Question 3
This law states that every particle attracts every other particle in the universe with a force that
is directly proportional to the product of their masses and inversely proportional to the square of the
distance between their centers
O Theory of General Relativity
O Hubble's Law of Cosmic Expansion
O Law of Universal Gravitation
O Archimede's Bouoyancy Principle
3 pts
Question 4
3 pts
Answers
Option A. The Law of Universal Gravitation, states that every particle attracts every other particle in the universe with a force that is directly proportional to the product of their masses and inversely proportional to the square of the distance between their centers.
Newton's law of gravitation, states that any particle of matter in the universe draws another with pressure varying at once as the manufactured from the loads and inversely as the rectangular.
Newton, the pressure of gravity appearing between the earth and another item is at once proportional to the mass of the earth, directly proportional to the mass of the item, and inversely proportional to the square of the distance that separates the centers of the earth and the item. Gravitational steady, G is unbiased of the nature of the particle, medium among the debris, and time. Its cost is constant anywhere within the Universe.
Learn more about Universal Gravitation here:-https://brainly.com/question/9373839
#SPJ9
Related Questions
Ethanol has a density of 0.789 g/cm3. What is the mass of 423 cm3 of ethanol? M = (D)(V)
334 g
536 g
.186 g
380 g
Answers
Answer:
The answer is 334 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question
volume of ethanol = 423 cm³
density = 0.789 g/cm³
So we have
mass = 0.789 × 423 = 333.747
We have the final answer as
334 gHope this helps you
Answer the question and you'll get brainilest!

Answers
Answer:
im just gonna explain how a speaker works and how it can make a window move. So a speaker has a crystal in it that when hit with and electric charge moves like a diaphragm that makes sound waves. the more electricity the slower it moves yet the slower it moves the bigger the movement. Anyway when that crystal moves it also moves the cardboard pice in front of it making a louder sound. The best way to think about it is a sound wave is like wind, its just moving air. so that moving air can push and pull something like a window pane. Cool Huh?
Explanation:
do any of the gases listed in the table above have molar masses larger than xe?
Answers
No, none of the gases listed in the table above have molar masses larger than Xe.
Unfortunately, the table you are referring to is not visible.
However, Xenon (Xe) is a heavy noble gas with a molar mass of 131.29 g/mol.
For comparison purposes, you would need to check the molar masses of the gases in your table to determine if any of them have larger molar masses.
Summary: Without access to the table, it is not possible to definitively answer your question, but it is important to compare the molar masses of the gases listed to that of Xenon (131.29 g/mol) to find out if any have larger molar masses.
Learn more about gases click here:
https://brainly.com/question/25736513
#SPJ11
Think about the experiment at the start of this lesson. The steel wool reaction is as follows: 4Fe(s)+3O2(g)→2Fe2O3(s).
How would you measure the amounts of each reactant used and the product that forms?
Answers
Answer:
I did this but I dont remember
Explanation:
kk
A metal cylinder has a mass of 6.20 g. The density of the cylinder is 21.0 g/mL. What is the volume
Answers
Answer:
\(\boxed {\boxed {\sf v \approx0.295 \ mL}}\)
Explanation:
We are asked to find the volume of a metal cylinder. We are given the mass and the density.
We know that density is the mass per unit volume of a substance. It is found using the following formula.
\(\rho= \frac {m}{v}\)
We know the density is 21.0 grams per milliliter and the mass is 6.20 grams. We substitute these values into the formula.
ρ= 21.0 g/mL m= 6.20 g\(21.0 \ g/mL = \frac{6.20 \ g}{v}\)
We are solving for the volume, so we must isolate the variable v. First, cross multiply. Multiply the first numerator by the second denominator, then the first denominator and the second numerator.
\(\frac {21.0 \ g/mL}{1} = \frac{6.20 \ g}{v}\)
\(21.0 \ g/ mL *v= 6.20 \ g * 1\)
\(21.0 \ g/ mL *v= 6.20 \ g\)
v is being multiplied by 21.0 grams per milliliter. The inverse operation of multiplication is division. Divide both sides of the equation by 21.0 g/mL.
\(\frac {21.0 \ g/ mL *v}{21.0 \ g/mL}= \frac{6.20 \ g }{21.0 \ g/mL}\)
\(v= \frac{6.20 \ g }{21.0 \ g/mL}\)
The units of grams cancel.
