Answers
Related Questions
The combustion of octane, C8H18, proceeds according to the reaction shown.
2C8H18(l)+25O2(g)⟶16CO2(g)+18H2O(l)
If 354 mol of octane combusts, what volume of carbon dioxide is produced at 15.0 ∘C
and 0.995 atm?
Answers
The concept ideal gas equation is used here to determine the volume of the carbondioxide. Combustion reactions are generally highly exothermic reactions. The volume of CO₂ is
A combustion is a chemical reaction in which a fuel undergoes oxidation as a result of the reaction with an oxidizing agent which causes the release of energy in the form of heat.
15.0 °C = 288 K
The ideal gas equation is:
PV = nRT
V = nRT / P
V = 354 × 0.0821 × 288 / 0.995 = 8412.3 L
To know more about combustion, visit;
https://brainly.com/question/14335621
#SPJ1
What is the chemical formula of CuH10N2O11.
Answers
A chemical formula tells us the number of atoms of each element in a compound. It contains the symbols of the atoms of the elements present in the compound as well as how many there are for each element in the form of subscripts.
Hope I helped :)
Calculate the amount of heat (kcal) released when 50.0g of steam at 100 degrees celsius hits the skin, condenses and cools to a body temperature of 37 degrees celsius
Answers
Answer:
\(Q=-126.1kJ\)
Explanation:
Hello,
In this case, by means of the released heat, we need to consider the cooling of water in two steps:
1. Condensation of steam at 100 °C.
2. Cooling of water from 100 °C to 37 °C.
Therefore, we need the enthalpy of condensation of water that is 40.65 2258.33 J/g and the specific heat that is 4.18 J/g°C for the same amount of cooled water to obtain:
\(Q=50.0g*[-2258.33\frac{J}{g}+4.18\frac{J}{g\°C}(37-100)\°C]\\\\Q=-126.1kJ\)
Best regards.
Washing soda, a compound used to prepare hard water for washing laundry, is a hydrate, which means that a certain number of water molecules are included in the solid structure. Its formula can be written as Na2CO3⋅χH2O where x is the number of moles of H2O per mole of Na2CO3 When a 2.558-g sample of washing soda is heated at 125∘C all the water of hydration is lost, leaving 0. 948 g of Na2CO3.What is the value of x?
Answers
Answer:
X = 10
Explanation:
First, we need to calculate the moles of Na₂CO₃ in the 0.948g. Then, the moles of water based on the difference of masses. X will be the ratio of moles of water and moles of sodium carbonate.
Moles Na₂CO₃ (Molar mass: 105.99g/mol):
0.948g * (1mol / 105.99g) = 8.944x10⁻³ moles
Moles H₂O (Molar mass: 18.02g/mol):
2.558g - 0.948g = 1.61g of water
1.61g * (1mol / 18.02g) =0.08935 moles
The ratio, X, is:
0.08935mol / 8.944x10⁻³ moles
X = 10Determine whether or not the mixing of each of the two solutions indicated below will result in a buffer.
Drag the appropriate items to their respective bins.

Answers
Sinve CH3NH2 is weak base and its salt is CH3NH3Cl, mixing 125.0 mL of CH3NH2 and 120.0 mL of CH3NH3Cl so produce a buffer.
What is a buffer?A buffer is a solution which resists changes to its pH when a small quantity of strong acid or base is added to it..
A buffer is made from a weak acid and its salt or a weak base and its salt.
From the given options, CH3NH2 is weak base and its salt is CH3NH3Cl.
Therefore, mixing 125.0 mL of CH3NH2 and 120.0 mL of CH3NH3Cl so produce a buffer.
Learn more about buffers at: https://brainly.com/question/1385846
Which of the following correctly describes a trend in atomic structure as you go down the elements in one group of the periodic table?
a: The atomic mass and the atomic number increase as you go down in the group.
b:The atomic mass increases and the atomic number stays constant as you go down in the group
c:The atomic mass stays constant and the atomic number increases as you go down in the group.
d:The atomic mass and the atomic number increase by 1 as you go down in the group.
Answers
groups are the vertical columns on the table. if you look at it, you can see that the atomic number and atomic mass increases as you go down.

