Answers
Answer:
D. Territory
Explanation:
Territory is defined as an area that is claimed by one or more individuals.
Territory is often used as a form of ownership of land or water mass between two parties. It is important as it gives each party a knowledge of their designated areas.
This validates it being an area that is claimed by one or more parties as organisms use that to protect their interest of food, water and mates for their survival.
Related Questions
In a science demonstration, a teacher mixed zinc (Zn) with hydrogen chloride (HCl) in a flask and quickly attached a balloon over the mouth of the flask. Bubbles formed in the solution and the balloon inflated.
What most likely occurred during this demonstration?
a.The Zn and HCl both retained their identity.
b.Either Zn or HCl, but not both, retained its identity.
c.Evaporation of one of the substances occurred.
d.One or more new substances formed.
Answers
Answer:
a. The Zn and HCl both retained their identity.
Students in a science class placed ice cubes in a cup. They were studying variables that affected how long it takes the ice to melt. Select the variable that
would most likely NOT affect the time it takes the ice to melt.
O Size of cup
O Size of ice cube
O Number of students
O Temperature of the room
Answers
Answer:
Number of students-this has nothing to do with the question, its called a distractor.
The variable that would most likely not affect the time it takes the ice to melt is the Number of students. The correct option is C.
What are the variables in the experiments?Variables are the different substances or objects that participate in the experiment directly or indirectly. They are substances that together form the experiment. These variables impact the result of the experiment.
There are two types of variables. They are Dependent and independent variables. Dependent variables can be controlled in the experiment, but independent variables can't be controlled.
Students are performing the experiment, so they cannot be the variables because their presence or absence would be to impact the experiment.
Thus, the correct option is C. Number of students.
To learn more about variables, refer to the link:
https://brainly.com/question/17344045
#SPJ2
Oxygen is a substance that dissolves in water. Fish absorb oxygen from the water. When dissolved oxygen levels are low, it can be hard for organisms to survive
Answers
Oxygen is a molecule that is essential for life. It is a colorless, odorless gas that makes up about 20% of the air we breathe.
What is molecule?A molecule is a group of two or more atoms that are held together by chemical bonds. They are the basic building blocks of all matter, and are composed of protons, neutrons, and electrons.
When oxygen is dissolved in water, it can be absorbed by aquatic organisms such as fish. These aquatic organisms use the oxygen to help them with their metabolic processes. When the levels of oxygen in the water are too low, the organisms are not able to survive and may die. Low oxygen levels can occur naturally due to environmental factors like temperature or seasonality, or they can be caused by human activities such as pollution. It is important to keep oxygen levels in the water high to ensure the health of aquatic ecosystems.
To learn more about molecule
https://brainly.com/question/1078183
#SPJ1
Which example has particles that can be drawn closer to occupy smaller volume? a. fruit juice b. block of wood c. air inside the syringe d. ice cube
Answers
The capacity of an object is measured by its volume. For instance, a cup's capacity is stated to be 100 ml if it can hold 100 ml of water in its brim. Here ice cube has particles that can be drawn closer to occupy smaller volume. The correct option is D.
Due to the strong intermolecular interactions, the solid molecules are very near to one another. Solids have a low volume and a high density as a result. Additionally, the solid molecules cannot be easily crushed due to the narrow intermolecular distance.
So here ice cube has small volume.
Thus the correct option is D.
To know more about volume, visit;
https://brainly.com/question/13807002
#SPJ1
how do you count atoms when there's leading coefficient?
Answers
Answer:
To find out the number of atoms: MULTIPLY all the SUBSCRIPTS in the molecule by the COEFFICIENT. (This will give you the number of atoms of each element.)
Explanation:
hi hope it helps
Calculate the volume of O2, at STP, required for the complete combustion of 125g octane (C8H18) to CO2 and H20
Answers
306.178 liters is the volume of O2 at STP, required for the complete combustion of 125g octane (\(C_{8}H_{18}\)) to \(CO_{2}\) and H20
To calculate the volume of \(O_{2}\) required for the complete combustion of octane (\(C_{8}H_{18}\)) to \(CO_{2}\) and \(H_{2}O\) at STP (Standard Temperature and Pressure), we need to consider the stoichiometry of the balanced chemical equation.
The balanced equation for the combustion of octane is:
\(C_{8}H_{18}\) + 12.5\(O_{2}\) -> 8\(CO_{2}\) + 9\(H_{2}O\)
From the equation, we can see that 1 mole of octane requires 12.5 moles of \(O_{2}\) to completely combust. The molar mass of octane (\(C_{3}H_{18}\)) is approximately 114.22 g/mol.
To calculate the moles of octane, we divide the given mass by the molar mass:
Moles of octane = 125 g / 114.22 g/mol ≈ 1.093 mol
Since the molar ratio between octane and \(O_{2}\) is 1:12.5, the moles of \(O_{2}\)required can be calculated as:
Moles of \(O_{2}\) = 1.093 mol * 12.5 ≈ 13.663 mol
Now, we can use the ideal gas law, PV = nRT, to calculate the volume of \(O_{2}\) at STP. At STP, the temperature is 273 K, and the pressure is 1 atm.
