-contemporary multiplier or the countercurrent mechanism is used to pay attention urine withinside the kidneys with the aid of using the nephrons of the human excretory system. The nephrons concerned withinside the formation of focused urine increase all of the manner from the cortex of the kidney to the medulla and are observed with the aid of using vasa recta.As already indicated, the loop of Henle is essential to the cappotential of the kidney to pay attention urine.
Read more about salt:
https://brainly.com/question/13818836
#SPJ4
4. Solve the following heat flow problem, being sure to show all your work (you may either type your
work or insert an image): Find the specific heat of 402 grams of graphite that absorbs 1136) of heat
energy as it changes temperature from 26°C to 30°C.
Answer should be 0.7J/gC
Answers
Answer:
0.70 J/g.°C
Explanation:
Step 1: Given data
Mass of graphite (m): 402 gHeat absorbed (Q): 1136 JInitial temperature: 26°CFinal temperature: 30 °CSpecific heat of graphite (c): ?Step 2: Calculate the specific heat of graphite
We will use the following expression.
Q = c × m × ΔT
c = Q / m × ΔT
c = 1136 J / 402 g × (30°C - 26°C)
c = 0.70 J/g.°C
How does shielding impact the strength of attraction between the nucleus and the valence electrons?
Answers
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
For the reaction
4PH3(g)↽−−⇀6H2(g)+P4(g)
the equilibrium concentrations were found to be [PH3]=0.250 M, [H2]=0.580 M,
and [P4]=0.750 M.
What is the equilibrium constant for this reaction?
c=
Answers
The equilibrium constant for the reaction given that the equilibrium concentration of [PH₃] = 0.250 M, [H₂] = 0.580 M, and [P₄] = 0.750 M is 7.3
How do I determine the equilibrium constant?From the question given above, the following data were obtained:
Equation: 4PH₃(g) ⇌ 6H₂(g) + P₄(g)Concentration of PH₃, [PH₃] = 0.250 MConcentration of H₂, [H₂] = 0.580 MConcentration of P₄, [P₄] = 0.750 MEquilibrium constant (K) =?The equilibrium constant for the reaction can be obtained as shown below:
Equilibrium constant = [Product]ᵐ / [Reactant]ⁿ
Where
m and n are coefficients of products and reactants respectivelyEquilibrium constant = [H₂]⁶[P₄] / [PH₃]⁴
Equilibrium constant = [(0.580)⁶ × 0.750] / (0.250)⁴
Equilibrium constant = 7.3
Thus, the equilibrium constant for the reaction is 7.3
Learn more about equilibrium constant:
https://brainly.com/question/16589765
#SPJ1
Ammonia can be prepared by the reaction of magnesium nitride with water. The prod- ucts are ammonia and magnesium hydroxide. When the equation is written and balanced, the coefficient of magnesium hydroxide is
Answer Options : 1,3,6,8
Answers
Answer:
3Explanation:
This is how to write the balanced equation for the reaction of magnesium nitride with water to create ammonia and magnesium hydroxide:
1. Let's begin with the improper equation:
NH3 + Mg(OH)2 from Mg3N2 + H2O
2. Correct the coefficients to bring the equation into balance:
6H2O + 3Mg3N2 2NH3 + 3Mg (OH)2
Magnesium hydroxide (Mg(OH)2) has a coefficient of 3.
Hope it helps! : )
A gas has a pressure of 6.5 atm at a temperature of 500. K. At what temperature will the pressure be 4.8 atm?
Answers
To solve this question we have use Gay-Lussac's Law:
\(\frac{P1}{T1}=\frac{P2}{T2}\)Where P1 and P2 are the pressures and T1 and T2 are the temperatures.
In this case, we know the values of P1, P2 and T1 and we have to find T2:
\(\begin{gathered} T2=T1\cdot\frac{P2}{P1} \\ T2=500K\cdot\frac{4.8atm}{6.5atm} \\ T2=369.23K \end{gathered}\)The pressure will be 4.8atm at 369.23K.
Vishwanath had some money. he spent 3 upon 4 part of money to buy goods for his birthday,1 upon 5 part of money give to his sister and the rest of Rs.40 to mother how much did he have at first
Answers
Answer:
The correct answer is - 800.
Explanation:
Given:
Total amount = ? or assume x
spend in buying birthday item = 3/4 of x
given to sister = 1/5 of x
remaining to mother = 40
solution:
the remaning amount = x- (3x/4+x/5) = 4=
=> x- 19x/20 = 40
=> x = 20*40
=> x = 800
thus, the correct answer is = 800
A molecule or ion that donates the hydrogen in a hydrogen bond is a hydrogen bond donor
a. True
b. False
Answers
Answer:
True
Explanation:
Hydrogen bonding is a type of intermolecular interaction that occurs when hydrogen is bonded to a highly electronegative atom.