\(v= \frac{6.20 }{21.0 mL}\)
\(v=0.2952380952\)
The original measurements of density and mass both have 3 significant figures, so our answer must have the same. For this number, 3 sig fig is the thousandth place. The 2 in the ten-thousandth place tells us to leave the 5.
\(v \approx0.295 \ mL\)
The volume of the metal cylinder is approximately 0.295 milliliters.
how do h2 receptor antagonists reduce the secretion of acids?
Answers
H₂ receptor antagonists reduce the secretion of acids by blocking the H₂ receptors on parietal cells in the stomach, thereby inhibiting the action of histamine and decreasing the production of gastric acid.
H₂ receptor antagonists, also known as H₂ blockers, are medications commonly used to treat conditions related to excessive gastric acid production, such as gastroesophageal reflux disease (GERD) and peptic ulcers. These drugs work by selectively binding to H₂ receptors present on parietal cells in the stomach.
When histamine, a naturally occurring chemical in the body, binds to H₂ receptors, it stimulates the parietal cells to produce and release gastric acid into the stomach. However, H₂ receptor antagonists competitively bind to the H₂ receptors, preventing histamine from binding and inhibiting its stimulatory effect.
By blocking the H₂ receptors and inhibiting histamine's action, H₂ receptor antagonists reduce the activation of parietal cells and subsequently decrease the secretion of gastric acid. This helps in alleviating symptoms associated with conditions caused by excess acid production, such as heartburn, acid reflux, and ulcers.
learn more about receptor antagonists Here:
https://brainly.com/question/9708933
#SPJ4
21. What is happening in terms of energy and attractive/repulsive forces when atoms come together and
form a bond?
Answers
Answer:
So A covalent bond consists of the mutual sharing of one or more pairs of electrons between two atoms. These electrons are simultaneously attracted by the two atomic nuclei. A covalent bond forms when the difference between the electronegativities of two atoms is too small for an electron transfer to occur to form ions.
Explanation:
words to know: covalent bond, electronegativities, and simultaneously
Covalent Bond: A chemical bond formed when electrons are shared between two atoms. Usually each atom contributes one electron to form a pair of electrons that are shared by both atoms.
Electronegativities: the degree to which an element tends to gain electrons and form negative ions in chemical reactions.
Simultaneously: at the same time.
hope this helps!
What weight of Y would be removed from water (the original solution with the original amount) with two successive extractions with 70-mL portions each of methylene chloride
Answers
To calculate the weight of Y that would be removed from the water solution with each extraction, we need to use the following steps:
Determine the initial concentration of Y in the water solution. The initial concentration can be calculated using the total mass of Y in the solution, the volume of the solution, and the number of moles of Y per mole of solution.
Determine the amount of Y removed during each extraction. The amount of Y removed can be calculated using the volume of the extraction solvent (in this case, methylene chloride) and the molar mass of Y.
Calculate the final concentration of Y in the water solution after each extraction. The final concentration can be calculated using the initial concentration, the amount of Y removed during each extraction, and the volume of the solution.
Here is the calculation:
The initial concentration of Y in the water solution can be calculated using the total mass of Y in the solution (m), the volume of the solution (V), and the number of moles of Y per mole of solution (n).
Y initial concentration = m / n
For example, if the total mass of Y in the solution is 1.0 g, the volume of the solution is 100 mL, and the number of moles of Y per mole of solution is 1, then the initial concentration of Y in the solution would be 1.0 g / 1 mol/100 mL = 1 g/mL.
The amount of Y removed during each extraction can be calculated using the volume of the extraction solvent (mL) and the molar mass of Y (M).
Y removed per extraction = mL * M
For example, if the volume of the extraction solvent is 70 mL and the molar mass of Y is 120 g/mol, then the amount of Y removed per extraction would be 70 mL * 120 g/mol = 8400 g.
The final concentration of Y in the water solution after each extraction can be calculated using the initial concentration, the amount of Y removed during each extraction, and the volume of the solution.
Final Y concentration after extraction = (initial Y concentration - Y removed per extraction) / V
For example, if the initial concentration of Y in the solution is 1 g/mL, the amount of Y removed per extraction is 8400 g, and the volume of the solution is 100 mL, then the final Y concentration after extraction would be (1 - 8400 g/mL) / 100 mL = 0.1 g/mL.
