QUICKLY PLEASE: What is true about 1. 0 mol Ca and 1. 0 mol Mg? (3 points)
Answers
Both 1.0 mol of calcium (Ca) and 1.0 mol of magnesium (Mg) contain the same number of atoms (Avogadro's number, 6.022 x 10²³ atoms), but they differ in mass and chemical properties.
In order to compare 1.0 mol Ca and 1.0 mol Mg, we must first understand the concept of a mole. A mole is a unit of measurement that represents 6.022 x 10²³ particles (atoms, molecules, ions, etc.). This number, known as Avogadro's number, allows us to compare amounts of different substances.
Although 1.0 mol Ca and 1.0 mol Mg both contain the same number of atoms, their masses are different. The molar mass of Ca is 40.08 g/mol, while the molar mass of Mg is 24.31 g/mol.
Therefore, 1.0 mol Ca has a mass of 40.08 g, and 1.0 mol Mg has a mass of 24.31 g. Additionally, Ca and Mg are both alkaline earth metals but possess different chemical properties, such as reactivity and electron configurations.
To know more about Avogadro's number click on below link:
https://brainly.com/question/28812626#
#SPJ11
Related Questions
Glucose is a sugar that can be reduced to produce a/an {blank}. Group of answer choices aldehyde alcohol ketone carboxylic acid
Answers
Glucose is a sugar that can be reduced to produce an alcohol (option B). Details about glucose reduction can be found below.
What is reduction in chemistry?Reduction is a reaction in which electrons are gained and valence is reduced.
Reduction can also mean the removal of oxygen or the addition of hydrogen to a substance.
Glucose is a chemical compound and sugar with the chemical formula of C6H12O6, however, it can undergo reduction reaction to produce alcohol/ethanol (C2H5OH) in a process called fermentation.
Learn more about reduction reaction at: https://brainly.com/question/19528268
#SPJ1
4) Calculate the net force *
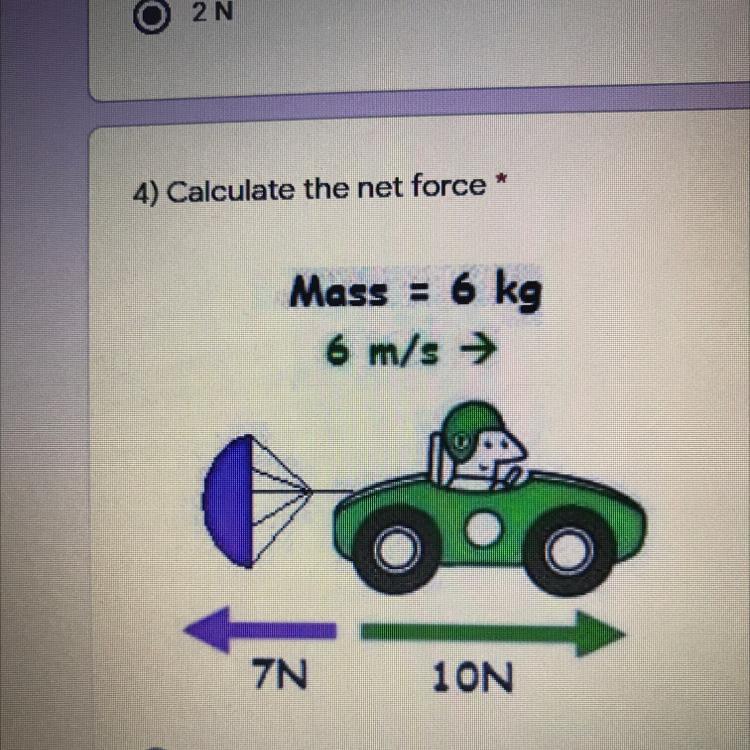
Answers
3 N
10-7= 3
the arrows are opposing each other.
Answer:
The net or resultant force depende on the direction we can easily understood the directions are opposite .Force is vector so if vectors are opposite we difference the two vectors.
10N-7N=3N
100. ml of buffer is prepared by mixing ha and the salt of ha. the ph of the buffer is 5.67. what is the ph of the buffer after 100. ml of distilled water is added?
Answers
The pH of the buffer after 100. ml of distilled water is added is 5.369.
The pH of the buffer is equal to the pKa of the weak acid.
