Answers
Answer:
8058.88
Explanation:
7.87 x 1024 = 8058.88
duh
Related Questions
Calcium carbonate is a common ingredient in antacids that reduces the discomfort associated with acidic stomach or heartburn. Stomach acid is hydrocholoric acid, HCl. What volume in milliliters (mL) of an HCl solution with a pH of 1.52 can be neutralized by 27.0 mg of CaCO3
Answers
Answer:
17.86mL of the HCl solution
Explanation:
The reaction of CaCO₃ with HCl is:
CaCO₃ + 2HCl → CaCl₂ + CO₂ + H₂O
The concentration of HCl with a pH of 1.52 is:
pH = 1.52 = -log [H⁺]
[H⁺] = 0.0302M = [HCl]
27.0mg = 0.0270g of CaCO₃ (Molar mass: 100.09g/mol) are:
0.0270g of CaCO₃ ₓ (1mol / 100.09g) = 2.70x10⁻⁴ moles of CaCO₃
Moles of HCl to react completely with these moles of CaCO₃ are:
2.70x10⁻⁴ moles of CaCO₃ ₓ (2 mol HCl / 1 mol CaCO₃) =
5.40x10⁻⁴ moles of HCl
As the concentration of HCl is 0.0302M, volume in 5.40x10⁻⁴ moles is:
5.40x10⁻⁴ moles of HCl * (1L / 0.0302mol) = 0.01786L =
17.86mL of the HCl solutionThe volume in milliliters (mL) of an HCl solution with a pH of 1.52 that can be neutralized by the given CaCO₃ is 17.87 mL
From the question,
We are to determine the volume of HCl that could be neutralized by the given CaCO₃
First, we will write the balanced chemical equation for the reaction
The balanced chemical equation for the reaction is
2HCl + CaCO₃ → CaCl₂ + CO₂ + H₂O
This means
2 moles of HCl is required to neutralize 1 mole of CaCO₃
Now, we will determine the number of moles of CaCO₃ present
Mass of CaCO₃ = 27.0 mg = 0.027 g
Using the formula
\(Number\ of\ moles = \frac{Mass}{Molar\ mass}\)
Molar mass of CaCO₃ = 100.0869 g/mol
∴ Number of moles of CaCO₃ present = \(\frac{0.027}{100.0869}\)
Number of moles of CaCO₃ present = 0.00026977 mole
Since
2 moles of HCl is required to neutralize 1 mole of CaCO₃
Then,
0.00053954 mole of HCl will be required to neutralize the 0.00026977 mole of CaCO₃
∴ 0.00053954 mole of HCl is required to neutralize the CaCO₃
Now, for the volume of HCl solution with a pH of 1.52 required
First,
We will determine the concentration of the HCl
From the given information
pH of the HCl = 1.52
Using the formula
pH = -log[H⁺]
Then,
1.52 = -log[H⁺]
∴ [H⁺] = 10^(-1.52)
[H⁺] = 0.0302 M
∴ The concentration of the HCl is 0.0302 M
Now, for the volume
Using the formula,
\(Volume = \frac{Number\ of\ moles}{Concentration}\)
∴ Volume of HCl required = \(\frac{0.00053954}{0.0302}\)
Volume of HCl required = 0.01787 L
Volume of HCl required = 17.87 mL
Hence, the volume in milliliters (mL) of an HCl solution with a pH of 1.52 that can be neutralized by the given CaCO₃ is 17.87 mL
Learn more here: https://brainly.com/question/14873607
A chemist has two solutions containing unknown salts in water. She determines that each has a solute concentration of 0.5 M. Which of the following will certainly NOT distinguish the two solutions from each other? choose one
-viscosity
-conductivity
-density
-vapor pressure
Answers
Viscosity, conductivity, and density are all physical properties that can be affected by solute concentration, but vapor pressure will not distinguish the two solutions from each other.
Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid or solid form. The vapor pressure of a solution depends on the vapor pressure of the pure solvent and the mole fraction of the solute. If two solutions have the same solute concentration, they will have the same mole fraction of solute and the same vapor pressure, even if they contain different salts.
Therefore, vapor pressure will not distinguish the two solutions from each other. However, viscosity, conductivity, and density can all be used to distinguish the two solutions. The viscosity of a solution depends on the size and shape of the solute molecules and their interactions with the solvent molecules. The conductivity of a solution depends on the presence of ions and their mobility. The density of a solution depends on the mass and volume of the solute and solvent. By measuring these properties, a chemist can determine the properties of the solute and determine if the two solutions are different.