Learn more about extraction Visit : brainly.com/question/18221689
#SPJ11
Can a chemical bond be formed between the hydrogen cations H +?
Answers
Answer:
yes
Explanation:
Answer:
Yes it can
Explanation:
1.what is the VSEPR number for T - shape molecular geometry?
2. what is the molecular geometry for the VSEPR number 660?
3. what is the molecular Geometry for the VSEPR number 321?
Answers
1) The VSEPR number for T-shaped molecular geometry is 5.
2) The geometry is octahedral
3) The geometry is Trigonal pyramidal
What is the VSEPR?The VSEPR (Valence Shell Electron Pair Repulsion) theory is used to predict the molecular geometry of a molecule based on the arrangement of electron pairs around the central atom.
For a T-shaped molecular geometry, there are three bonding pairs of electrons and two non-bonding pairs of electrons around the central atom. Therefore, the VSEPR number for T-shaped molecular geometry is 5.
The VSEPR number is the sum of the number of atoms bonded to the central atom and the number of lone pairs of electrons on the central atom.
Learn more about VSEPR:https://brainly.com/question/29756299
#SPJ1
Barium metal was quantitatively precipitated from a 1. 52 g sample of bacl2∙2h2o. The mass of the barium that was collected was 0. 844 g. Calculate the experimental mass percent of barium in the sample.
Answers
To calculate the experimental mass percent of barium in the sample, we need to use the following formula:
Experimental Mass Percent = (Mass of Barium / Mass of Sample) x 100
Given that the mass of the barium collected was 0.844 g and the mass of the sample was 1.52 g, we can substitute these values into the formula to calculate the experimental mass percent:
Experimental Mass Percent = (0.844 g / 1.52 g) x 100
Simplifying the equation:
Experimental Mass Percent = 0.555 x 100
Calculating the value:
Experimental Mass Percent = 55.5%
So, the experimental mass percent of barium in the sample is 55.5%. The experimental mass percent of barium in the sample is 55.5%. To calculate the experimental mass percent, we divided the mass of barium collected by the mass of the sample and multiplied the result by 100. This gives us the proportion of barium in the sample, expressed as a percentage. To calculate the experimental mass percent of barium in the sample, we used the formula (Mass of Barium / Mass of Sample) x 100. Given that the mass of barium collected was 0.844 g and the mass of the sample was 1.52 g, we substituted these values into the formula to calculate the experimental mass percent. By dividing 0.844 g by 1.52 g, we obtained the proportion of barium in the sample. Multiplying the result by 100, we converted this proportion into a percentage. The final result, 55.5%, represents the experimental mass percent of barium in the sample. This calculation allows us to quantify the amount of barium present in relation to the total sample mass.
To know more about barium visit:
https://brainly.com/question/2572464
#SPJ11
A sample of n sludge specimens is selected and the ph of each one is determined. the one-sample t test will then be used to see if there is compelling evidence for concluding that true average ph is less than 7.0. what conclusion is appropriate in each of the following situations?
Answers
For the sample of n sludge specimens is selected and the conclusion and the pH of each one is determined.
A) The null hypothesis is rejected. Sufficient evidence is present for the fact that the true average pH is less than 7.0.
B) The null hypothesis is rejected. Sufficient evidence is present for the fact that the true average pH is less than 7.0.
C) The null hypothesis is rejected. Sufficient evidence is present for the fact that the true average pH is less than 7.0.
D) The null hypothesis is rejected. Sufficient evidence is present for the fact that the true average pH is less than 7.0.
E) The null hypothesis is rejected. Sufficient evidence is present for the fact that the true average pH is less than 7.0.
The null hypothesis in inferential statistics is that two possibilities are always equal. It shows that the underlying assumption is that the observed difference is just the result of chance. It is possible to estimate the probability of the null hypothesis is correct using statistical tests.
Learn more about pH from the link given below.
https://brainly.com/question/23136085
#SPJ4
Which statement about Niels Bohr's atomic model is true?
Answers
Correct answer is Each orbit has a specific energy level.
According to Bohr's atomic model There is a certain energy for each orbit, with the inner orbit having the lowest energy. As you move farther from the nucleus, the energy of the orbits increases. We can say that the energy of the electrons are quantized if we state that they can only have certain energies.The ground state, also known as the lowest orbit, is where the electron typically resides.A lower energy level to a higher energy level is reached by an atom's electrons by gaining the necessary energy, and a higher energy level to a lower energy level by losing energy.Inner energy levels have low energy and outer or higher energy levels have high energy.Therefore, Correct statement is Each orbit has a specific energy level.
