Answers
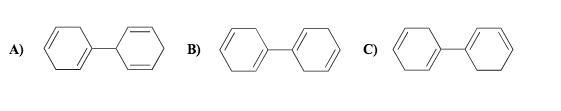
Hello. You forgot and show the compounds. The compounds are in the attached image.
Answer:
I < II < III
Explanation:
The stability of the presented compounds must be analyzed, taking into account the free electrons to form arrangements between the atoms. In this case, the molecules of greater stability follow the concept of valence and are characterized as those that manage to reach a large number of elements that manage to obey the octet rule.
Related Questions
How many sig figs are in 0.0003422
Answers
Answer:
4
Explanation:
The zeros before a non zero digit do not count as significant figures so there are 4 sig figs in the number
HELPPPP FASTTTTTTT
An inflated balloon is left outside overnight. It has a volume of 1.7 4 L when the temperature is 20.2 degC and the pressure is 1.02 atm. At what temperature will the balloon have a volume of 1.56 L if the pressure falls to 0.980 atm?
What gas law will you use to solve this problem?
options
A. boyles gas law
B. charles gas law
C. Gay lussacs gas law
D. combined gas law
E. ideal gas law
What is your reason for choosing the gas law?
options
A.moles are in the problem
B. there are 2 variables that are changing
C. there are 3 variables that are changing
In this problem, is the volume increasing or decreasing?
What is the unknown that you are solving for?
options.
A.T1
B.T2
What temperature will you use in your calculations?
A. 20.2 degc
B. 273 k
C. 293.2 K
What is the final temperature after the volume and pressure decrease in this problem?
options.
A. 253k
B. 314k
C. 353 K
Answers
Explanation:
1.The gas law that we will use to solve this problem is the combined gas law.
2.We will choose the combined gas law because there are three variables that are changing in this problem: volume, pressure, and temperature.
3The volume is decreasing in this problem, from 1.74 L to 1.56 L.
The unknown that we are solving for is T2, the temperature at which the balloon will have a volume of 1.56 L.
4We will use the temperature in Kelvin for our calculations. To convert from Celsius to Kelvin, we add 273.15 to the Celsius temperature. Therefore, the temperature we will use in our calculations is 293.35 K (20.2°C + 273.15).
5To find the final temperature, we can use the combined gas law equation:
(P1 x V1)/T1 = (P2 x V2)/T2
where P1 = 1.02 atm, V1 = 1.74 L, T1 = 293.35 K, P2 = 0.980 atm, and V2 = 1.56 L.
Substituting the values into the equation and solving for T2, we get:
T2 = (P2 x V2 x T1)/(P1 x V1)
= (0.980 atm x 1.56 L x 293.35 K)/(1.02 atm x 1.74 L)
= 268.06 K
Therefore, the final temperature after the volume and pressure decrease is 268.06 K, which is approximately 253 K (option A).
can someone help me with my chemistry homework please???