Assuming that the dilution with 100 mL of distilled water does not significantly affect the buffer capacity or the dissociation of the weak acid, the total volume of the buffer after dilution is 200 mL, and the concentration of HA and A- is reduced by a factor of 2.
The pKa of the weak acid remains the same, so we can use the Henderson-Hasselbalch equation again to calculate the new pH of the buffer,
pH = pKa + log([A-]/[HA])
Substituting [A-] = [HA]/2, we get:
pH = 5.67 + log(1/2) = 5.67 - 0.301
pH = 5.369
To know more about the pH, here
brainly.com/question/15289714
#SPJ4
What is evaporation ?In what way it is different from boiling? What is the effect
of humidity on the rate of evaporation?
Define Latent heat of fusion? Why does steam cause more severe burning as
compared to boiling water at 1000C ?
Answers
Evaporation is the process by which a liquid converts into a gas or vapor at a temperature below its boiling point. Latent heat of fusion is the amount of heat required to convert a solid into a liquid without any change in temperature. Steam causes more severe burning as compared to boiling water at 100°C due to the large amount of latent heat of vaporization.
On the other hand, boiling is the process by which a liquid turns into a gas or vapor at a temperature equal to or above its boiling point. In other words, when the liquid is heated to a temperature equal to or greater than its boiling point, it turns into a gas or vapor. The effect of humidity on the rate of evaporation is that if the air around the liquid is already humid, then the rate of evaporation will be slower because the air is already saturated with water molecules.
Latent heat of fusion is the amount of heat required to convert a solid into a liquid without any change in temperature. In other words, it is the amount of heat required to break the intermolecular forces between the molecules of a solid to convert it into a liquid.
Steam causes more severe burning as compared to boiling water at 100°C because steam contains a large amount of latent heat of vaporization. When steam comes in contact with the skin, it releases a large amount of latent heat of vaporization, which causes more severe burns as compared to boiling water at 100°C.
Evaporation is the process of turning a liquid into a gas or vapor at a temperature below its boiling point. Boiling, on the other hand, is the process of turning a liquid into a gas or vapor at a temperature equal to or above its boiling point. Humidity affects the rate of evaporation. Latent heat of fusion is the amount of heat required to convert a solid into a liquid. Steam causes more severe burning as compared to boiling water at 100°C due to the large amount of latent heat of vaporization.
To know more about Evaporation visit:
brainly.com/question/28319650
#SPJ11
What is the mass of 9.3 x 1024 molecules of glucose, C6H12O6? (C6H12O6; 180.18 g/mol)

Answers
2.78 × 10⁴⁹ is the mass of 9.3 x 1024 molecules of glucose, C6H12O6
Define a mole.
A mole is equivalent to 6.022 x 10^23 of that substance (such as atoms, molecules, or ions). The term "Avogadro's number" or "Avogadro's constant" refers to the integer 6.022 1023. To convert between mass and the quantity of particles, use the mole idea.
Your body utilizes glucose, a type of sugar obtained from the foods you consume, as an energy source. It is referred to as blood glucose or blood sugar as it passes through your circulation to reach your cells. The hormone insulin transports glucose from the circulation into the cells for use as fuel and storage.
Number of molecules (n) equals given mass / molar mass
Number of moles Equals Number of molecules* 6.022 1023
Amount of moles is equal to 9.3 x 1024 / 6.022 x 1023 = 1.54 x 1047
Molar mass is equal to Number of moles × Molar mass
= 1.54 × 10⁴⁷ × 180.156
= 2.78 × 10⁴⁹
To learn more about Avogadro's number use:
https://brainly.com/question/14138110
#SPJ1
What is the ratio of hydronium ion concentrations in solution at the pH that results in the highest MP activity to that which results in the lowest MP activity?
see previous pic.
Answers
The ratio of hydronium ion concentrations in solution at the pH that results in the highest MP (Metalloprotein) activity to that which results in the lowest MP activity can be calculated using the equation for pH, which is -log[H+]. Since pH and [H+] are inversely proportional, a higher pH value indicates a lower [H+] concentration. Therefore, the ratio of hydronium ion concentrations would be the inverse of the ratio of pH values.
In simpler terms, if the pH resulting in the highest MP activity is 8 and the pH resulting in the lowest MP activity is 4, then the ratio of hydronium ion concentrations would be 10^-8/10^-4, which simplifies to 10^-4. This means that the hydronium ion concentration at the pH resulting in the lowest MP activity is 10,000 times higher than that at the pH resulting in the highest MP activity.