Learn more about Bohr's model here:
https://brainly.in/question/1438012
#SPJ9
The complete question is mentioned below:
Which statement about Niels Bohr’s atomic model is true?
a) Higher orbits have lower energies.
b) Each orbit has a specific energy level. c) Electrons can exist in any energy level. d) Orbits close to the nucleus have no energy.
Can energy be destroyed?
Answers
Answer: No
Explanation:
combining which of the following substances with germanium will cause the germanium to emit free electrons? question 19 options: a) bismuth b) gallium c) indium d) aluminum
Answers
The substance combined with germanium that will cause the germanium to emit free electrons is the aluminum. Correct answer: letter D.
Adding aluminum to germanium will cause the germanium to emit free electrons. This is because aluminum has one more electron in its outermost shell than germanium does. When the two atoms are combined, the extra electron from the aluminum will fill the outermost shell of the germanium atom, and the germanium atom will then become unstable and emit an electron.
What are aluminum and germanium?Aluminum and germanium are elements of the periodic table. Some similarities between the two are:
Both are metals with similar properties. Both are good conductors of electricity and heat. Both are used in the electronics industry.Learn more about the germanium:
https://brainly.com/question/488982
#SPJ4
Think about chemicals you may have in your home and list as many as you can think of:
Answers
Sucrose; C12H22O11
C9H8O4; acetyl salicylic acid
H2O2; hydrogen peroxide,
NaOH; sodium hydroxideExplanation:
A fixed mass of oxygen gas occupies 300cm cube at 0 degree centigrade. what volume would the gas occupy at 15 degree centigrade
Answers
Answer:
Volume occupied by oxygen gas at 15 degree centigrade is equal to \(316.5\) centimeter cube
Explanation:
Assuming Pressure is constant.
\(\frac{V_1}{T_1} = \frac{V_2}{T_2}\)
where T1 and T2 are temperature in Kelvin
Substituting the give values we get-
\(\frac{300}{273} = \frac{V_2}{288}\)
\(V_2 = \frac{288*300}{273} \\V_2 = 316.5\)
Volume occupied by oxygen gas at 15 degree centigrade is equal to \(316.5\) centimeter cube
13. What physical property is characteristic of all of the elements in the group located in the
rightmost column of the periodic table?
A.
very high melting point
B.
gas state at room temperature
C. good conductor of electric current
D. extremely brittle solid at room temperature
Answers
Answer:
B. gas state at room temperature
Explanation:
HELP ITS DUE IN AN HOUR!!
What is a lattice position?
Explain
Answers
Explanation:
Crystallography. an arrangement in space of isolated points (lattice points ) in a regular pattern, showing the positions of atoms, molecules, or ions in the structure of a crystal.
If an atom experiences sufficient thermal activation, it can move to a neighboring lattice position.4 If the vibration frequency of the atom is v and the atom has Z nearest neighbors, the total number of jump attempts is vZ. However, only a small fraction of the attempts will be successful, with a probability depending on the ratio between the necessary activation energy for a single jump QD and the thermal activation kBT. The effective jump frequency ΓD is then
(5.6)
With each successful jump, the atom travels one atomic distance λ and the total traveling distance in unit time is thus ΓDλ. Substituting the jump frequency ΓD into the expression for the root mean square displacement of a random walker [equation (5.5)] and using the spatial coordinate r leads to
How to determine how long a branch is with mass and torque.
Answers
Answer:
First determine the lever arm and then multiply it times the applied force.
The equation τ = m(r^2)α is the rotational analog of Newton's second law (F=ma)
Hope this helps.
If Half- life of an isotope is 30 days and it was assumed that
the person ate 100 Bq of isotope. Using the GI track model
information, calculate the number of transformations in
Stomach
Answers
If Half- life of an isotope is 30 days and it was assumed that the person ate 100 Bq of isotope, there are 50 transformations in the stomach.
The radioactive decay of a sample of an isotope can be characterized by the half-life of that isotope. When a radioisotope undergoes decay, its nucleus becomes unstable, and it emits particles or energy to become more stable. The half-life of an isotope is the time it takes for half of the original sample to decay. The question states that the half-life of an isotope is 30 days, and the person ingested 100 Bq of isotope. It also says to calculate the number of transformations in the stomach using GI track model information .