Learn more about Viscosity:
brainly.com/question/30577668
#SPJ4
How many moles of NaHCO3
are in 27.5 g NaHCO3
?
Answers
Which of the following is an example of a molecule? A. Cl(chlorine) B. HCl (hydrochloric acid) C. Hydrogen D. Bromine
Answers
Answer:
hydrogen
Explanation
a molecule of hydrogen is the simplest possible molecule . its consists of two protons and two electrons heid together by electrostatic forces. like atomic hydrogen the assemblage can exist in a number of energy levels
What is a neutralization reaction?
Answers
The attraction that nonpolar molecules have for each other is primarily caused by the presence of what?
Answers
(a) Van der Waal's forces. Van der Waal's forces are weak electrostatic interactions between non-polar molecules.
These weak forces arise from the fluctuating dipoles within the molecules, which cause temporary charges to develop and attract each other. This attraction leads to the formation of weak intermolecular bonds, which are mainly responsible for the attraction between non-polar molecules. These forces are weak in comparison to other intermolecular forces, such as hydrogen bonding and ionic bonds, but are still important for the stability of non-polar molecules and their ability to dissolve in other non-polar solvents.Van der Waals forces are attractive intermolecular forces between molecules caused by the fluctuating dipole moments of molecules.
learn more about ionic bonds Refer:brainly.com/question/11527546
#SPJ1
complete question:The attraction that non-polar molecules have for each other is primarily caused by —
(a) Van der Waal's forces (b) Difference in electronegativities
(c) Hydrogen bonding (d) High ionisation energy
Researchers investigated the influence of environmental pH on the activity of peroxidase, an enzyme that catalyzes the conversion of hydrogen peroxide to water and oxygen gas. In an experiment, the researchers added a hydrogen peroxide solution containing guaiacol to several identical test tubes and adjusted the solution in each test tube to a different pH. The researchers included the guaiacol because it caused the solutions to change color as the reactions proceeded, which the researchers relied on for measuring reaction rates. Finally, the researchers added the same amount of peroxidase to each test tube and measured the rate of each reaction at 23°C. The results of the experiment are represented in Figure 1. One of the researchers proposes using oxygen gas production to measure reaction rates. Which of the following statements best justifies the use of the proposed modification as a way of creating an appropriate control for the investigation?
O the experiment can be repeated without hydrogen peroxide, which will help eliminate an uncontrolled variable. O the experiment can be repeated without hydrogen peroxide, which will help eliminate an uncontrolled variable. O the experiment can be repeated without peroxidase, which will introduce a second independent variable. O the experiment can be repeated without peroxidase, which will introduce a second independent variable. O the experiment can be repeated without guaiacol, which will reveal the effect of guaiacol on the reaction rates. O the experiment can be repeated without guaiacol, which will reveal the effect of guaiacol on the reaction rates. O the experiment can be repeated without water, which will reveal whether the reaction can occur inside a living cell.
Answers
In studying the influence of environmental pH on peroxidase activity, researchers can repeat the experiment without guaiacol, which will reveal the effect of guaiacol on reaction rates.
The proposal to use the production of gaseous oxygenOne of the investigators' proposal to use oxygen gas production to measure reaction rates leads to a change in the dependent variable.
In the first experiment the dependent variable was the volume of guaiacol and in the second experiment the dependent variable will be oxygen production.
Learn more about experiments at https://brainly.com/question/30055326
#SPJ4

During a volcanic eruption, lava flowed at a rate of 37 m/min. At this rate how far in kilometers
can lava travel in 45 minutes?
Answers
Which of these metals is the MOST reactive?
Lithium
Copper
Gold
Calcium
Answers
Answer:
The correct answer to you your question is Potassium
Explanation:
Potassium is the most reactive
We use less than 1% of the water on Earth for
Answers
Answer: drinking, bathing.
Explanation:
Record the mass of the object based on the reading from the triple-beam balance.
The mass of the object is
grams.
Answers
Answer:Typically, the reading scale of the middle beam reads in 100 gram increments, the far beam in 10 gram increments, and the front beam can read from 0 to 10 grams. The triple beam balance can be used to measure mass directly from the objects, find mass by difference for liquid, and measure out substances.
Explanation:
You've probably heard that some types of bacteria can cause infections and make you sick.
That's true, but that's not all bacteria do. There are an estimated 5 nonillion bacteria on the
planet. 5 nonillion is a 5 with 30 zeros after it, like this:
5,000,000,000,000,000,000,000,000,000,000.