The reason for this is that metalloproteins are sensitive to changes in pH, as they rely on specific amino acids residues to bind to metal ions. A change in pH can disrupt these interactions and reduce MP activity. Therefore, maintaining a stable pH is crucial for optimal MP activity.
To know more about amino acids click this link
brainly.in/question/10752843
#SPJ11
what is the chemical symbol for the group 6a (16) element that lies in period 4?
Answers
The group 6A (16) elements are located in the sixth column or group of the periodic table. These elements include oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and polonium (Po).
To determine which element in group 6A lies in period 4, we can count down the rows of the periodic table to locate the fourth row. This row is also referred to as the fourth period. Moving horizontally across the fourth row, we find that sulfur (S) is the element in group 6A that is located in period 4.
Therefore, the chemical symbol for the group 6A (16) element that lies in period 4 is S, which stands for sulfur. Sulfur is a nonmetal with atomic number 16, atomic mass 32.06, and a melting point of 115.21 °C. It is widely used in the production of sulfuric acid and in the vulcanization of rubber.
Learn more about elements here:
https://brainly.com/question/30858299
#SPJ11
Your friend has just started a mining company and has asked you to help and talk to his newly recruited staff about identification of rocks and minerals
Prepare a presentation (not more than 200 words) to be delivered to the staff about rocks and minerals and how they can be identified.
Answers
Answer:
Good day everyone, I am here to talk to you about rocks and minerals and how to identify them.
First, let's define what rocks and minerals are. Rocks are made up of two or more minerals and can be classified into three main types: igneous, sedimentary, and metamorphic. Minerals, on the other hand, are naturally occurring inorganic substances with a definite chemical composition and crystal structure.
Now, when it comes to identifying rocks and minerals, there are a few key things to look out for. One is the color - minerals can come in a wide range of colors, and certain colors can indicate specific types of minerals. Another factor to consider is the texture - does the rock feel smooth or rough, and are there any visible grains or crystals? Additionally, the hardness of a mineral can be a helpful clue, as can its reaction to acid or other chemicals.
There are also various tools and techniques that can be used to aid in the identification process, such as a magnifying glass, streak test, and acid tests. It's important to use caution when handling certain minerals, as some can be toxic or contain harmful substances.
What volume of a 0.100 M AgNO3 solution is required to completely react with the CaCl2 in 3.00 L of a 0.100 M CaCl2 solution? The balanced chemical equation is:
Answers
Based on the balanced chemical equation 2\(Ag(NO)_{3}\)(aq) + \(CaCl_{2}\)(aq) → 2AgCl(s) + \(Ca(NO)_{3}2\)(aq), the volume of a 0.100 M \(Ag(NO)_{3}\)solution required to completely react with the \(CaCl_{2}\) in 3.00 L of a 0.100 M \(CaCl_{2}\) solution is 3.00 L.
To determine the volume of the \(Ag(NO)_{3}\) solution required to react with the \(CaCl_{2}\) solution, we need to consider the balanced chemical equation and the stoichiometry of the reaction. The balanced chemical equation is:
2\(Ag(NO)_{3}\)(aq) + \(CaCl_{2}\)(aq) → 2AgCl(s) + \(Ca(NO)_{3}\)2(aq)
From the equation, we can see that 2 moles of \(Ag(NO)_{3}\) React with 1 mole of \(CaCl_{2}\) to produce 2 moles of AgCl. Given that the \(CaCl_{2}\) solution has a concentration of 0.100 M and a volume of 3.00 L, we can calculate the number of moles of \(CaCl_{2}\)present: moles of \(CaCl_{2}\) = concentration of \(CaCl_{2}\) × volume of \(CaCl_{2}\) solution
= 0.100 M × 3.00 L
= 0.300 moles
According to the stoichiometry of the equation, 1 mole of CaCl2 reacts with 2 moles of \(Ag(NO)_{3}\). Therefore, the number of moles of \(Ag(NO)_{3}\)required to react with the given amount of \(CaCl_{2}\) is also 0.300 moles. Now, we can calculate the volume of the \(Ag(NO)_{3}\) solution using its concentration:
volume of \(Ag(NO)_{3}\) solution = moles of \(Ag(NO)_{3}\)/ concentration of \(Ag(NO)_{3}\)
= 0.300 moles / 0.100 M
= 3.00 L
Hence, 3.00 L of the 0.100 M \(Ag(NO)_{3}\) solution is required to completely react with the \(CaCl_{2}\) in 3.00 L of the 0.100 M \(CaCl_{2}\)solution based on the balanced chemical equation and stoichiometry of the reaction.