Since the isotope has a half-life of 30 days, we can use the following formula to find the number of transformations in the stomach:` N = N₀ (1/2)^(t/T₁/₂)`where: N₀ = initial number of nuclei N = final number of nuclei (after time t)T₁/₂ = half-life of the isotope The isotope has a half-life of 30 days, so T₁/₂ = 30 days. The question doesn't specify how long the person has had the isotope in their stomach, so we'll assume it's been there for one half-life, or 30 days. Therefore, t = 30 days.
Substituting into the formula:` N = 100 (1/2)^(30/30)`Simplifying:` N = 100 (1/2)^1`Evaluating:`N = 50`So after 30 days in the stomach, the person would have 50 Bq of the isotope left. Therefore, the number of transformations in the stomach is the difference between the initial number of transformations (100 Bq) and the final number of transformations (50 Bq):`Number of transformations in stomach = 100 - 50 = 50 transformations. Therefore, there are 50 transformations in the stomach.
More on isotope: https://brainly.com/question/28039996
#SPJ11
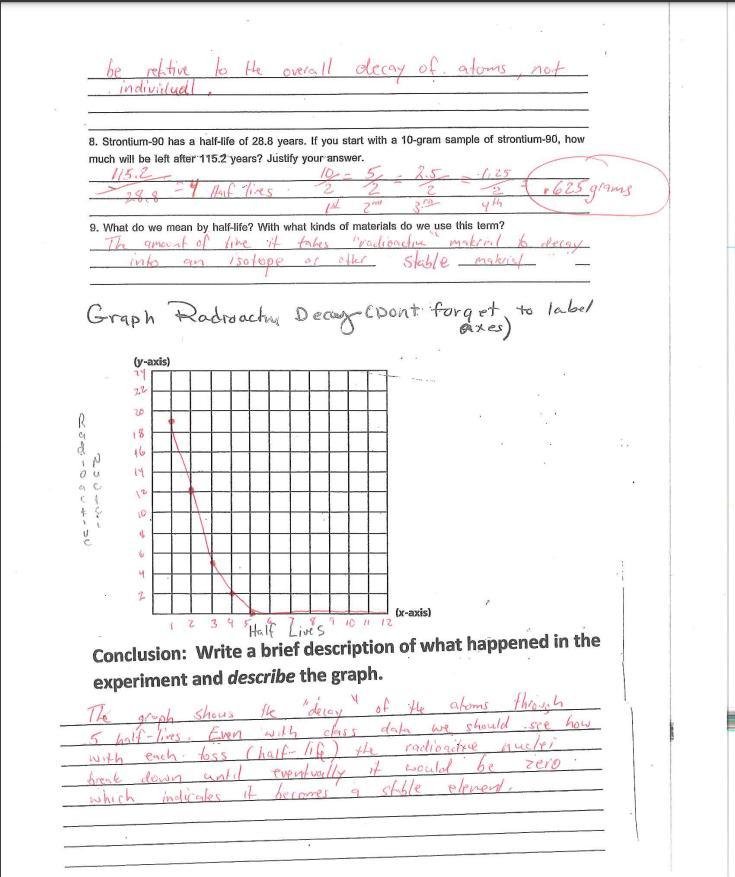
6. Is there any way to predict when a specific piece of candy will land marked
side up or "decayed?" If you could follow the fate of an individual atom in a
sample of radioactive material, could you predict when it would decay?
Explain.
Answers
Answer:
No, each piece of candy has a 50% chance of decaying.No, just like the candy, each atom has a 50% chance of decaying each half-life.Explanation:

How many moles are in 1.2x10^3 grams of ammonia, NH3?
Answers
Glaciers can form in all these areas except

Answers
Answer:
the answer is over water D
PLS HELP ASAPP NEED RN
1. Compare the spatial arrangements of Zn atoms in the reactants to the spatial arrangements of the hydrogen
gas molecules in the products.
2. Calculate the mass of the hydrogen gas produced if the reaction goes to completion producing 2.05 g of
ZnCl2.
3. Draw a balanced particle model that shows how the reactants changed into the products.

Answers
Let us take our minds back to the kinetic theory of matter. Recall that the particles that compose matter are in constant random motion and the solids are arranged in a definite pattern while the particles of a gas are in random motion.