Thermal Energy and Kinetic Molecular Theory Quick Check
Answers
The Kinetic Molecular Theory is a scientific model that states atoms in a compound are found in a constant state of motion (movement).
What is the Kinetic Molecular Theory?The Kinetic Molecular Theory is a scientific model that states atoms in a compound are found in a constant state of motion (movement).
Thermal energy refers to the movement of particles and therefore both concepts are interrelated.
In conclusion, the Kinetic Molecular Theory is a scientific model that states atoms in a compound are found in a constant state of motion (movement).
Learn more about the Kinetic Molecular Theory here:
https://brainly.com/question/134712
#SPJ1
Solve for the missing values?
Help me plz!

Answers
Substituting this in to the angle 7x (which is equal to y), we get y= 84 degrees.
We also see that 24 + y + x = 180 since it is along a straight line. So 24 + 84 + z = 180 and then z = 72 degrees.
The chemical process in which small organic molecules called monomers bond together to form a chain is called __________.
Answers
you’re welcome <3
Answer:
polymerization
Explanation:
any process in which relatively small molecules, called monomers, combine chemically to produce a very large chainlike or network molecule, called a polymer.
yup help plssss.......
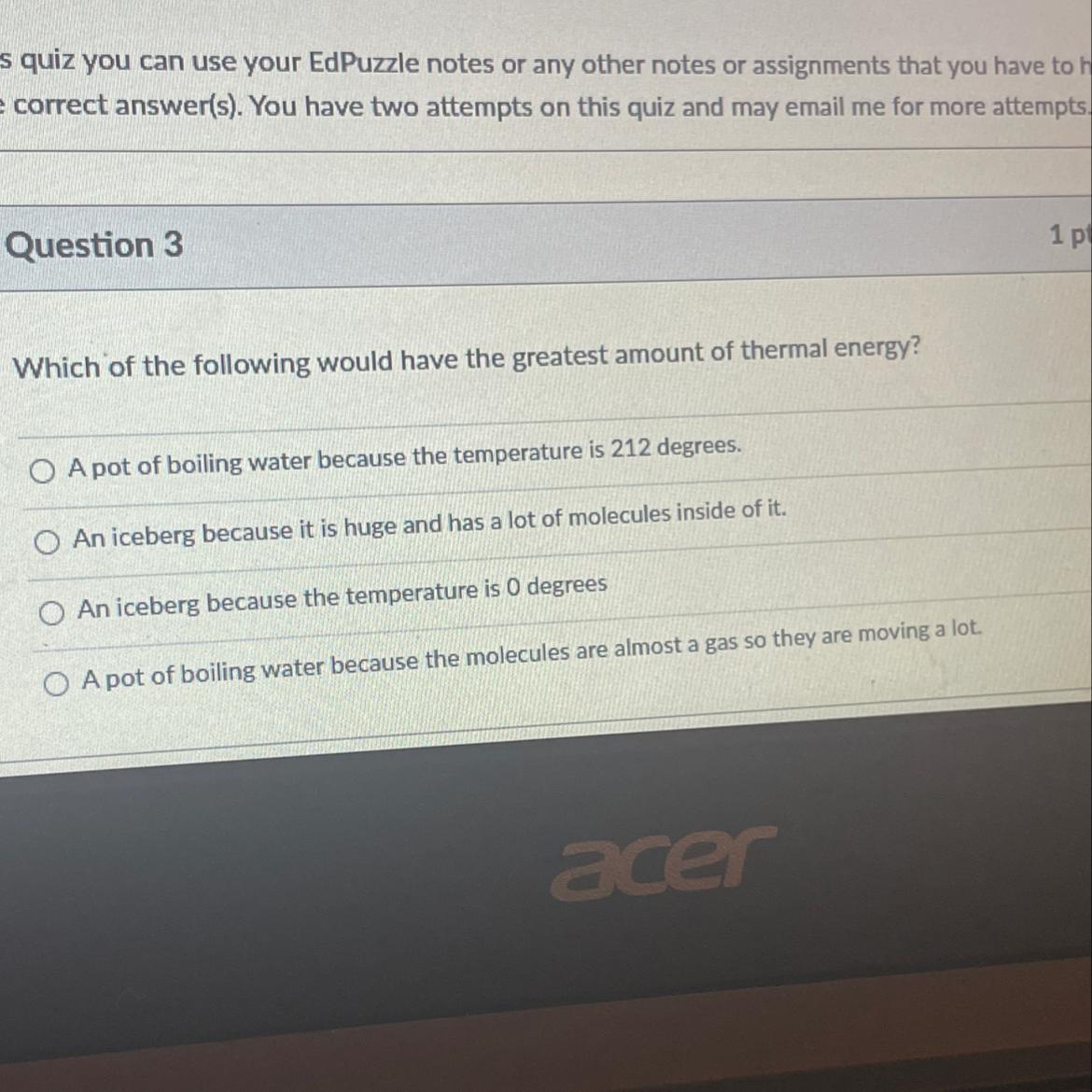
Answers
Answer:
D
Explanation:
because it's so hot that it's almost to the point of changing to another element
Answer:
To be honest with you I think it is A or D
Explanation:
bc I said so
The Ksp for LaF3 is 2 x 10^-19. What is the solubility of LaF3 in water in moles per liter?