Answers
1.) Lithium and Sulfide:
Formula: \(\bold{Li_{2}S}\)Ion Charges: \(\bold{Li~1+,~Li~1+,~S~2-}\)2.) Lithium and Chlorine:
Formula: \(\bold{2LiCl}\)Ion Charges: \(\bold{Li~1+, Li~1+,Cl~1-,Cl~1-}\)3.) Lithium and Oxygen:
Formula: \(\bold{Li_{2}O}\)Ion Charges: \(\bold{Li~1+,Li~1+,O~2-}\)4.) Lithium and Nitrogen:
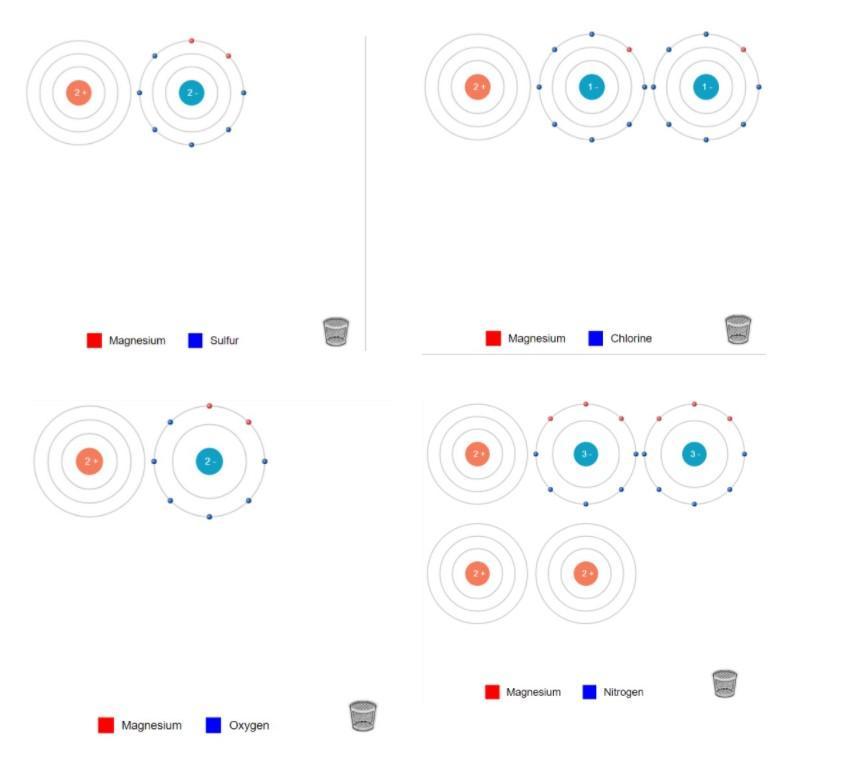
Formula: \(\bold{Li_{3}N}\)Ion Charges: \(\bold{Li~1+,Li~1+,Li~1+,N~3-}\)5.) Magnesium and Sulfur:
Formula: \(\bold{MgS}\)Ion Charges: \(\bold{Mg~2+,S~2-}\)6.) Magnesium and Chlorine:
Formula: \(\bold{MgCl_2}\)Ion Charges: \(\bold{Mg~2+,Cl~1-,Cl~1-}\)7.) Magnesium and Oxygen:
Formula: \(\bold{MgO}\)Ion Charges: \(\bold{Mg~2+,O~2-}\)8.) Magnesium and Nitrogen:
Formula: \(\bold{Mg_3N_2}\)Ion Charges: \(\bold{Mg~2+,Mg~2+,Mg~2+,N~3-,N~3-}\)Explanation:______________________________
Lithium and Sulfur: In order to make Lithium Sulfide, There must be 2 Lithium and 1 Sulfur. You transfer the electrons from both Lithium's to the Sulfur.Lithium and Chlorine:In order to make Lithium Chloride, There must be 2 Lithium and 2 Chlorine. You transfer the electrons from both Lithium's to the Chlorines, (One electron for each chlorine.)Lithium and Oxygen:In order to make Lithium Oxide, There must be 2 Lithium and 1 Oxygen. You transfer the electrons from both Lithium to Oxygen. Lithium and Nitrogen:In order to make Lithium Nitride, There must be 3 Lithium and 1 Nitrogen. You transfer the electrons from all 3 Lithium to Nitrogen. Magnesium and Sulfur:In order to make Magnesium Sulfide, There must be 1 Magnesium and 1 Sulfur. You transfer the both electrons from Magnesium to Sulfur. Magnesium and Chlorine:In order to make Magnesium Chloride, There must be 1 Magnesium and 2 Chlorine. You transfer on electron to each Chlorine. Magnesium and Oxygen:In order to make Magnesium Oxide, There must be 1 Magnesium and 1 Oxygen. You transfer both electrons from Magnesium to Oxygen. Magnesium and Nitrogen:In order to make Magnesium Nitride, There must be 3 Magnesium and 2 Nitrogen. You transfer 3 electrons from Magnesium to Nitrogen.______________________________

if salt and sand is mixed with distilled water, what will be the residue and what will be the filtrate?