Many of those 5 nonillion bacteria help you much more than they harm you. In fact, bacteria
have many very important functions for life on Earth. In the soil, bacteria help decompose
dead organisms and cycle nitrogen through the ecosystem, which plants rely on. What else
do bacteria do? Bacteria in your digestive system help you digest your food. We even use
some bacteria to make the foods we eat. In yogurt, probiotics are often called "good"
bacteria. They ferment the milk.
AB
If soil did not have bacteria in it, then
A
plants would probably not have enough nitrogen.
B
we probably could not digest our food.
C
plants would probably never die.
D
we could not eat yogurt.
Answers
Answer:
(A) Plants would probably not have enough nitrogen.
Explanation:
According to the passage, bacteria help us digest our food and make yogurt. But it is the bacteria in the soil specifically that "Cycle nitrogen through the ecosystem, which plants rely on"
Name the following compounds NH4CI
Answers
The compound NH4Cl or ammonium chloride is composed of two ions: ammonium ion (NH4+) and chloride ion (Cl-). The ammonium ion is a polyatomic cation made up of one nitrogen atom and four hydrogen atoms, while the chloride ion is a monatomic anion made up of one chlorine atom.
In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor. hydrogen+oxygen⟶water
If you burn 46.2g of hydrogen and produce 413g of water, how much oxygen reacted?
mass of oxygen:
Answers
Answer:
ok, here is your answer
Explanation:
AI-generated answer
To find the mass of oxygen that reacted, we need to use the Law of Conservation of Mass, which states that in a chemical reaction, the mass of the reactants equals the mass of the products.
First, we need to find the number of moles of hydrogen that reacted:
Molar mass of hydrogen (H₂) = 2.016 g/mol
Number of moles of H₂ = mass/molar mass = 46.2 g/2.016 g/mol = 22.92 mol
Next, we need to use the balanced chemical equation to find the number of moles of water produced:
hydrogen + oxygen → water
2H₂ + O₂ → 2H₂O
From the equation, we can see that for every 2 moles of H₂, 1 mole of O₂ is required to produce 2 moles of H₂O. Therefore, the number of moles of O₂ required to produce 22.92 moles of H₂O is:
Number of moles of O₂ = 1/2 x 22.92 mol = 11.46 mol
Finally, we can find the mass of oxygen that reacted by using its molar mass:
Molar mass of oxygen (O₂) = 32.00 g/mol
Mass of oxygen = number of moles x molar mass = 11.46 mol x 32.00 g/mol = 366.72 g
Therefore, the mass of oxygen that reacted is 366.72 g.
mark me as brainliest4. Which is a mixture whose individual particles are larger than those in a solution but still too small to be easily seen?
Answers
COLLOID is the answer to that
Answer: Colloids
Explanation: they can not be separated easily but scatter light
Granite is part of the crust layer of the earth.
True
False
Answers
Answer:
That would be True
Explanation:
granite is a part of the crust layer.
Calculate the missing cell potential. 2.20 V 0.77 V -0.44 FeO42- Fe3+ Fe2+ Fe Eo Will Fe3+ disproportionate. Give reasons
Answers
The value of missing cell potential is - 0.04 V
What is cell potential ?
The cell potential (Ecell), is the measure of the potential difference between two half cells in an electrochemical cell.
Fe(III) / Fe(II) potential involves different chemical species in acidic and basic solution.
2FeO²⁻ + 3ClO⁻ + 2OH⁻ --> FeO₄²⁻ + 3Cl⁻ + H₂O
FeO₄²⁻ can easily oxidise water under acidic conditions.
Hence, The value of missing cell potential is - 0.04 V
Learn more about cell potential here ;
https://brainly.com/question/1313684
#SPJ1
HELP WITH MY 2 QUESTION
1-What type of packaging is used for milk?
2-How do the physical and chemical properties (material, reactivity, shape, hardness, color)
Answers
Answer:
Answer to number one
Explanation:
Polyethylene terephthalate (PET) is a plastic material used for milk packaging.
How much water has to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M?
Answers
Approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
To find the amount of water that needs to be evaporatedThe relationship between the initial and final concentrations and volumes must be taken into account.