As such, the zinc has its atoms in a fixed pattern while the hydrogen atoms are roaming freely in the gaseous state.
Given that;
Amount of the acid = 10.95 g/36.5 g/mol = 0.3 moles
Amount of the zinc = 1g/65 g/mol = 0.015 moles
Given the reaction equation; \(Zn(s) + 2HCl(aq) ------ > ZnCl_{2} (aq) + H_{2}(g)\)
1 mole of Zn reacts with 2 moles of acid
0.015 moles of zinc reacts with x moles of acid
x =0.015 moles * 2 moles / 1 mole
= 0.03 moles
Thus, the zinc is the limiting reactant.
1 moles of the zinc produces 1 mole of the hydrogen
The amount of the hydrogen produced = 0.03 moles * 2 g/mol
= 0.06 moles of hydrogen
Learn more about stoichiometry:https://brainly.com/question/9743981
#SPJ1

please help me with this or I may get an F in chemistry :(

Answers
Answer:
Subconscious - psychic activity just below the level of awareness
Election - the act of electing someone
Explanation:
I hope this helped enough, good luck in chemistry!
How to identify polar molecules
Answers
Answer:
Polar molecules occur when there is an electronegativity difference between the bonded atoms. Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out
Explanation:
YOU MUST SHOW ALL STEPS OF THE G.U.E.S.S. METHOD CORRECTLY. NO UNITS=WRONG ANSWER
Q: wave is traveling with a frequency of 2424 Hz and has a wavelength of 0.5 meters. What speed is this wave going?
Answers
Answer:
The equation for wave speed can be used to calculate the speed of a wave when both wavelength and wave frequency are known. Consider an ocean wave with a wavelength of 3 meters and a frequency of 1 hertz. The speed of the wave is: Speed = 3 m x 1 wave/s = 3 m/s.
SO... take your meters and hezert and do tha same
Explanation:
Plz mark me as brainlyist
What do you understand by the terms radial node and nodal plane, as applied to AO wavefunctions? Illustrate your answer using the 2s and 2p AOs. Explain why radial nodes arise from the radial part of the wavefunction, whereas nodal planes arise from the angular part of the wavefunction
Answers
In the context of atomic orbital (AO) wavefunctions, the terms "radial node" and "nodal plane" refer to different aspects of the wavefunction's behavior.
A radial node is a region in the AO wavefunction where the probability of finding an electron is zero along the radial direction. In other words, it represents a spherical shell where the electron is unlikely to be found. The number of radial nodes is determined by the principal quantum number (n) of the orbital. For example, the 2s orbital has one radial node, while the 2p orbital has no radial nodes.
On the other hand, a nodal plane is a flat plane within the AO wavefunction where the probability of finding an electron is zero along a particular direction. It represents a surface that divides the orbital into two regions of opposite phases. The number of nodal planes is determined by the angular quantum numbers (l and m) of the orbital. For example, the 2s orbital has no nodal planes, while the 2p orbital has one nodal plane (the xz or yz plane).
Radial nodes arise from the radial part of the wavefunction because they depend on the distance from the nucleus. The radial part determines the distribution of the electron density as a function of distance, and the nodes correspond to regions where the density drops to zero.
On the other hand, nodal planes arise from the angular part of the wavefunction because they depend on the orientation and shape of the orbital. The angular part describes the angular distribution of the electron density around the nucleus, and the nodal planes correspond to regions where the phase of the wavefunction changes sign.
In summary, radial nodes are related to the distance from the nucleus and arise from the radial part of the wavefunction, while nodal planes are related to the orientation and shape of the orbital and arise from the angular part of the wavefunction. The 2s orbital has one radial node and no nodal planes, while the 2p orbital has no radial nodes and one nodal plane.
learn more about radial node here
https://brainly.com/question/31829965
#SPJ11
what do plants need to live?
put a mark next to the ones that plants need to live
sunlight
soil
water
plant food from a garden store
sugar
carbon dioxide
minerals
fertilizer
leaves
oxygen
chlorophyll
vitamins
i'll mark brainliest for whoever has the right answer or who ever answers it!
Answers
Answer:
Explanation:
sunlight
soil
water
sugar
carbon dioxide
minerals
leaves
vitamins