Answers
Answer:
salt and sand
Explanation:it is what it is
Protons and neutrons have opposite, but equal magnitude, charges. An atom contains the same number of protons and electrons Neutrons and electrons are found in the nucleus of an atom. Protons have about the same mass as electrons. Electrons make up most of the mass of an atom. which one is true about subatomic particles
Answers
Answer:
the true statement is an atom contains the same number of electron and protonExplanation:
let us see the behaviour of the sub atomic particles
nutrons and protons found in the nucleus of an atoman atom have the same number of electron and proton but they have different chargenutron is chargeless particlemost mass of the atom concentrated in the nucleus of an atomelectrons have almost 0 mass from an atommass of proton and mass of nutron are equvalent or almost equalthere are many properties of subatomic particles i have listed some of them above .
I think it is help ful for youWhen KNO3 dissolved in water, what is the intermolecular
attraction between NO3-ions and H2O molecules?
Answers
Answer:Ion-dipole
Explanation:
How many grams of NaCl

Answers
You would recover 36.525g of NaCl after evaporating all of the water.
How to find the how many grams of NaCl that would be recover when all water is evaporated off of this solution?To find the grams of NaCl that would be recovered after evaporating all the water, we can use the following formula:
mass = moles * molar mass
Where:
Moles = Molarity * Volume
Molarity = 0.250 M
Volume = 2500.0 mL = 2.5 L
Molar mass of NaCl = 58.44 g/mol
mass = 0.250 M * 2.5 L * 58.44 g/mol
mass = 36.525 g
Learn about evaporation here https://brainly.com/question/2013258
#SPJ1
ILL GIVE BRAINLY PLEASE HELP!!! What type of transport across the cell (plasma) membrane requires energy?
active transport
bilayer
passive transport
concentration gradient
Answers
Active transport requires energy to transport the molecules across the cell membrane. Thus, Option A is correct.
Active transport is the transport of molecules from a lower concentration to a higher concentration across the cell (plasma) membrane. As this process is against the concentration gradient, it requires cellular energy to transport the molecules or ions. Active transport involves Primary active transport and secondary active transport.
Passive transport involves the movement of molecules from a higher to lower concentration gradient and thus does not require energy and is slower than active transport.
Therefore, only active transport requires energy for the transportation of molecules across the cell membrane.
To learn more about active transport,
brainly.com/question/12133248
1. Mr. Brown weighs 670 newtons and he climbed a ladder that was 5 meters
high. How much work did he do?
Answers
Work = 670 * 5
Work = 3350 Nm
1. How many grams are there in 1.5 x 10^25 molecules of CO2?
Answers
Answer: 1.1 kg
Explanation:
Mw CO2 is 44g —> 6.02214076*10^23 molecules
1.5*10^25 molecules —> 1.1 kg
What would the approximate age of an
igneous rock that contains only 1/4 of its
original carbon-14 (half-life of carbon is
5700 years)
Answers
Explanation:
Carbon-14 has a half life of 5730 years, meaning that 5730 years after an organism dies, half of its carbon-14 atoms have decayed to nitrogen atoms. Similarly, 11460 years after an organism dies, only one quarter of its original carbon-14 atoms are still around.
What will occur when potassium reacts with sulfur?
Answers
calculate the gravimetric factor of Fe in Fe2O3
Answers
i hope this calculation will help you

Help me out
On another planet, the isotopes of titanium have the given natural abundances.

Answers
The average atomic mass of titanium on the given planet is approximately 46.68164 atomic mass units (u). The average atomic mass may vary depending on the specific isotopic composition of titanium found on different celestial bodies or regions.