Know more about moles here:
https://brainly.com/question/29367909
#SPJ8
four hydrogen nuclei do not fuse directly into a helium nucleus
Answers
The particles/elements created by the reactions in the Sun are positron (A), neutrino (B), gamma ray (E), and helium (F).
In the process of nuclear fusion in the Sun, hydrogen nuclei combine to form helium through a series of reactions. These reactions involve the conversion of hydrogen nuclei (protons) into helium nuclei (two protons and two neutrons). However, the overall reaction also produces other particles and energy in the form of gamma rays.
During the fusion process, positrons (A) are created as a result of the conversion of protons into neutrons. Neutrinos (B) are also produced as a byproduct of the fusion reactions. They are elusive particles that have very little interaction with matter and can pass through most materials unaffected. Gamma rays (E) are high-energy photons that are released during the fusion reactions. Finally, helium (F) is the end product of the fusion process, where four hydrogen nuclei combine to form a helium nucleus.
Overall, the fusion reactions in the Sun produce positrons, neutrinos, gamma rays, and helium as the main particles/elements.
To learn more about nuclear fusion click here: brainly.com/question/14019172
#SPJ11
In the Sun, four hydrogen nuclei do not fuse directly into a helium nucleus. The overall reaction involves several steps, and other particles are also produced in the process.
Study this animation of the reactions that occur in the Sun, and then select all the particles/elements that are created by the reaction.
Answers(
A. positron
B. neutrino
E. gamma ray
F. helium)
Does axons have insulation
Answers
Answer: Yes they do;
Explanation:
Axons, the long projections of neural cells that make up our peripheral nervous system's nerves, are comparable to power wires in that they have dense electrical insulation and can easily transmit impulses from the body and signals from the brain to a toe, for example.
What are the lines around the nucleus called?
Answers
Answer:
am can u pliz tell me more about ur qtn
Explanation:
like a nuclear of a what
maybe a plant or and animal
Answer:
Orbiting electrons
Explanation:
PLEASE HELP ASAP!! {40 POINTS}
Classify EACH possible hypothesis about a medical aloe Vera plant as falsifiable or non-falsifiable
A) Aloe Vera gel is the best natural skin moisturizer.
B) Aloe Vera gel can heal wounds by boosting cell renewal.
C) Aloe Vera juice tastes better than carrot juice.
D) Drinking aloe juice can reduce the risk of lung cancer.

Answers
Answer:
The correct answer is -
FALSIFIABLE:
Aloe vera gel can heal wounds by boosting cell renewal.
Drinking aloe juice can reduce the risk of lung cancer.
NON-FALSIFIABLE:
Aloe vera gel is the best natural skin moisturizer.
Aloe vera juice tastes better than carrot juice.
Explanation:
Falsifiable is the ability or chances of any hypothesis, claim or statement to be proved wrong. In such a hypothesis, it is possible to carry an experimental observation that disproves the idea in claim or question.
In the given examples
Aloe vera gel can heal wounds by boosting cell renewal.
Drinking aloe juice can reduce the risk of lung cancer.
There is no observation in the favor of the claim so there more likely to be falsifiable whereas, Aloe vera gel is the best natural skin moisturizer.
Aloe vera juice tastes better than carrot juice are more non-falsifiable as these are based on personal choice or experimental observation.
Answer:
FALSIFIABLE IS *DOWNARROW
Aloe vera gel can heal wounds by boosting cell renewal.
Drinking aloe juice can reduce the risk of lung cancer.
NON-FALSIFIABLE IS *DOWNARROW*
Aloe vera gel is the best natural skin moisturizer.
Aloe vera juice tastes better than carrot juice.
Explanation:
yes
Which of the compounds below will dissociate in water? Check all that apply.
A.) Ba(NO3)2
B.) CaSO4
C.) CO2
D.) H2CO3
E.) Mg3(PO4)2
Answers
Answer: The answers are A, B, and E.
Explanation: Got it wrong on Edge and that's what it showed.