We define the term ''hydrogen bond donor'' as the molecule that supplies the hydrogen atom in the hydrogen bond.
Hence, it is true that the molecule or ion that donates the hydrogen in a hydrogen bond is a hydrogen bond donor
ASAP PLEASE!!!B. Complete the drawing for the sample reaction below to show the law of conservation of
mass, when XY is produced.
+
->
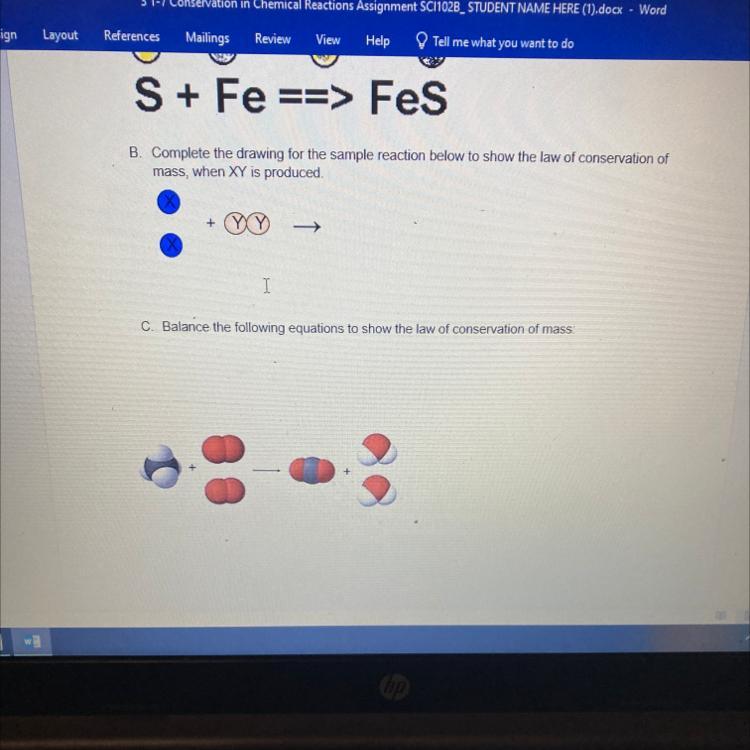
Answers
The complete reaction, according to the law of conservation of mass is:
XX + YY → 2XY
The Law of Conservation is a fundamental principle in chemistry and physics. It states that in a closed system, mass cannot be created or destroyed during a chemical reaction or a physical change. The total mass of the substances involved before the reaction or change must equal the total mass of the substances after the reaction or change.
This principle is based on the understanding that atoms are not created or destroyed, but they can combine or separate to form different substances.
Learn more about the law of mass conservation, here:
https://brainly.com/question/28711001
#SPJ1
The meaning of the word symptom:
Answers
The word "symptom" refers to a specific manifestation or indication of a condition, disease, or disorder that is experienced or observed by an individual.
Symptoms are subjective or objective changes in the body's normal functioning that may be recognized as abnormal, uncomfortable, or problematic. Symptoms can manifest in various ways depending on the nature of the underlying condition. They can be physical, such as pain, rash, cough, fever, or fatigue, indicating an illness or injury affecting the body. Symptoms can also be psychological, such as anxiety, depression, or confusion, reflecting disturbances in mental health.
Symptoms serve as important clues for medical professionals to identify and diagnose diseases or disorders. They provide valuable information about the nature, severity, and progression of an illness, helping healthcare providers formulate appropriate treatment plans. Additionally, symptoms may also be important for individuals to self-assess their own health status and seek appropriate medical attention.
It is essential to note that symptoms alone may not provide a definitive diagnosis, as they can overlap across different conditions. Further evaluation, including medical tests and examinations, is often necessary to confirm a diagnosis and determine the appropriate course of action.
for more such questions on symptom
https://brainly.com/question/21078887
#SPJ8
which element has the electrons configuration 1s22s22p63s23p64s23d104p2
Answers
The element with the electron configuration 1s22s22p63s23p64s23d104p2 is Silicon (Si).
Explanation:
The electron configuration of an element describes the arrangement of electrons in its atoms. The numbers and letters in the configuration represent the energy levels (n), sublevels (s, p, d, f), and the number of electrons in each sublevel.
In this case, the electron configuration of the element is:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2
Breaking this down, we can see that the element has:
- 2 electrons in the 1s sublevel
- 2 electrons in the 2s sublevel
- 6 electrons in the 2p sublevel
- 2 electrons in the 3s sublevel
- 6 electrons in the 3p sublevel
- 2 electrons in the 4s sublevel
- 10 electrons in the 3d sublevel
- 2 electrons in the 4p sublevel
Based on the number of electrons in the outermost energy level (valence electrons), we can determine that this element is in group 14 of the periodic table. Looking at the periodic table, we can see that the
More than half the total present volume of ocean water resides in what ocean?