To calculate the average atomic mass of titanium on the given planet, we need to consider the natural abundances and masses of each isotope of titanium.
The average atomic mass is calculated by multiplying the natural abundance of each isotope by its respective mass and summing them up.
Let's perform the calculation step by step:
Step 1: Multiply the abundance of each isotope by its mass:
(73.700% * 45.95263 u) + (15.000% * 47.94795 u) + (11.300% * 49.94479 u)
Step 2: Calculate the individual contributions from each isotope:
= (0.737 * 45.95263) + (0.150 * 47.94795) + (0.113 * 49.94479)
Step 3: Add up the individual contributions:
= 33.84765431 + 7.1921925 + 5.64179347
Step 4: Sum up the contributions:
= 46.68164 u
Therefore, the average atomic mass of titanium on the given planet is approximately 46.68164 atomic mass units (u).
It's important to note that the calculation assumes the provided natural abundances are accurate and representative of the titanium isotopes on that planet.
for more questions on atomic mass
https://brainly.com/question/30390726
#SPJ8
The element neon (Ne), which is used in signs, differs from both fluorine (F) and sodium (Na) by one proton each. Sodium, an alkali metal, and fluorine, a halogen, are both
extremely reactive. In contrast, neon, which is a noble gas, is highly unreactive. Use the
information contained on a periodic table to explain the difference in their chemical
properties.
Answers
Answer:
Explanation:
Atoms generally tend to achieve there octet configuration (i.e have there outermost shell completely filled) and become stable. They do this by participating in chemical bonding (majorly by transferring or sharing electrons). Atoms (of elements) that have very few electrons on there outermost shell (like the group 1 atoms; example is sodium) and atoms that have almost completely filled outermost shell (like the group 7 elements; example is fluorine) are highly reactive because it is easier to lose an electron to become stable and also easier to gain an electron to become stable. However, elements in group zero of the periodic table do not participate in bonding and resist chemical reactions because they have a completely filled outermost shell and are hence stable.
Since, the groups of the periodic table shows the number of electrons in the outermost shell of each member (of a particular group), the chemical properties of each group is usually almost similar.
The radius of a potassium
atom is 0.227 nm. Express this
radius in the unit centimeters.
Answers
Answer:
0.227 nm can also be written as
0.227 * \(10^{-9}\)m
and we know that 1 cm = 1m * 100
so we will multiply by with 100:
0.227 * \(10^{-7}\) cm
2.27 * \(10^{-8}\) cm
The radius of a potassium atom is 0.227 nm. In centimeters, the radius of potassium is 2.27 × 10⁻⁸ cm
Potassium is an element on the periodic table with the atomic number 19. It has white, lustrous, and shiny color. They exhibit a low melting point and are a great conductor of electricity.
Given that:
The radius of a potassium atom is 0.227 nm
Using the standard conversion analysis:
Since 1 nanometer (nm) = 1.0 × 10⁻⁷ centimeters (cm)
Then, 0.227 nm is:
= \(\dfrac{(0.227 \ nm \times 1.0 \times 10^{-7} (cm))}{1 \ nm}\)
= 2.27 × 10⁻⁸ cm
Therefore, we can conclude that the radius of a potassium atom in centimeters is 2.27 × 10⁻⁸ cm
Learn more about conversion rates here:
https://brainly.com/question/21630826?referrer=searchResults
The bright-line spectra of four elements, G,J, L, and M, and a mixture of at
least two of these elements are given below.
Which elements are present in the mixture?
M
Mixture
750
750
G and J
G and L
M, J, and G
M, J, and L
700
700
650
650
Bright-Line Spectra
600
600
550 500
550
Wavelength (nm)
500
450
450
400
400
.
Answers
Based on the given bright-line spectra and the observed wavelengths in the mixture's spectrum, the elements G and J are the ones present in the mixture.