There are two types of chemical compound one is covalent compound and other is ionic compound, covalent compound formed by sharing of electron and ionic compound formed by complete transfer of electron. Therefore, the correct option are option A,B,D,E.
What is chemical Compound?Chemical Compound is a combination of molecule, Molecule forms by combination of element and element forms by combination of atoms in fixed proportion.
An ionic compound is a metal and nonmetal combined compound. Ionic compound are very hard. They have high melting and boiling point because of strong ion bond.
The compound that is ionic in nature can be dissociated very easily in water. Since ionic compounds are polar in nature, they readily dissolve in water. among given option Ba(NO\(_3\))\(_2\), CaSO\(_4\), H\(_2\)CO\(_3\) and Mg\(_3\)(PO\(_4\))\(_2\) will dissociate in water.
Therefore, the correct option are option A,B,D,E.
To learn more about chemical compound, here:
brainly.com/question/26487468
#SPJ2
What is the percent by volume of ethanol in gasohol when 95 ml of ethanol is added to sufficient gasoline to make 1.0 l of gasohol?
Answers
The percentage of volume of ethanol is 9.5%.
What is volume by volume percentage?Volume/volume percentage (v/v% or percent v/v) is a unit used to express how much of a material is present in a solution. It is defined as the volume of the solute divided by the sum of the volumes of the solution, multiplied by one hundred. Examples: The average alcohol concentration (v/v%) of wine is 12 percent.
The formula used to get the volume of ethanol in percentages is
Percent of volume of ethanol = (volume of solute / total volume) * 100
Percent volume of Ethanol = (volume of ethanol / total volume) * 100
= 95mL / 1000mL * 100
= 9.5%
to know more go to - https://brainly.com/question/14589084
#SPJ4
What is the percent of O in
CO2?
(C = 12.01 amu, O = 16.00 amu)
[? ]%
Answers
Answer:
show me a pic?? then I'll lyk
Explanation: Because carbon atoms are only 1/3 of the compound since the other 2 atoms are oxygen.
Four identical balls are put into the cylinder. The volume reading is now 34cm². What is the volume of each ball?
Answers
If the volume reading is now 34cm². The volume of each ball is 7.49 cm^3.
How to find the volume?Assuming that the cylinder is perfectly round and has a height of 1 cm, we can use the formula for the volume of a cylinder and the volume of a sphere to solve this problem.
The volume of the cylinder is given by:
V_cylinder = πr^2h
where r is the radius of the cylinder and h is the height. Since the volume reading is 34 cm^3 and the height is 1 cm, we can solve for the radius:
34 = πr^2(1)
r^2 = 34/π
r ≈ 3.09 cm
Now, we can use the volume of a sphere formula to find the volume of each ball. The volume of a sphere is given by:
V_sphere = (4/3)πr^3
Since the four balls are identical, we can divide the volume of the cylinder by 4 to get the volume of each ball:
V_ball = V_cylinder/4
V_ball = (πr^2h)/4
V_ball = (π(3.09)^2(1))/4
V_ball ≈ 7.49 cm^3
Therefore, the volume of each ball is approximately 7.49 cm^3.
Learn more about volume here:https://brainly.com/question/463363
#SPJ1
Compared to compounds with network structures, compounds arranged in molecules (covalent bonds) have ____________ melting points.
Answers
Explanation:
.... have lower melting points.
6NaBr+1AlO3=3Na2O+2AlBr3 How many grams of NaBr would be needed in order to make 23.5 grams of AlBr3
Answers
Answer:
23.5 grams of AlBr3 will be produced by 27.20 grams of NaBr
Explanation:
The balanced equation here is
6NaBr + 1AlO3 = 3Na2O + 2AlBr3
6 moles of NaBr are required to produce 2 moles of AlBr3
Mass of one mole of NaBr = 102.894 g/mol
Mass of one mole of AlBr3 = 266.69 g/mol
Mass of 6 moles of NaBr = 6*102.894 g/mol
Mass of two moles of AlBr3 = 2*266.69 g/mol
6*102.894 g NaBr produces 2*266.69 g of AlBr3
23.5 grams of AlBr3 will be produced by (6*102.894)/(2*266.69 )*23.5 = 27.20 grams of NaBr
Which of the following is an example of using creativity while doing background research?