Answers
The solubility of\(LaF_3\) in water is 3.04 x 10^-6 mol/L.
The solubility of \(LaF_3\) in water can be determined using the Ksp expression:
\(Ksp = [La^{3+}][F^-]^3\)
Where \([La^{3+}]\)and \([F^-]\) are the molar concentrations of the \(La^{3+}\) and \(F^-\) ions in the solution.
Since each \(LaF_3\) formula unit dissociates into one \(La^{3+}\) ion and three \(F^-\) ions, the molar solubility of \(LaF_3\) can be represented as x. Thus, the molar concentrations of \(La^{3+}\) and \(F^-\) ions in the solution can be written as x and 3x, respectively.
Substituting these values into the Ksp expression gives:
Ksp = x*(3x)^3 = 27x^4
Now, we can solve for x:
x = (Ksp/27)^(1/4)
= (2 x 10^-19 / 27)^(1/4)
= 3.04 x 10^-6 mol/L
For more question on solubility click on
https://brainly.com/question/24057916
#SPJ11
Place the steps necessary to balance a chemical equation correctly in order, starting with the first step at the top of the list. n Place these in the proper order. O Do a final check to make sure the equation is balanced. O Place reactants on the left of the reaction arrow, and products on the right. O Balance the atoms. O Adjust the coefficients such that there are the smallest whole-number coefficients
Answers
In a chemical equation, the numbers 2, 4, 1, and 3 must be balanced in the correct sequence.
2. Position the reactant to the left and the products to the right of the reaction arrow.
4. Maintain Atom Equilibrium.
1. Modify the coefficients to have the fewest whole-number coefficients possible.
3. Verify that the equation is balanced one last time.
Chemistry relies on balancing chemical equations to guarantee that the rule of conservation of mass is upheld. The reactants are written on the left side of the arrow and the products are on the right in the first step. There must be an equal amount of atoms on both sides of the equation.
Changing the coefficients in front of each molecule is accomplished. These coefficients must be as little as whole numbers are able to be. The equation must be balanced, which means that each element's number of atoms on either side of the equation must be equal. We must go back and change the coefficients until the equation is balanced.
Learn more about the balanced chemical equation at
https://brainly.com/question/28294176
#SPJ4
The question is -
List the steps required to successfully balance a chemical equation in ascending order, with the first step at the top.
1. Verify that the equation is balanced one last time.
2. Position the products on the right and the reactants on the left of the reaction arrow.
3. Maintain atom balance.
4. Modify the coefficients to have the fewest whole-number coefficients possible.
7.no .
with steps please anyone
no spam

Answers
Molar mass=65g/mol
Now
\(\boxed{\sf No\:of\:moles=\dfrac{Given\:Mass}{Molar\:Mass}}\)
\(\\ \rm\longmapsto No\:of\;Moles=\dfrac{1.3}{65}=0.02mol\)
Chromium is manufactured by heating a mixture of chromium(III) oxide with aluminium powder.
Cr2O3(s) + 2Al(s) → 2Cr(s) + Al2O3(s)
a=Calculate the mass of aluminium needed to react with 50 g of Cr2O3.
b=Calculate the mass of chromium produced from 50 g of Cr2O3.
c=Calculate the mass of chromium produced from 5 kg of Cr2O3.
d=Calculate the mass of chromium produced from5 tonnes of Cr2O3.