Given: Initial concentration \((C^1) = 1 M Initial volume (V^1) = 250 mL\)
\((C^2) = 3 M final concentration\)
We can use the equation:
\(C^1 * V^1 = C^2 * V^2\)
Where:
\(V^2\)is the final volume of the solution
Rearranging the equation to solve for V2:
\(V^2 = (C^1 * V^1) / C^2\)
Substituting the given values:
\(V^2 = (1 M * 250 mL) / 3 M\)
\(V^2 = 250 mL / 3\)
\(V^2\) ≈ \(83.33 mL\)
To find the amount of water that needs to be evaporated, we subtract the final volume from the initial volume:
Amount of water to be evaporated = \(V^1 - V^2\)
Amount of water to be evaporated = 250 mL - 83.33 mL
Amount of water to be evaporated ≈ 166.67 mL
Therefore, approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
Learn more about Initial concentration here: brainly.com/question/30720317
#SPJ1
A 5.0 g sample of metal was heated from 10°C to 40. °C. It absorbed 43.7 J of energy as heat. What is the specific heat of this piece of metal?
Answers
Answer:
Balls
Explanation:
Cuz
PLEASE HELP QUICKLY!!!
HI gas is removed from the system
at equilibrium below. How does the
system adjust to reestablish
equilibrium?
51.8 kJ + H₂(g) + 1₂(g) = 2HI(g)
A. The reaction shifts to the right (products) and the concentrations
of I, and H₂ decrease.
B. The reaction shifts to the left (reactants) and the concentrations
of H₂ and I increase.
C. The reaction shifts to the right (products) and the concentrations
of I, and H₂ increase.
D. The reaction shifts to the left (reactants) and the concentration of
HI increases.

Answers
Answer:
A. The reaction shifts to the right (products) and the concentrations of I and H₂ decrease.
Explanation:
If gas is removed from the system at equilibrium, the system will try to compensate for the loss by shifting the reaction in a direction that produces more gas molecules. This is known as Le Chatelier's principle, which states that a system at equilibrium will respond to a disturbance by shifting in a way that minimizes the effect of the disturbance.
In this case, since gas is being removed from the system, the reaction will shift to the side that produces more gas molecules. Looking at the balanced equation, we can see that 2HI(g) has a greater number of gas molecules compared to H₂(g) and I₂(g). Therefore, the system will shift to the right (products) to produce more HI(g) and reestablish equilibrium.
which physical change is endothermic
Answers
Answer:The physical changes that are endothermic are melting, vaporization and sublimation.
Explanation:
A student says that since the atomic theory is just a theory, it should not be considered useful. Which statement best argues against the student's opinion? (2 points) Scientific theories change over time. Scientific theories are the results of many experiments and observations. Scientists often do not agree about specific details of scientific theories. Scientists often propose competing theories. Scientific theories do not become Scientific Laws.
Answers
Answer:
Scientific theories are the results of many experiments and observations.
Explanation:
I think this is ti sorry if I'm wrong :|
Stephan’s mother cuts a twig from a rose bush and plants it in the soil. After a few days, Stephan observes a new plant growing. Which characteristic does the growth of the new plant depict?
Answers
The growth of the new plant depicts the asexual reproduction characteristic. The characteristic that describes the growth of the new plant in Stephan's mother cutting a twig from a rose bush and planting it in the soil is asexual reproduction.
Asexual reproduction is the mode of reproduction by which organisms generate offspring that are identical to the parent's without the fusion of gametes. Asexual reproduction is a type of reproduction in which the offspring is produced from a single parent.
The offspring created are clones of the parent plant, meaning they are identical to the parent.The new plant in Stephan’s mother cutting a twig from a rose bush and planting it in the soil depicts the process of asexual reproduction, which is the ability of a plant to reproduce without seeds. In asexual reproduction, plants can reproduce vegetatively by cloning themselves using their roots, bulbs, or stems.
Know more about characteristic here:
https://brainly.com/question/28790299
#SPJ8
How many mL of 2.25M H2SO4 are needed to react completely with 69.9g BaO2

Answers
Answer:
4 millllllermeeters jb
Which of the following is NOT an example of kinetic energy?
A.an apple hanging from a tree branch
B.a roller coaster climbing a hill
C.a car rolling down a hill
D.a pencil falling
Answers
Answer: A car rolling down a hill
Explanation: because kinetic energy is the force of some being pulled or pushed
Answer: A. An apple hanging from a tree branch
Explanation:
Kinetic energy is associated with movement
Assume that the temperature is held constant and determine the volume of the helium at 792 mmHg ? Express your answer to three significant figures and include the appropriate units.
Answers
The volume of the helium gas at 792 mmHg is 1652 mL.
What is the relationship between the volume and pressure of a gas at constant temperature?The relationship between the volume and pressure of a gas at constant temperature is given by Boyle's law.
Boye's law states that the volume of a given mass of gas is inversely proportional to its pressure provided the temperature is kept constant.
Mathematically, Boyle's law is given below as follows:
P₁V₁ = P₂V₂where:
P₁ = the initial pressure of the gasV₁ = the initial volume of the gasP₂ = the final pressure of the gasV₂ = the final volume of the gasSolving for the final volume of the gas, V₂
V₂ = P₁V₁ / P₂
P₁ = 719 mmHg
V₁ = 1820 mL
P₂ = 792 mmHg
final volume, V₂ = 719 * 1820 / 792
final volume, V₂ = 1652 mL
Learn more about Boyle's law at: https://brainly.com/question/1696010
#SPJ1
Complete question:
A sample of helium occupies 1820 mL at 719 mmHg. Part A Assume that the temperature is held constant and determine the volume of the helium at 792 mm Hg? Express your answer with the appropriate units.
a student mixed 20 grams of salt into a beaker with 200 milliliters of warm water. then, the student set the cup of saltwater on a windowsill undisturbed for one week. what changes did the student observe? include what happened when salt was mixed with warm water and what most likely happened to the saltwater after one week.
Answers
Answer:
Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Explanation:
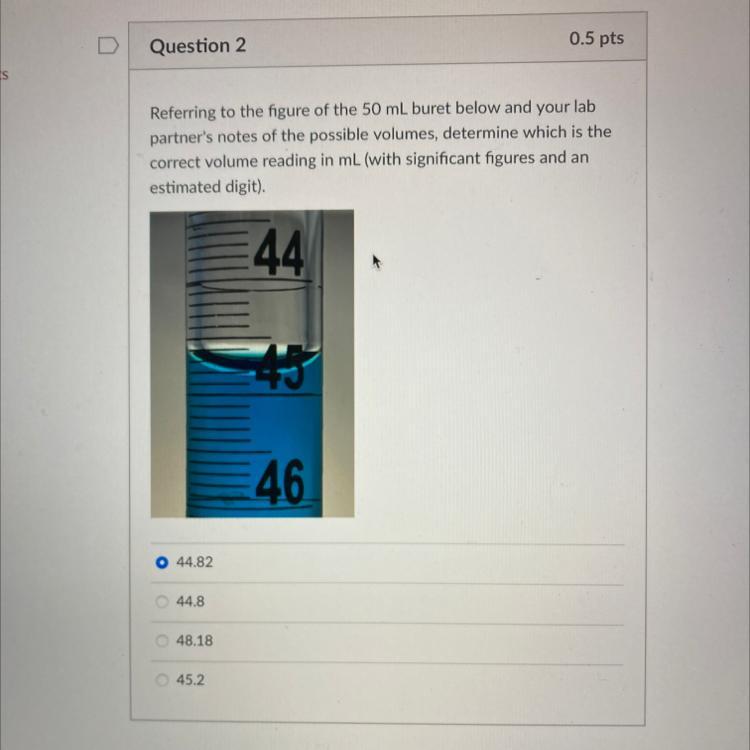
Referring to the figure of the 50 mL buret below and your lab
partner's notes of the possible volumes, determine which is the
correct volume reading in mL (with significant figures and an
estimated digit).
44
O 44.82
44.8
48.18
45.2
46
Answers
In this case, we can see that the reading of the burette is simply 45.2
How to read the burette?One of the apparatus that could be used to measure the volume of a liquid is the burette. It is a long tube that is graduated in milliliter and could be used to put the titrant when one is carrying out a volumetric analysis.
If you want to read the measurement on a burette, then you have to look at the position of the lower meniscus of the burette. In this case, we can see that the reading of the burette is simply 45.2
Learn more about burette:https://brainly.com/question/2957407
#SPJ1
Calculate the percent composition of H3PO4
Answers
The percent composition of hydrogen, phosphorous and oxygen in phosphoric acid is 3.06 %,31.60%,65.31 % respectively.
What is percent composition?Percent composition is defined as a convenient way to record concentration of solution.It is a expression which relates solute to solvent as,mass of solute/mass of solution ×100.There are two types of percentage composition percent weight by volume and percent volume by volume .
Percent composition of hydrogen=3/97.99×100=3.06%.
Percent composition of phosphorous=30.97/97.99×100=31.60%
Percent composition of oxygen=64/97.99×100=65.31%
Learn more about percent composition,here:
https://brainly.com/question/17505281
#SPJ1