Organizing data in tables
Validating results by repetition
Drawing a conclusion
Writing a hypothesis
Answers
Answer:
B
Explanation:
Answer:
D. Writing a hypothesis
Explanation:
does any solid ba(io3)2 form when 7.5mg of barium chloride is dissolved in 500ml of 0.023m sodium iodate?
Answers
A solid Ba(IO₃)₂ will form when 7.5mg of barium chloride is dissolved in 500ml of 0.023m sodium iodate.
To determine whether a solid Ba(IO₃)₂ will form when 7.5 mg of barium chloride is dissolved in 500 mL of 0.023 M sodium iodate, we need to compare the solubility product (Ksp) of Ba(IO₃)₂ to the ion product (Q) at the given conditions.
The Ksp of Ba(IO₃)₂ is 1.5 × 10⁻⁹ at 25°C.
The ion product, Q, is calculated by multiplying the concentrations of the ions in solution raised to their stoichiometric coefficients. In this case, Ba₂⁺ and IO₃⁻ are in a 1:2 ratio, so:
Q = [Ba₂⁺][IO₃⁻ ]²
The concentration of Ba₂⁺ is determined by the amount of barium chloride dissolved in the solution:
0.0075 g BaCl₂ x (1 mol BaCl₂/208.23 g) x (1 mol Ba₂+/1 mol BaCl₂) / 0.5 L = 1.804 × 10⁻⁵ M Ba₂⁺
The concentration of IO₃⁻ is given as 0.023 M.
Plugging these values into the equation for Q:
Q = (1.804 × 10⁻⁵)(0.023)² = 9.87 × 10⁻⁹
Since Q > Ksp, the ion product exceeds the solubility product and a solid Ba(IO₃)₂ is expected to form.
To know more about the Barium chloride, here
https://brainly.com/question/31193853
#SPJ4
How many liters of F2 do you have if you are given 100 grams of F2?
Answers
Calculate the equilibrium constant and free energy change of given following reaction for Daniell cell at 298 K temperature. Zn (s)+Cu (aq)2+⇌Zn (aq)2+ +Cu (s)
Cell potential =1.1 volt (F=96500 coulomb)
Answers
The equilibrium constant (K) for the given reaction is \(1.26 * 10^{35}\), and the standard free energy change (ΔG°) is approximately -212,300 J/mol.
To calculate the equilibrium constant (K) and the free energy change (ΔG°) for the given reaction in the Daniell cell, we can use the Nernst equation:
ΔG° = -nFE°
where:
ΔG° is the standard free energy change
n is the number of electrons transferred in the balanced equation
F is Faraday's constant (96500 C/mol)
E° is the standard cell potential
Given that the cell potential (E°) is 1.1 V, we can determine the number of electrons transferred by looking at the balanced equation:
\(Zn (s) + Cu^{2+} (aq) < -- > Zn^{2+} (aq) + Cu (s)\)
In this case, 2 electrons are transferred.
Now we can calculate ΔG°:
ΔG° = -nFE° = -(2)(96500 C/mol)(1.1 V) = -212,300 J/mol
To calculate the equilibrium constant (K), we can use the equation:
ΔG° = -RTln(K)
At 298 K, we can rearrange the equation to solve for K:
K = exp(-ΔG° / RT)
Substituting the values:
K = exp(-(-212,300 J/mol) / (8.314 J/(mol·K) × 298 K)) ≈ exp(80.81)
≈ \(1.26 * 10^{35}\)
To learn more about equilibrium constant click here https://brainly.com/question/30620209
#SPJ11
Nitric acid, HNO3, i produced by a proce that allow nitrogen dioxide to react with water. How many mole of nitrogen dioxide, NO2 react with 3. 56 g of H2O ?
Answers
3.56 grams of water will react with 1 mole of nitrogen dioxide (NO2). Therefore, 3.56 grams of water will react with 0.051 moles of nitrogen dioxide (NO2).
When nitrogen dioxide (NO2) reacts with water, it forms nitric acid (HNO3). The reaction equation is:
2NO2 + H2O → HNO3 + NO
The reaction is exothermic, so it releases energy in the form of heat. This can lead to an increase in temperature and pressure if the reaction is carried out in an enclosed container.
The rate of the reaction is affected by several factors, including temperature, pressure and the concentration of the reactants. Increasing the temperature and pressure can speed up the reaction, but this can also lead to a decrease in the reaction's efficiency.