Note: 1 tonne = 1,000,000 g
Ar: Cr = 52, O = 16, Al = 27
Answers
Why do farmers spray water over their crops before a frost?
Answers
Answer:
When water freezes and turns into ice, it releases latent heat. Then, the ice that builds up on the plant will insulate it from the colder surrounding air temperatures. Because of this, some growers choose to spray their crop with water before the freeze occurs.
Explanation:
Which of the following describe how water mixes in a thermohaline current? Select the two correct answers.
a. Cold water sinks under warmer water.
b. Less salty water sinks under saltier water.
c. Warm water sinks under colder water.
d. Saltier water sinks under less salty water.
Answers
Two correct answers which describe how water mixes in a thermohaline current are A. Cold water sinks under warmer water. And D. Saltier water sinks under less salty water.
What is Thermohaline Current?Thermohaline Current is caused by variations in the seawater's surface density from pole to equator. Variations in temperature (thermal) and salinity (haline) affect the equator-to-pole surface. On Earth's climate, the thermohaline ocean currents have a significant impact on circulating heat across the planet.
In the thermohaline current, warmer, fresher water masses are less dense and float, while colder, saltier water masses are more dense and sink. Due to its higher density, this cold, salty water sinks.
So, it is obvious that the correct answers are:
A. Cold water sinks under warmer water.
D. Saltier water sinks under less salty water.
Here to learn more about Thermohaline Current:
https://brainly.com/question/1176119
#SPJ4
Calculate the energy of a photon having a wavelength of 7.71 x 10^-5 m.
Answers
Answer:
Calculate the energy of a photon having a wavelength of 7.71 x 10^-5 m.
Explanation:
Based on the information in the table of standard reduction potentials below, select the best species to use for the
cathode and anode that would yield the largest potential.
What Eᵒcell (V) would result using these species?

Answers
Answer:
best species to use for the
cathode and anode that would yield the largest potential.
Explanation:
The best cathode will be Al ³⁺ + e --> Al and the best anode is Ce⁴⁺ + e --> Ce³⁺. The E °cell is +3.37 emf.
What is cathode?Cathode is defined as the electrode of a polarized electrical device where a regular current exits. A Daniell galvanic cell, for instance, can be transformed into an electrolytic cell by switching the direction of the current, in which case the copper electrode serves as both the positive terminal and the anode.
Anode is defined as an electrode of a polarized electrical device that allows regular current to flow through it. The anode is the electrode into which current travels. The anode is often the positive side.
E ᵒcell = +1.70 - ( - 1.66 )
E ᵒcell = +1.70 + 1.66
E ᵒcell = +3.37 emf
Thus, the best cathode will be Al ³⁺ + e --> Al and the best anode is Ce⁴⁺ + e --> Ce³⁺. The E °cell is +3.37 emf.
To learn more about cathode and anode, refer to the link below:
https://brainly.com/question/4052514
#SPJ2
Which of the following is a way to decrease rate of dissolution of a solid in a liquid?
a
increase surface area
b
decrease agitation
c
increase temperature
(40 points)
Answers
A way to decrease the rate of dissolution of a solid in a liquid is to decrease agitation. Therefore, option B is correct.
What do you mean by the term dissolution ?A solute in a gaseous, liquid, or solid phase can dissolve in a solvent to create a solution through the process of dissolution.
The greatest concentration of a solute that may dissolve in a solvent at a specific temperature is known as solubility. The solution is deemed saturated when the solute concentration reaches its maximum.
Solubility and solution rate both decrease with rising temperature. The greatest quantity of a solute that can dissolve in a specific amount of solvent or solution at a specific temperature is the solute's solubility.
Thus, option B is correct.
To learn more about the dissolution, follow the link;
https://brainly.com/question/23851972
#SPJ1
how many CO grans are produced from 1.80 mol of sulfur dioxide2
Answers
Answer:
A mol (approximately)represents the number 6.02 X 10^^23. Mols become useful when we learn that, for any element on the periodic table, 6.02 X 10^^23 atoms of that element have a mass equal to the atomic mass in grams. So, on the periodic table carbon has an atomic mass of 12.011. That means: 12.011 grams of carbon is made up of 6.02 X 10^^23 atoms.