From the given bright-line spectra and the spectrum of the mixture, we can determine the elements present in the mixture by comparing the specific wavelengths observed. Examining the bright-line spectra, we can identify that G has a distinct wavelength at 650 nm, J at 600 nm, L at 550 nm, and M at 500 nm.
Looking at the spectrum of the mixture, we can observe two prominent wavelengths, 650 nm and 600 nm. These correspond to the wavelengths of G and J, respectively. Since the spectrum of the mixture does not exhibit the wavelengths specific to L (550 nm) or M (500 nm), we can conclude that only G and J are present in the mixture.
Therefore, based on the given bright-line spectra and the observed wavelengths in the mixture's spectrum, the elements G and J are the ones present in the mixture.
This analysis relies on the principle that each element has characteristic wavelengths at which they emit light. By comparing the observed wavelengths in the mixture's spectrum with those of the individual elements, we can determine the elements present in the mixture.
Know more about wavelengths here:
https://brainly.com/question/10750459
#SPJ8
From its position in the periodic table, determine which atom in each pair is more electronegative: (a) N or P (b) N or Ge (c) S or F (d) Cl or S (e) H or C (f) Se or P (g) C or Si
Answers
Answer:
p, get, s, s, c, se, SIse hhgggggggg I no
Answer:
1) Cl
2) O
3) O
4) S
5) N
6) P
7)N
Explanation:
Calculate the density of a material that has a mass of 60g and a volume of 15 ml.
Need help ASAP
Answers
Answer:
4 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question we have
\(density = \frac{60}{15} \\ \)
We have the final answer as
4 g/mLHope this helps you
You are conducting a kinetics experiment to find the rate law of a reaction.
You add the following amounts to a test tube. What is the concentration of the Oxalic
Acid?
.
• 6.00 mL of 0.525 M Oxalic Acid
. 4.00 mL of distilled water
2.00 mL of 0.200 M KMnO4
Answers
The concentration of Oxalic Acid is 0.2625 M.
A kinetic experiment is conducted to determine the rate law of a reaction. The concentration of Oxalic Acid can be calculated using the given amount of the reactants and the volume of the test tube. A balanced chemical equation can be used to find the stoichiometric ratio of the reactants in the given reaction.The balanced chemical equation for the reaction is:5 H2C2O4 + 2 KMnO4 + 3 H2SO4 → 10 CO2 + 2 MnSO4 + 8 H2OThe stoichiometric ratio between Oxalic Acid and Potassium Permanganate is 5:2. The Oxalic Acid is the limiting reactant, and Potassium Permanganate is in excess.The amount of Oxalic Acid in the solution can be calculated using the formula:molarity = moles of solute / volume of solution in L.The moles of Oxalic Acid can be calculated using the formula:moles of H2C2O4 = Molarity of H2C2O4 x Volume of H2C2O4 in L= 0.525 M x 0.006 L= 0.00315 moles.
The volume of the solution after the addition of the reactants is:6.00 mL of 0.525 M Oxalic Acid + 4.00 mL of distilled water + 2.00 mL of 0.200 M KMnO4= 12.00 mLThe concentration of Oxalic Acid in the solution can be calculated using the formula:Molarity of H2C2O4 = moles of H2C2O4 / volume of solution in L= 0.00315 moles / 0.012 L= 0.2625 M.
for such more questions on Acid
https://brainly.com/question/27915098
#SPJ8
what is effects of azeotrope system?
Answers
Answer:
An azeotrope or a constant boiling point mixture is a mixture of two or more liquids whose...Such a system of solvents is known as a heteroazeotrope. ... that will affect the volatility of one of the azeotrope constituents more than another.
Explanation:
Azeotrope, in chemistry, a mixture of liquids that has a constant boiling point because the vapor has the same composition as the liquid mixture. The boiling point of an azeotropic mixture may be higher or lower than that of any of its components.
Which of the following would cause an increase in the magnetic force
between two magnets?
A. Decreasing the separation between the two magnets
B. Increasing the separation between the two magnets
C. Decreasing the amount of excess charge on the first magnet
D. Increasing the amount of excess charge on the first magnet
Answers
Decreasing the separation between the two magnets will result in increase in the magnetic force between two magnets.
What is a Magnet?This is referred to a substance which produces magnetic field and result in the attraction and repulsion of certain types of things.
Increase in the separation will reduce the magnetic force and vice versa which is why option A was chosen.
Read more about Magnet here https://brainly.com/question/14997726
#SPJ1
A vessel contains 2.00 mol of He, 4.50 mol of Kr, and 0.50 mol of N2 gases. If the partial pressure of He is 0.120 atm, what is the total pressure inside the vessel?
Answers
Considering the Dalton's partial pressure, the total pressure inside the vessel is 0.42 atm.
Dalton's partial pressureDalton's law states that the total pressure of a gas mixture is equal to the sum of the pressures that each gas would exert if it were alone:
\(P_{T}\)= P₁ + P₂ + ... + Pₙ
where n is the amount of gases present in the mixture.
This relationship is due to the assumption that there are no attractive forces between the gases.
Dalton's partial pressure law can also be expressed in terms of the mole fraction of the gas in the mixture. So in a mixture of two or more gases, the partial pressure of gas A can be expressed as:
\(P_{A}\)=\(x_{A}\)\(P_{T}\)
Total pressure inside the vesselIn this case, you know:
Amount of moles of He= 2 molesAmount of moles of Kr= 4.50 molesAmount of moles of N₂= 0.50 molesTotal amount of moles= Amount of moles of He + Amount of moles of Kr + Amount of moles of N₂= 2 moles + 4.50 moles + 0.50 moles= 7 molesPartial pressure of He= 0.120 atmThe partial pressure of gas He can be expressed as:
\(P_{He}\)=\(x_{He}\)\(P_{T}\)
Then, calculate the mole fraction of He as:
\(x_{He}\)= Amount of moles of He÷ Total amount of moles
\(x_{He}\)= 2 moles÷ 7 moles
\(x_{He}\)= 2/7
the total pressure can be calculated as:
0.120 atm= 2/7×\(P_{T}\)
0.120 atm÷ 2/7=\(P_{T}\)
0.42 atm= \(P_{T}\)
Finally, the total pressure is 0.42 atm.
Learn more about Dalton's partial pressure:
brainly.com/question/14239096
brainly.com/question/25181467
brainly.com/question/14119417
#SPJ1
Whats the answer giving brainliest have a good day lol help

Answers
Answer: i would say north pole because the sunlight has to change directions to reach it if that makes since
Explanation: sorry if im wrong
Cation and anion if FeF2
Answers
How do models help scientists study atomic composition?
Answers
Models can be used to represent a process or show the composition of a material. For example, a model of an atom shows what matter is made out of and how it is composed. Scientists have been using models since ancient times to help explain the world around us. Models help you understand something that you cannot see or touch by making it visible or giving it a form that you can physically explore. In this case, the model lets scientists explore atoms without having to use an electron microscope. This method provides them with up-close images from different angles so they can learn about their properties in detail.
How many liters of carbon dioxide gas are in 18.6 moles of CO2 at STP?
Answers
Answer:
416.876
Explanation:
Uhmm I am so so very sorry if this is incorrect
A student planned to make copper sulfate crystals from excess copper oxide and dilute sulfuric acid.
The equation for the reaction is:
CuO(s) + H,SO (aq) -, CuSO (aq) + H20(1)
This is the method used.
1. Add 25 cm° of dilute sulfuric acid to a conical flask.
2. Gently warm the dilute sulfuric acid.
3. Add excess copper oxide to the dilute sulfuric acid.
4. Stir the mixture.
5. Heat to evaporate all the water from the mixture.
Suggest two improvements to the method
Explain why each improvement is needed.
A student plans a method to prepare pure crystals of copper sulfate.