Learn more about Nitric acid:
https://brainly.com/question/22698468
#SPJ4
What is the formula mass of copper(ii) fluoride?
a. 146.10
b. 165.10
c. 101.55
d. 90.00
e. none of the above
Answers
The correct option is (c) 101.55.
Copper fluoride (CuF) -Copper(II) Fluoride Dihydrate is slightly soluble in water and has uses in ceramics and in fluxes used for brazing and soldering.Iodide ions are strong reducing agents. Therefore, Copper (II) Iodide reduces to insoluble copper (I) iodide. Thus making CuI2, CuI. CuI is not stable, so it doesn't exist in solution.Copper(II) fluoride is an inorganic compound with the chemical formula CuF2. It is a white crystalline, hygroscopic solid with a rutile-type crystal structure, similar to other fluorides of chemical formulae MF2 (where M is a metal).What is copper fluoride used for?
Copper fluoride is used in ceramics and in fluxes for brazing and soldering. It is only marginally soluble in water. Fluoride compounds have a wide range of uses in modern science and technology, from etching and oil refining to synthetic organic chemistry and the production of medications.Learn more about copper fluoride
brainly.com/question/13130550
#SPJ4
prokaryotic organisms are all ______ , or single- celled, organisms
Answers
Answer:
multi-celled
Explanation:
prokaryotic organisms are all, multi-celled, or single- celled, organisms
how is the purity of substances important?
Answers
Answer:
Pure substances have the ability to form predictable outcomes from their chemical reactions. Chemists tend to use pure substances when conduction chemial research as impure substances can mess up their experiments.
:quilibrium:
1. Define equilibrium when the equation
Use the equation below to answer the following equilibrium questions:
H₂O (g) + CO (g) =H₂(g) + CO₂(g) + 42 KJ
2. In the reaction above, what could happen that causes the equilibrium to shift to the right?
3. In the reaction above, what could happen to cause the equilibrium to shift to the left?
4. If pressure was increased, what direction would equilibrium shift?
5. If heat was added, what direction would equilibrium shift. What would happen to the concen
6.
If CO was added, what direction would equilibrium shift. What would happen to the concent
chiometry:
the following equation to answer the questions that follow:
Answers
Equilibrium is a state in a chemical reaction where the rate of the forward reaction is equal to the rate of the reverse reaction, resulting in no net change in the concentrations of reactants and products. It is represented by a double arrow (⇌) in chemical equations. In the given equation: H₂O (g) + CO (g) ⇌ H₂(g) + CO₂(g) + 42 KJ
To shift the equilibrium to the right, one or more of the following could occur:
Increasing the concentration of H₂ or CO₂
Decreasing the concentration of H₂O or CO
Increasing the pressure
Removing some of the products (H₂ and CO₂)
Decreasing the temperature
To shift the equilibrium to the left, one or more of the following could occur:
Decreasing the concentration of H₂ or CO₂
Decreasing the pressure
Increasing the temperature
If pressure is increased, the equilibrium will shift in the direction that produces fewer moles of gas. In this case, since there are fewer moles of gas on the right side of the equation (H₂ and CO₂), the equilibrium will shift to the right. If heat is added, the equilibrium will shift in the endothermic direction to absorb the additional heat. In this case, the forward reaction is endothermic (42 KJ on the right side), so the equilibrium will shift to the right to consume the added heat.
If CO is added, the equilibrium will shift to the right to consume the additional CO.The concentration of H₂O and CO₂ will increase, while the concentrations of H₂ and CO will decrease until a new equilibrium is reached.
for such more questions on reaction
https://brainly.com/question/11231920
#SPJ8
what type of crystal/stone is this? i’ve had it for a while and i’m not sure what it is exactly

Answers
Answer:
that is called a smooth pebbles
When two or more different elements bond together, compounds are formed. The smallest parts of these compounds that retain their original properties are called molecules. All atoms contain negatively charged electrons that orbit around a positively charged nucleus. The nucleus contains positively charged ____________ and neutrally charged ____________ . It is the ____________ charged ____________ that give atoms properties that are favorable in forming chemical bonds.
Answers
Answer:
protons, neutrons, negatively, electrons
Explanation:
protons are positive, neutrons are neutral. the entire basis of bonding is chemistry is the distribution of electrons, which are negatively charged particles surrounding the nucleus.