The above question is tricky.
If the question considers 1 molecule of SO2 as a particle, then the answer is 1.80 * 6.02 X 10^^23
If the question considers the S as one particle, and the O2 as 2 more particles, then the answer is: 3 * 1.8 * 6.02 X 10^^23.
Explanation:
hope it helps U
Which statement explains why NaBr is classified as a compound?
1.
Na and Br are chemically combined in a fixed proportion.
2.
Na and Br are both nonmetals.
3
NaBr is a solid
298 Kand standard pressure.
4.
NaBr dissolves in H20 at 298 K.
Submit
Hide Toolbar
Answers
Answer:1
Explanation:i know cuz I got it right
NaBr is classified as a compound because sodium and bromine are chemically combined in a fixed proportion.
Explanation:
Element is defined as the simplest form of a substance that cannot be divided further by any physical means.For example oxygen (\(O_2\)), coal (carbon) etc.A compound is defined as the form of a substance in which two or more different elements are chemically combined together in a fixed proportion.For example sodium chloride (NaCl), nitric acid (\(HNO_3\))A compound can be further divided into a simple substance.So, from this, we can conclude that NaBr is classified as a compound because sodium and bromine are chemically combined in a fixed proportion.
Learn more about elements and compounds here:
brainly.com/question/16084453?referrer=searchResults
brainly.com/question/184321?referrer=searchResults
Determine the ratio of electrostatic potential energies for CsF and Csl.
lon
lonic Radius (pm)
Cst
170
F-
133
1
220
Answers
Answer:
The answer is "1.29".
Explanation:
Formula for electrostatic potential energy:
\(\to V=\frac{K Q_1 \times Q_2}{r}\)
where
\(\to K = \text{electrostatic constant}\\\\\to Q= \text{Charge on Cation}\\\\\)
The Charge on Cation is \(C_{s}^{+}\) is cation in both \(C_sI\) and \(C_sF\) were same:
\(\to V\ \alpha \ \frac{1}{r}\)
\(\to \frac{V(C_sF)}{V(C_sI)} = \frac{r(C_sI)}{r(C_sF)}\)
\(= \frac{r(C_s^{+}) +r(I^{-})}{r(C_s^{+}) +r(F^{-})} \\\\=\frac{170+220}{170+133} \\\\=\frac{390}{303} \\\\ =1.2871 \\\\ =1.29\)
We expect NaCl to have a lower melting point
than Nal since the smaller the ion (CI-¹), the
lower the bond energy (and the melting
point).
Select one:
True
Or False
Answers
It is false because NaCl has a higher bond energy than Nal.
What determines melting point?The melting point of a substance is determined by the strength of the intermolecular forces between its atoms or ions. In the case of NaCl, the strong electrostatic attraction between the positively charged Na+ ions and the negatively charged Cl- ions results in a relatively high melting point.
In contrast, NaI is a ionic compound with a lower bond energy between the atoms. So, it has lower melting point in comparision to NaCl. So the statement is False, NaCl has a higher melting point than NaI.
Find out more on melting point here: https://brainly.com/question/40140
#SPJ1
The mass of a carbon atom in grams is approximately 12 amu, or 2.0 × 10-23 g.
To make things easy we could ask, how many carbon atoms would reach an easy-to-remember mass, 12 g? (12 g is easy to remember because it's already on the periodic table for carbon!)
Let's do it! Calculate the number of carbon atoms we would need to reach 12 g on a lab balance.
It may be helpful to consider the following equation to solve:
(mass of carbon atom in grams) × N = 12 g of carbon (reading on balance)
Where N is the number of atoms needed to reach 12 g of carbon on the lab's balance. (check within this question for the other information you need)
How many carbon atoms, N, are needed to reach 12 grams on the scale?
Answers
Therefore, we need 6.0 × 1022 atoms of carbon to reach 12 g on the lab balance.