Answers
The Pacific Ocean is the largest ocean in the world based on water volume, totaling some 660 million cubic kilometers and is almost equally divided into the North and South Pacific waters.
Name the following compounds
H
H
Н
CH
H
=
C-C-
-ċ-
H
-C
0-I
H-C-
H
H
H
Answers
Ionic compounds can be made from a metallic ion and a nonmetallic ion. Metallic ions (M) are positive (cations). Nonmetallic ions (N) are negative (anions). x stands for the number of metallic ions and y stands for the number of nonmetallic ions. For each equation given below, enter the number in the space provided to correctly answer the question.
x(+1M) + (–2N) = 0
What does x have to be?
(+2M) + y(–1N) = 0
What does y have to be?
x(+2M) + y(–3N) = 0
What do x and y have to be? x =
and y =
Answers
The x and y shows the number of atoms that participate or taking part in the chemical reaction.
What does x and y indicate?The x and y are the number of atoms that is bonded to make a stable compound because for bonding suitable number of atoms are needed to make bond.
So we can conclude that x and y shows the number of atoms that participate or taking part in the chemical reaction.
Learn more about ionic here: https://brainly.com/question/2687188
#SPJ1
Answer:x(+1M) + (–2N) = 0
What does x have to be? ⇒ 2
(+2M) + y(–1N) = 0
What does y have to be? ⇒ 2
x(+2M) + y(–3N) = 0
What do x and y have to be? x = ⇒ 3
and y = ⇒ 2
Explanation: )
Indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium after changing the concentration of the reactant or product. An up arrow indicates an increase in concentration, a down arrow indicates a decrease in concentration, and leaving it blank means there is no change in the concentration. after the concentration of Br₂ is decreased after the concentration of HBr is increased H₂(g) + Br₂(g) O 0 □ ↓ Answer Bank ↑ 2HBr(g) 0 □

Answers
The concentration of species in the reaction changes the reaction in the following ways: decrease carbon dioxide = forward direction of reaction.
The direction of the reaction can be assessed by the following. On increasing the concentration of the reactant the reaction processes in the forward direction. On increasing the concentration of product the reaction processes in the backward direction.
The given equilibrium is:
2CO(g) + O2(g) ↔ 2CO2(g)
increase CO = forward direction of reaction
increase oxygen = forward direction of reaction
decrease CO = no change in equilibrium as reaction not processes.
decrease oxygen = no change in equilibrium as reaction not processes.
increase carbon dioxide= reverse direction
decrease carbon dioxide = forward direction of reaction.
For more information about the direction of reaction, refer to the link: brainly.com/question/2400156
#SPJ9
write a short note on importance of air
Answers
Why is there an imbalance in the carbon cycle?
Answers
In science class, the students planned and conducted an investigation to learn about specific heat capacity. They collected data and created the following data table:
Data Table
Material | Specific heat capacity (cal/g/°C)
Dry soil | 0.2
Water | 1
Oil | 0.4
Use these data and the concept of hydrogen bonding to explain why water has such a high specific heat capacity. Then, apply this idea to explain why coastal areas and those near large bodies of water have much more moderate climates than inland areas.
Answers
The presence of large bodies of water helps to stabilize temperature changes in coastal areas.
Water has a high specific heat capacity due to the presence of hydrogen bonding between its molecules. Hydrogen bonding occurs when the positively charged hydrogen atom of one water molecule is attracted to the negatively charged oxygen atom of another water molecule. This bonding is stronger than the typical intermolecular forces found in other substances.
Hydrogen bonding in water requires a significant amount of energy to break, which leads to the high specific heat capacity of water. This means that water can absorb and store a substantial amount of heat energy without a significant increase in temperature. Conversely, when water loses heat, it releases a significant amount of energy before its temperature decreases.
In coastal areas and regions near large bodies of water, the high specific heat capacity of water plays a crucial role in moderating climates. Water acts as a heat sink, absorbing heat during the day and releasing it at night. This leads to milder temperature fluctuations compared to inland areas, which have lower specific heat capacities. As a result, coastal regions experience cooler summers and warmer winters, providing a more moderate climate overall.
The presence of large bodies of water helps to stabilize temperature changes in coastal areas, providing a buffering effect and contributing to the moderation of the climate.
for more such question on temperature visit
https://brainly.com/question/4735135
#SPJ8
Can you help me with this homework question please. I

Answers
1) Rate of a reaction. This is the speed at which a reaction takes place. At the beginning of a reaction, we have pure reactants and no products. This makes the reaction take place at maximum speed which gradually decreases as it reaches the equilibrium. At this point, the forward and backward rates are the same.
The rate of the forward reaction is at its maximum when pure NO gas is first placed in the container.
.
1. When you transfer energy into a substance, the temperature
a. Increases
b. Decreases
C. Stay the Same
Answers
Answer:
increase
Explanation: