question 7 a data analyst is creating a visualization in tableau public. they want to keep it private from other users until it is complete. which icon should they click? 1 point close eye source private
Answers
To keep the data private from the public, the user should use the c)eye icon.
The Eye icon helps users to keep their visualizations private from other users. When clicked, it makes the visualization inaccessible to other users.
This helps ensure that the visualization is only visible to the user who created it and not to anyone else. Additionally, the Eye icon also helps prevent accidental deletion or modification of the visualization.
When the visualization is private, it is not visible to other users until the Eye icon is clicked again to make the visualization public. This makes it easier for the user to control who can access their visualization and helps protect their data.
Therefore, the correct option is c and the icon is the eye.
To know more about data science and interpretation, click below:
https://brainly.com/question/13104055
#SPJ4
Related Questions
If you try to place the compound on the pan over the flame, what could possibly happen? Explain your answer. [2 marks] anyone please help me!!:(
Answers
Answer:
it will probably flame up or explode or maybe start boiling
when planning this synthesis, the next logical step is to identify the carbon atoms of the original starting material. on the structure provided, mark the carbon atoms that most likely came from acetylene.
Answers
In order to identify the carbon atoms that most likely came from acetylene in the synthesis process, we need to understand a bit more about the properties of acetylene and its reactions.
Acetylene is a hydrocarbon with the chemical formula C2H2, meaning it is composed of two carbon atoms and two hydrogen atoms. When acetylene undergoes a reaction known as hydroboration-oxidation, it can be transformed into a variety of organic compounds, including alcohols and aldehydes.
In order to determine which carbon atoms in the starting material most likely came from acetylene, we would need to know more about the specific synthesis process being used. However, it's possible that the carbon atoms that came from acetylene would be the ones directly attached to the carbon-carbon triple bond in the molecule.
Without more information, it's difficult to say for sure which specific carbon atoms in the starting material came from acetylene. However, by understanding the properties and reactions of acetylene, we can make some educated guesses about which atoms might be involved in the synthesis process.
To know more about acetylene : https://brainly.com/question/15346128
#SPJ11
What does the atomic number of an element represent?
A. number of isotopes
B. number of protons
C. number of bonds
D. number of atoms
Answers
Answer:
protons
Explanation:
btw you protons and electrons are always the same
prcAnswer:
e c. proton
Explanation:
i pueslist
what is the name of the compoud H2Co2
Answers

What might happen if the mouse was removed from this
food web? Please be specific and mention ALL organisms
affected.
I’ll give u brainliest hurry
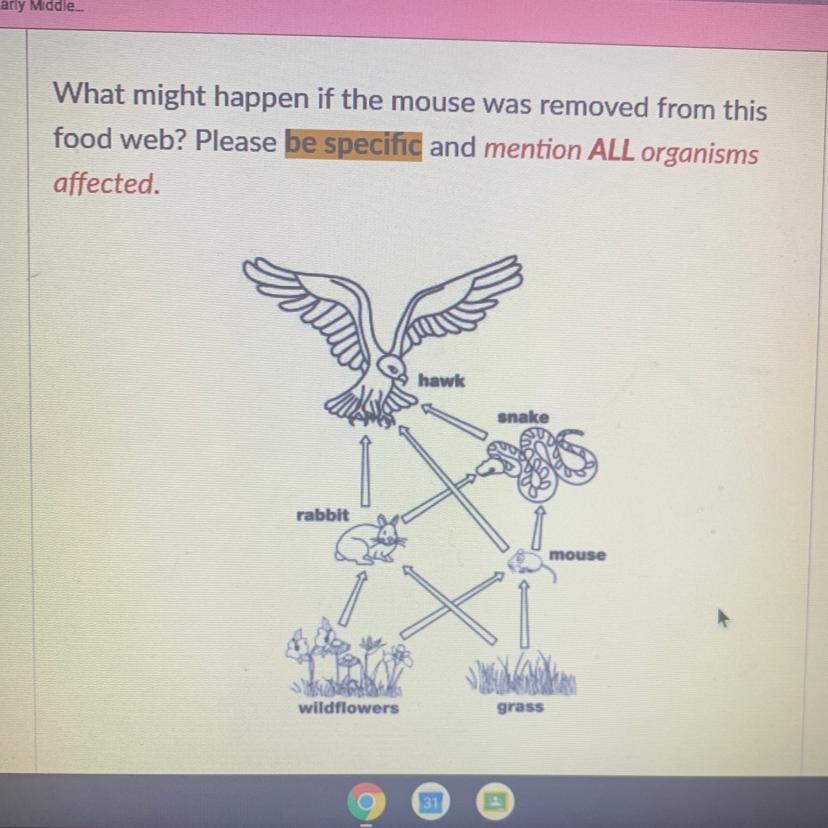
Answers
Answer:
They would most likely starve and die UNLESS they could move to another habitat or tend to eating other sources of food. All the other animals in the food web would die too, because their food supplies would have gone. The populations of the consumers would fall as the population of the producer fell.
When a organism is removed, the organism who eats or hunt them will decrease some because they lost one of the food source even though they still have other food sources. This new organism will brake the balance of the ecosystem so their food sources will decrease by having too many predators that hunt and eat them.
In food chain's if one source from it is ever removed it would mess up the entire chain causing issue
25 Points! A flashlight uses the chemical energy stored in a battery. This chemical energy is eventually converted into which two forms of energy?
A.) Mechanical and nuclear
B.) Radiant and thermal
C.) Thermal and mechanical
D.) Nuclear and electrical
Answers
Answer:A.) Mechanical and nuclear
Explanation:
iv,e got nuthing sorry im only a kid i have to leared that yet
answer:
B) Radiant and Thermal
explanation:
"In a flashlight, chemical energy in the batteries is converted to electrical energy when the circuit including the batteries, switch and light bulb is completed. The electrical energy is converted to light (electromagnetic / radiant energy) and some heat (thermal energy) by the light bulb." -https://www.crosbyisd.org/cms/lib6/TX02216626/Centricity/Domain/392/6_9C-EnergyFormsConversions.pdf
A student found a 500 cm3 sample of vinegar. Knowing that vinegar is a solution of ethanoic acid (CH3COOH) and water, this student decided to take a 25.0 cm3 sample, dilute it with 25.0 cm3 of water and then react it with sodium hydroxide. The dilute sample required 37.0 cm3 of 0.850 mol dm–3 sodium hydroxide solution to react entirely with it via the following reaction:
CH3COOH(aq) + NaOH(aq) → NaCH3COO(aq) + H2O(l)
Calculate the concentration of ethanoic acid in the original sample of vinegar.
Answers
Answer:
1.26mol/dm3
Explanation:

10 moles of potassium hydroxide in 5.16 L of solution
Answers
Answer:
The molarity is 8.52M
Explanation:
molality = moles of solute/kg of solvent = 5.2mol/kg
moles of solute = 5.2mole
mass of solute = moles of solute × MW = 5.2 × 56.106 = 291.8g
mass of solvent = 1kg = 1000g
Mass of solution = mass of solute + mass of solvent = 291.8g + 1000g = 1291.8g
Density of KOH = 2120g/L
Volume of solution = mass/density = 1291.8/2120 = 0.61L
Molarity = number of moles of solute/volume of solution = 5.2/0.61 = 8.52M
The concentration in the solution is 1.94 mol/L
From the question,
We are to determine the concentration of 10 moles of potassium hydroxide in 5.16 L of solution.
From the formula
Number of moles = Concentration × Volume
Then, we can write that
\(Concentration = \frac{Number\ of\ moles}{Volume}\)
From the question,
Number of moles of potassium hydroxide (KOH) = 10 moles
Volume of solution = 5.16 L
∴ Concentration of the solution = \(\frac{10}{5.16}\)
Concentration of the solution = 1.94 mol/L
Hence, the concentration in the solution is 1.94 mol/L
Learn more here: https://brainly.com/question/19196652
Here is the complete question:
Calculate the concentration in each of the following solutions:
1. 10 moles of potassium hydroxide in 5.16 L of solution
NO FILES PLZ HELP
Which of the following statements describes a chemical change?
Select one:
a. A gas is given off when a liquid boils.
b. A new substance is formed with different properties.
c. A solid dissolves in a liquid.
O d. A solid forms when a liquid freezes.
Answers
all of the other reactions can be reversed and do not change the make up or atoms of the solution
please rank brainliest if possible :)
wgat would haooeb ti ag abd ck us solid sodiun chloride is added to a saturated solution of silver chloride
Answers
According to the equilibrium law, this should move the AgCl equilibrium to the left (i.e., favor the reverse reaction) until the system finds equilibrium once more. This will increase the concentration of Cl-(aq) ions in the solution.
The following equilibrium is established by an AgCl solution that is saturated:
Ag+(aq) + Cl- =>AgCl(s) (aq)
NaCl will entirely separate into ions when added to the solution:
Na+(aq) + Cl=> NaCl(s) (aq)
According to the equilibrium law, this should move the AgCl equilibrium to the left (i.e., favor the reverse reaction) until the system finds equilibrium once more. This will increase the concentration of Cl-(aq) ions in the solution.
What is seen is that as an AgCl precipitate forms, both [Ag+] and [Cl-] will decrease.Because part of the Ag ions precipitated out, the [Ag+] in the new system will be lower than it was in the original saturated solution, while the [Cl-] ions will be higher (because you added more when you added NaCl). There will have been some precipitation of that excess Cl-, but not all of it. The result will be the same as the Ksp of AgCl if these new ion concentrations are entered into the Ksp equation.
learn more about saturated solution here
https://brainly.com/question/4529762
#SPJ4
the question you are looking for is
What would happen to the Ag+ and Cl- concentrations if solid NaCl were dissolved in a saturated solution of AgCl in water?
how many moles of ammonia can be produced from the reaction of 56.0 grams of nitrogen with 72.0 grams of hydrogen?
Answers
4 moles of ammonia can be produced from the reaction of 56.0 grams of nitrogen.
What is mole?
The International System of Units uses the mole (symbol: mol) as the unit of substance amount. How many elementary entities of a particular substance are present in an object or sample is determined by the quantity of that substance. It is specified that the mole contains exactly 6.02214076×10²³ elementary entities.
The reaction of hydrogen and nitrogen is
N₂ (s) + 3 H₂ (g) → 2 NH₃
The mass of nitrogen is 28.
The mass of hydrogen is 2
The mass of 3 H₂ is 6 .
The mass of ammonia is 14 + (3×1) = 14 + 3 = 17
The mass of 2 NH₃ is 34.
28 grams of nitrogen produced 2 moles of ammonia.
1 grams of nitrogen produced 2/28 moles of ammonia.
56 grams of nitrogen produced (2 × 56)/28 moles of ammonia.
= 4 moles
To learn more about substance, click on the below link:
https://brainly.com/question/14180294
#SPJ4
3. How many seconds would it
take o radio wave to travel
from the planet Mars to
Earth when they are the
closest?
(Closest distance from Mars
to Earth = 54.6 million
kilometers)
Answers
Answer:
270 sec . ( = 4 min . 30 sec . ) Explanation: Any radio wave is moving at the speed of light, regardless of the frequency, so the frequency should be superfluous information. The speed is light is close to 300 , 000 k m s I take the distance you are given, to be 8.2 ⋅ 10 7 k m (since 8.2 ⋅ 107 k m doesn't make good sense - it's less than 1000 km) i.e. 82 , 000 , 000 km.
Before we actually compute this, we can notice that this is a little more than half the distance of the earth from the sun (150 mill. km), a distance the light uses 500 sek. = 8 min 20 sec. to travel, so we should get about 4 1 2 min for the radio wave to reach Mars from the earth.
Actual calculation: 82 , 000 , 000 km/300 , 000 k m s = 820 3 s = 273.3 s As the distance is given with 2 significant figures, we round this off to 270 sec = 4 min 30 sec.
Explantation:
Hope this helps =)
Four different people are using sound wave technology. Which describe the form of technology most likely being used by each person?
Answers
Answer:
Its D
Explanation:
Ali is using a specialized headphone that cancels the extra noise in the atmosphere.
Megan is using a transmitter to convert electrical signals into radio waves.
Kristoff is using a microphone to convert sound waves into the electrical signals.
Jenna is using the radio to convert radio waves into sound waves.
Answer:
D. Ali is using specialized headphones, Megan is using a transmitter, Kristoff is using a microphone, and Jenna is using a radio.
Explanation:
6. Sometimes a tire that appears to be fine may be unsafe because: A. There may be perforations too small to see B. The manufacturing date may be incorrect C. Chemical reactions may have deteriorated the rubber
Answers
Answer:
C. Chemical reactions may have deteriorated the rubber
Explanation:
A tire ages with time and most of the time this happens due to chemical reactions in the rubber parts. This could happen faster due to heat and sun.
As a tire is deteriorating or aging, a chemical change happens. What happens is that more chemical bonds are going to be produced with time between the molecules. We call this the process of oxidation. This actually bad for the tire, because the rubber would turn out to be stuffs d strong causing the tires thread to separate and also disintegration would occur.
You need to prepare 100.0 mL of a pH 4.00 buffer solution using 0.100M benzoic acid (pK
a
=4.20) and 0.240M sodium benzoatc. How many milliliters of each solution should be mixed to prepare this buffer? benzoic acid:
Previous question
Answers
To prepare the pH 4.00 buffer solution, you should mix approximately 61.35 mL of the 0.100 M benzoic acid solution with 38.65 mL of the 0.240 M sodium benzoate solution.The ratio of benzoic acid to sodium benzoate in the buffer solution using the Henderson-Hasselbalch equation.
To prepare a pH 4.00 buffer solution using benzoic acid and sodium benzoate, we need to calculate the appropriate volumes of the 0.100 M benzoic acid and 0.240 M sodium benzoate solutions.
First, we need to determine the ratio of benzoic acid to sodium benzoate in the buffer solution. The Henderson-Hasselbalch equation can help us with this calculation:
pH = pKa + log([A-]/[HA])
Given that the pH is 4.00 and pKa is 4.20, we can rearrange the equation:
log([A-]/[HA]) = pH - pKa
log([A-]/[HA]) = 4.00 - 4.20
log([A-]/[HA]) = -0.20
Next, we take the antilog of -0.20 to find the ratio of [A-] to [HA]:
[A-]/[HA] = antilog(-0.20)
[A-]/[HA] = 0.63
The ratio of [A-] to [HA] is 0.63.
Now, let's calculate the volumes of each solution needed. Let's assume x represents the volume (in mL) of the 0.100 M benzoic acid solution and y represents the volume (in mL) of the 0.240 M sodium benzoate solution.
Since the total volume is 100.0 mL, we have the equation: x + y = 100
Considering the ratio of [A-] to [HA] as 0.63, we can write the equation: y/x = 0.63
Solving these two equations simultaneously will give us the volumes of each solution:
x + y = 100
y/x = 0.63
By substituting y = 0.63x from the second equation into the first equation, we get:
x + 0.63x = 100
1.63x = 100
x = 61.35 mL (rounded to two decimal places)
Substituting this value back into the equation x + y = 100, we find:
61.35 + y = 100
y = 38.65 mL (rounded to two decimal places)
Therefore, to prepare the pH 4.00 buffer solution, you should mix approximately 61.35 mL of the 0.100 M benzoic acid solution with 38.65 mL of the 0.240 M sodium benzoate solution.
To know more about the Henderson-Hasselbalch equation, click here, https://brainly.com/question/31732200
#SPJ11
What does the 2 mean in the formula 5Mg3(PO4)2? There are two elements in magnesium phosphate. There are two molecules of magnesium phosphate. There are two magnesium ions in a molecule of magnesium phosphate. There are two phosphate ions in a molecule of magnesium phosphate.
Answers
Answer: In this compound, phosphorous and oxygen act together as one charged particle, which is connected to magnesium, the other charged particle.
Explanation:
Balance the following chemical equation (if necessary):
FeCl3(aq) + Na₂S (aq) → Fe₂S3 (S) + NaCl(aq)
Answers
The correct balanced chemical equation is FeCl₃(aq) + Na₂S(aq) → 3Fe₂S₃(s) + 3NaCl(aq)
To achieve a chemical equation's balance:
Fe₂S₃ (s) + NaCl (aq) FeCl₃ (aq) + Na₂2S (aq)
Let's start by balancing the atoms of iron (Fe). The iron atoms are already balanced, since there are two on the reactant side and two on the product side.
Let's now balance the atoms of sodium (Na). Two sodium atoms are present on the reactant side, hence two sodium atoms are required on the product side. By adding a coefficient of 2 in front of NaCl, we may do this:
Let's balance the sulfur (S) atoms lastly.
FeCl3(aq) + Na2S(aq) → 3Fe2S3(s) + 3NaCl(aq)
Learn more about chemical equation, here:
https://brainly.com/question/14457720
#SPJ1
What is the molar concentration a a 12 % sodium chloride solution (MW 58.5)
Answers
The molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To determine the molar concentration of a 12% sodium chloride solution, we need to convert the given percentage concentration into molarity.
First, we need to understand that the percentage concentration refers to the mass of the solute (sodium chloride) relative to the total mass of the solution.
In this case, a 12% sodium chloride solution means that there are 12 grams of sodium chloride in 100 grams of the solution.
To convert this into molar concentration, we need to consider the molar mass of sodium chloride, which is 58.5 g/mol.
We can start by calculating the number of moles of sodium chloride in 12 grams:
Moles of sodium chloride = mass of sodium chloride / molar mass of sodium chloride
Moles of sodium chloride = 12 g / 58.5 g/mol = 0.205 moles
Next, we calculate the volume of the solution in liters using the density of the solution. Since the density is not provided, we assume a density of 1 g/mL for simplicity:
Volume of solution = mass of solution / density
Volume of solution = 100 g / 1 g/mL = 100 mL = 0.1 L
Finally, we calculate the molar concentration (Molarity) by dividing the number of moles by the volume in liters:
Molar concentration = moles of solute / volume of solution
Molar concentration = 0.205 moles / 0.1 L = 2.05 M
Therefore, the molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To learn more about molarity click here: brainly.com/question/31545539
#SPJ11
5. Which set of coefficients will balance this chemical
equation?
_C2H4(g) +
O2(g) → 2CO2(g) + 2H2O(g)
A. 1.2
B. 1.3
C. 2.3
D. 2.5
Answers
Answer: The answer is B 1,3
How many moles are there in 54g of H2O?
3
1
6.022 x 1023
162
Answers
Please help me in Q3 it’s not understandable to me

Answers
Answer:
A- mixture
B- element
C- compound
Explanation:
A mixture is composed of two or more substances. Since it is not entirely homogeneous throughout, then it can not melt or boil at a fixed temperature.
An element is any substance that cannot be decomposed into simpler substances by ordinary chemical processes. This describes substance B.
A pure chemical element has a fixed melting and boiling point.
A compound is composed of one or more chemical elements. A compound may be broken down into its components by certain processes.
The Calories on a food label indicate the
amount of energy the food contains in
one serving. Which form of energy is
contained in food?
Answers
Answer:
chemical energy
Explanation:
Helpppppppppppppppppppp

Answers
Answer:
option B it was captured by earth's gravity
Explanation:
please Brinlmark me please
A 1-year-old girl with a hyperlipoproteinemia and lipase deficiency has the following lipid profile:
cholesterol: 300 mg/dL
LDL: increased
HDL: decreased
triglycerides: 200 mg/dL
chylomicrons: present
A serum specimen from this patient that was refrigerated overnight would most likely be:
Answers
A serum specimen from a 1-year-old girl with hyperlipoproteinemia and lipase deficiency that was refrigerated overnight would most likely be usable for a lipid profile.
A serum specimen from a 1-year-old girl with hyperlipoproteinemia and lipase deficiency that was refrigerated overnight would most likely be usable for a lipid profile. Lipid profile is a blood test that measures the amount of cholesterol, triglycerides, and other lipids present in your blood. A lipid profile test can help diagnose hyperlipoproteinemia and lipase deficiency.
It helps determine the total amount of lipids, including cholesterol, in your blood.A serum specimen is a blood sample that has been collected and separated from red blood cells. Serum specimens are refrigerated overnight because they are stable at low temperatures. This helps prevent changes in the sample's lipid profile.
The lipid profile results for the patient described above indicate that the patient has a high cholesterol level, an increased level of LDL cholesterol, a low level of HDL cholesterol, and a high level of triglycerides.
The results of this lipid profile can be used to diagnose and monitor the patient's condition. A follow-up test will be required after the treatment has been given in order to monitor its efficacy and check whether the lipid profile is within the normal range.
Learn more about lipid -
brainly.com/question/28437379
#SPJ11
What are produced when a base is mixed with water?
hydrogen ions
hydroxide ions
oxygen atoms
oxygen ions
Answers
Answer:
The answer is hydrogen ions
How many grams are in 3 moles of Rf?
O 785
0 784
0786
O 783
Answers
EASY PLEASE HELP !!!!!!

Answers
Answer: B.) Salt and water
Explanation: A neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of H+ ions and OH- ions to generate water.
Hope this helps :)
Answer:
B. Salt and water
Explanation:
have a nice day!!
some assumptions from the kinetic molecular theory are listed below. which one is most frequently cited to explain diffusion of a gas?
Answers
Some assumptions from the kinetic molecular theory , one is most frequently cited to explain diffusion of a gas is the gas consist of tiny particles moving in the random straight line motion.
The assumption of the kinetic molecular theory are :
the gases are made up of tiny particles and these tiny particles are in random motion.there is no force of attraction between the gas particles.the temperature of the gas id depend on the kinetic energy og the gas particles.Thus, the gas is the gas consist of tiny particles moving in the random straight line motion explain the diffusion of a gas.
To learn more about kinetic molecular theory here
https://brainly.com/question/5954988
#SPJ4
ASAP. Magnetic field lines cannot be observed using a compass or iron filings.
True or false
Answers
Answer:
false
Explanation:
magnetic field lines can be accurately observed using *iron filling*
The isotopes of Madeupium (Ma) and their abundance in nature are shown below. Which is the most likely estimate of what the atomic mass is close to?
Ma-74 (74.333 - 85%)
Ma-75 (74.999 - 10%)
Ma-76 (75.700 - 5%)
A. 74 g
B. 75 g
C. 76 g
Answers
Answer:
A = 74 g
Explanation:
Given data:
Ma-74 (74.333 - 85%)
Ma-75 (74.999 - 10%)
Ma-76 (75.700 - 5%)
Atomic mass close to = ?
Solution:
1st of all we will calculate the average atomic mass.
Average atomic mass = (abundance of 1st isotope × its atomic mass) +(abundance of 2nd isotope × its atomic mass) + (abundance of 3rd isotope × its atomic mass) / 100
Average atomic mass = (85×74.333)+(10×74.999)+(5×75.700) /100
Average atomic mass = 6318.305 +749.99+ 378.5 / 100
Average atomic mass= 7446.795/ 100
Average atomic mass = 74.467 amu
The average atomic mass is closer to the 74 g.