What is carbon ?Carbon is an element found in nature that is essential for life on Earth. It is an abundant, non-metallic, and highly versatile element that is used in a variety of ways in many different fields. Carbon is found in all living organisms and is the basis of all life on Earth. It is also found in rocks, soil, and the atmosphere. Carbon is the fourth most abundant element in the universe and is the basis for organic compounds that form the basis of all living things. In industry, carbon is used to produce steel, glass, plastics, and many other materials. Carbon is also used in the manufacture of fuels and is a component of the chemical processes necessary for life. Carbon is also used in batteries, semiconductors, and solar cells.
To learn more about carbon
https://brainly.com/question/26789123
#SPJ1
What are two dangers associated with
nuclear fission?
Answers
Answer:
Nuclear energy produces radioactive waste
A major environmental concern related to nuclear power is the creation of radioactive wastes such as uranium mill tailings, spent (used) reactor fuel, and other radioactive wastes. These materials can remain radioactive and dangerous to human health for thousands of years
Explanation:
1. A balloon is inflated to a pressure of 2.55 atm at a temperature of 25 °C. What temperature
(in °C) is required to maintain the same volume if the pressure decreases to 1.39 atm?
Answers
Answer :
-111°C
Hope it helps

The final temperature of the gas in the balloon is equal to -110.6°C.
What is Gay Lussac's law?Gay-Lussac's law can be described as when the volume of the gas is kept constant then the pressure (P) is directly proportional to the absolute temperature (T in kelvin) of the gas.
The mathematical representation of Gay Lussca's law can be written as follows:
P/T = k
The pressure (P) of a gas is always directly proportional to the temperature (T) of the gas.
P ∝ T (where volume is constant)
\(\frac{P_1}{T_1} =\frac{P_2}{T_2}\)
Where P₁, T₁, P₂, and T₂ are the initial and final pressure and temperature.
The initial temperature of the balloon, T₁ = 25 °C = 25 + 273 = 298 K
The initial pressure of the balloon, P₁ = 2.55 atm
The final pressure of the balloon, P₂ = 1.39 atm
Substituting temperatures and pressures of the gas in the balloon in the above equation:
2.55/298 = 1.39/T₂
T₂ = 162.4 K
T(K) = 273 + T(°C)
T(°C) = 162.4 - 273 = - 110.6°C
Therefore, the final temperature of the gas inside the balloon is -110.6°C.
Learn more about Gay Lussac's law, here:
brainly.com/question/11387781
#SPJ2
Carbon disulfide burns in oxygen to yield carbon dioxide and sulfur dioxide according to the following chemical equation. CS2 + 3O2 → CO2 + 2SO2
If 1.00 mol CS2 reacts with 1.00 mol O2, identify the limiting reactant.
How many moles of excess reactant remain?
How many moles of each product are formed? (you need to give 2 answers)

Answers
In the given reaction, carbon disulfide acts as an excess reagent and oxygen acts as a limiting reagent.
Do the limiting reactant calculation.a) Oxygen is the limiting reactant.
b) 0.667 moles of carbon disulfide will still be present.
c) The formation of 0.333 moles of carbon dioxide and 0.667 moles of sulfur dioxide.
provided here,
To make 1 mole of carbon dioxide and 2 moles of sulfur dioxide from 1 mole of carbon disulfide, 3 moles of oxygen are required.
1.00 ol of carbon disulfide is the number of moles.
One mole of oxygen is equal to 1.00 mol.
The limiting reactant is oxygen. It will be totally eaten. (1.00 mol).
(B) An excess reactant is carbon disulfide. It will respond with
Oxygen reacts with 0.33 moles of carbon disulfide.
The extra reagent still present:
0.667 moles There will be a residue of carbon disulfide.
Determine the moles of each product:In order to make one mole of carbon disulfide and one mole each of carbon dioxide and sulfur dioxide, you need three moles of oxygen.
The carbon dioxide equivalent for 1 mole of oxygen is 1 mole/3, or 0.333 moles.
Sulfur dioxide is equal to 0.667 moles per 1.00 mol of oxygen (1.00 mol/(3/2)) mol.
Since carbon disulfide is an excess reagent in the above reaction, oxygen is a limiting reagent.
For more information on limiting agent kindly visit to
https://brainly.com/question/29505468
#SPJ1
can someone help me with this?
please tell me what arrows to add and I will give brainiest

Answers
Within 5 years the kinetic energy needed for the phase change of methane is increased by some factors. Hence, the arrow for change in kinetic energy for the phase change can be used here.