The student's method is:
1. Add one spatula of calcium carbonate to dilute hydrochloric acid in a beaker.
2. When the fizzing stops, heat the solution with a Bunsen burner until all the liquid is gone.
The method contains several errors and does not produce copper sulfate crystals.
Explain the improvements the student should make to the method so that pure crystals of copper sulfate are produced.

Answers
The student's method for preparing pure crystals of copper sulfate contains errors and does not produce the desired outcome.
Use copper oxide instead of calcium carbonate: The student should add copper oxide (CuO) to the hydrochloric acid instead of calcium carbonate. Copper oxide reacts with hydrochloric acid to form copper chloride, which can then be converted to copper sulfate through a subsequent reaction with sulfuric acid.
Add sulfuric acid to the copper chloride solution: After the copper chloride solution is formed, the student should add sulfuric acid to it. This reaction between copper chloride and sulfuric acid will yield copper sulfate and hydrochloric acid. The student should ensure that the correct stoichiometric ratio is maintained to maximize the yield of copper sulfate crystals.
Crystal formation: The student should allow the solution to cool slowly after the reaction with sulfuric acid. This promotes the formation of larger, well-defined copper sulfate crystals.
Filtration and drying: Once the crystals have formed, the student should filter the solution to separate the solid crystals from the remaining liquid. The filtered crystals should then be thoroughly dried to remove any remaining water, resulting in pure copper sulfate crystals.
By following these improvements, the student can obtain pure crystals of copper sulfate.
For more such questions on copper sulfate visit:
https://brainly.com/question/17439051
#SPJ8
What part of the scientific method involves controlling variables while testing a hypothesis? (2 points) Select one: a. Analyzing data b. Conducting an experiment c. Drawing a conclusion d. Making observations
Answers
Answer:
D
Explanation:
best choice, makes the most sense
In the scientific method, conducting an experiment involves designing and performing controlled experiments to test a hypothesis and the correct option is option B.
Scientists manipulate the independent variable while controlling other variables (dependent and controlled variables) to observe and measure the effect on the dependent variable.
By controlling variables, scientists can isolate the factors that influence the outcome, ensuring that any observed changes are a result of the manipulated variable and not other unrelated factors. This step allows for the collection of reliable data, which is essential for drawing meaningful conclusions based on the evidence gathered from the experiment.
Thus, the ideal selection is option B.
Learn more about Scientific method, here:
https://brainly.com/question/17309728
#SPJ3
KCIO3 -> KCI + 02
How many moles of KCI are produced if 6743 grams of KCIO3 decomposes?
Answers
55.03 moles of KCI are produced when 6743 grams of \(KClO_{3}\) decomposes
To determine the number of moles of KCl produced when 6743 grams of \(KClO_{3}\) decomposes, we need to use the concept of molar mass and the balanced chemical equation.
First, let's calculate the molar mass of \(KClO_{3}\)
The molar mass of potassium (K) is approximately 39.10 g/mol.
The molar mass of chlorine (Cl) is approximately 35.45 g/mol.
The molar mass of oxygen (O) is approximately 16.00 g/mol.
So, the molar mass of \(KClO_{3}\) is:
(39.10 g/mol) + (35.45 g/mol) + (3 * 16.00 g/mol) = 122.55 g/mol.
Now, we need to calculate the number of moles of \(KClO_{3}\):
Number of moles = Mass / Molar mass
Number of moles = 6743 g / 122.55 g/mol = 55.03 mol.
According to the balanced chemical equation:
2\(KClO_{3}\) -> 2 KCl + 3 O2,
we can see that for every 2 moles of \(KClO_{3}\), we obtain 2 moles of KCl.
Therefore, the number of moles of KCl produced will be equal to the number of moles of \(KClO_{3}\) since the ratio is 1:1. Thus, 55.03 moles of KCl will be produced.
Know more about molar mass here:
https://brainly.com/question/837939
#SPJ11