What is kinetic energy change ?The phase change of a substance is the change from one state or phase to the other such as from solid to liquid, liquid to vapor etc. The minimum energy needed to overcome the barrier potential for a physical and chemical change is called its activation energy or activation potential.
A substance need kinetic energy to move apart its molecules which are strongly connected in liquid phase to convert it into vapor phase. If the activation potential increases, the substance need more kinetic energy.
Here, if the kinetic energy needed for the phase change of liquid methane increases. That is the reason why liquid methane in Titan not evaporated for some years.
Here the actual change in kinetic energy is increasing, thus, the methane gas is needed to gain more energy to evaporate. Hence, you have to add the first arrows given in the data.
Find more on activation potential:
https://brainly.com/question/12563191
#SPJ9
Part B
Next, you’ll test your hypothesis from part A by examining the reaction times of vinegar and baking soda in water at four different temperatures. You’ll carry out the reaction using water at room temperature (about 25°C), 40°C, 60°C, and 80°C. Make sure that you use the same amounts of vinegar and baking soda for all three three trials.
Gather all the materials, and perform these steps for each trial:
Heat at least
cup (60 milliliters) of water to the required temperature (refer to the data table). Water may be heated on a stove, on a hot plate, or in a microwave oven.
Measure and record the actual temperature of the water.
Measure 1 tablespoon (15 milliliters) of the water into the cup.
Add
teaspoon (1.5 grams) baking soda to the water, and stir until it is dissolved. The solution will be clear.
Measure 1 tablespoon (15 milliliters) of vinegar, but do not pour it into the cup yet.
Very quickly, do all of the following:
a. Pour the measured vinegar into the cup.
b. Start the stopwatch.
c. Stir or carefully swirl the substances in the cup.
The chemical reaction will produce bubbles. You’ll be able to see the bubbles and hear them pop. Watch and listen for when the reaction stops. When it looks and sounds like it has finished, stop the stopwatch.
Record the reaction time in the data table.
Discard the solution down the drain, and rinse the cup.
Repeat steps 1–9 of this procedure, doing three trials for each water temperature. Record the average temperature and reaction time for each set of the three trials. Read this math review to know how to calculate average of a data set.
Answers
The reaction time decreases as the temperature increases of the reaction mixture increases.
A sample record of results is:
Temperature (°C) Trial 1 Trial 2 Trial 3 Average25°C 11 seconds 11 seconds 11 seconds 11 seconds40°C 8 seconds 8 seconds 8 seconds 8 seconds60°C 5 seconds 5 seconds 5 seconds 5 seconds80°C 3 seconds 3 seconds 3 seconds 3 secondsWhat is the effect of an increase in temperature on reaction time?An increase in temperature leads to an increase in reaction rate or a decrease in reaction time.
The increase in temperature provides more thermal energy to the reactant molecules, which leads to an increase in the average kinetic energy of the molecules. As a result, more reactant molecules have sufficient energy to overcome the activation energy barrier and undergo successful collisions, leading to an increased reaction rate.
Learn more about reaction time at: https://brainly.com/question/26142029
#SPJ1
A solution is made by dissolving 38.81 grams of nickel (II) sulfate, NiSO4, in enough water to make 0.467
liters of solution. Calculate the molarity of this solution.
Answers
The molarity of the NiSO₄ solution made by dissolving 38.81 grams of nickel (ii) sulfate, NiSO₄, in enough water to make 0.467 liters of solution is 0.535 M
How do i determine the molarity of the solution?First, we shall obtain the mole of 38.81 grams of nickel (ii) sulfate, NiSO₄. Details below:
Mass of NiSO₄ = 38.81 grams Molar mass of NiSO₄ = 154.75 g/molMole of NiSO₄ = ?Mole of NiSO₄ = mass / molar mass
= 38.81 / 154.75
= 0.25 mole
Now, we shall determine the molarity of the solution. Details below:
Mole of NiSO₄ = 0.25 moleVolume of solution = 10.467 LMolarity of solution = ?Molarity of solution = mole / volume
= 0.25 / 0.467
= 0.535 M
Thus, the molarity of the solution is 0.535 M
Learn more about molarity:
https://brainly.com/question/16073358
#SPJ1