Using the molar volume of an ideal gas at STP (22.4 L/mol), the volume of \(O_{2}\) required is:
Volume of \(O_{2}\) = Moles of \(O_{2}\) * Molar volume = 13.663 mol * 22.4 L/mol ≈ 306.178 L
Therefore, approximately 306.178 liters of \(O_{2}\) at STP would be required for the complete combustion of 125 grams of octane (\(C_{8}H_{18}\)) to \(CO_{2}\) and \(H_{2}O\)
Know more about combustion here:
https://brainly.com/question/10458605
#SPJ8
In which of the following intermolecular interactions will polar molecules, such as water, commonly participate? (Mark all that apply A. Hydrogen bonding with non-polar molecules B. Hydrogen bonding with polar molecules C. Van der Waal interaction with polar molecules D. Electrostatic interaction with lonic compounds
Answers
B. Hydrogen bonding with polar molecules is the inter molecular interactions will water participate commonly with polar molecules.
Why does water pull polar molecules toward it?Polarity causes the water molecules to attract each other. Water is polar despite possessing a zero net charge because of the structure of its molecules. The molecule's hydrogen ends are positive, and its oxygen ends are negative. Water molecules are attracted to other polar molecules and to one another as a result.
What polar molecules are drawn to water?The polarity of water enables hydrogen bonds to form whenever adjacent water molecules are drawn to each other by their opposing charges. Other polar molecules and ions, such as many biomolecules including sugars, nucleic acids, and certain amino acids, are also attracted to or attracted by water.
Learn more about polar molecule here:
brainly.com/question/15173422
#SPJ4
PLEASE HELP ITS A TEST QUESTION!!!! The particles that make up gases are close or apart from each other?
Answers
Convert 1897cm into km
Answers
Answer:
1.897 km
Explanation:
1897÷1000=1.897km
you would convert it in to 0.019 km
How many moles of NaCl can be produced from 2.5 moles of BaCl2.
Answers
The number of moles of NaCl that can be produced from 2.5 moles of BaCl₂ is 5.
What is the number of moles of NaCl produced?
To determine the number of moles of NaCl that can be produced from 2.5 moles of BaCl2, we first need to balance the chemical equation for the reaction between BaCl₂ and Na₂SO₄:
BaCl₂ + Na₂SO₄ -> 2NaCl + BaSO₄
From the balanced equation, we can see that 1 mole of BaCl₂ reacts with 2 moles of NaCl.
Therefore, we can use a mole ratio to calculate the number of moles of NaCl that can be produced from 2.5 moles of BaCl₂:
2.5 moles BaCl₂ × (2 moles NaCl / 1 mole BaCl₂) = 5 moles NaCl
Learn more about number of moles here: https://brainly.com/question/14357742
#SPJ1
The complete question is below:
How many moles of NaCl can be produced from 2.5 moles of BaCl2, when BaCl₂ reacts with Na₂SO₄?
How many molecules are there in 985 mL of nitrogen at 0.0 degrees C and 1.00x10^-6 mm Hg?
(It would be better if work or steps to solve problem are given but if not its fine).
Answers
Answer:
3.48 x 1013 N2 molecules
step-by-step explanation:
Lets set up our equation first
P = 1.00 x 10-6 mm Hg T = 0.0° C + 273 = 273 K
We are given the V = 985 mL ,
R = 0.0821 L·atm/mol·K
Now use the ldeal gas law, but we are solving n, amount of substanvce
PV = nRT, we will change this equation to ;
n = PV/RT
n = 1.00 x 10-6 mm x 1 atm/760 mm x 985 mL x 1 L/103
mL/
(0.0821 L·atm/mol·K x 273 K) = 5.78 x 10-11 moles N2
nmolecules = 5.78 x 10-11 moles N2 x 6.02 x 1023 N2 molecules/1 mol N2
2) Copper metal can be recovered from an ore, CuCO3, by the decomposition reaction:
2CuCO3 (s)-→ 2Cu(s) + 2CO2 (g) + O2 (g)
What is the mass of a sample of "impure" ore if it is 47.5 %by mass CuCO3 and produces
350.0 grams of Cu? (Assume complete decomposition of CuCO3.) (5 points).
Answers
736.84g is the mass of a sample of "impure" ore if it is 47.5 %by mass CuCO3 and produces 350.0 grams of Cu.
What is CuCO3?Copper(II) carbonate or cupric carbonate is a chemical compound with formula CuCO ₃. At ambient temperatures, it is an ionic solid consisting of copper(II) cations Cu²⁺ and carbonate anions CO²⁻ ₃.
What is an ore?Ore is a naturally occurring rock or silt that has precious minerals in it that may be extracted, processed, and sold for a profit. These minerals are usually metals. Mined ore is then processed or refined, frequently by smelting, to remove the valuable metals or minerals.
To find the total mass of CuCO3 follow the given steps:
Given:
Mass percent=47.5% by mass
Mass of Cu =305.0 g
mass percent = (mass of chemical ÷ total mass of compound) x 100.
Rearranging the above eq.
Total mass of compound= mass of chemical /mass percent*100
Total mass of compound =350/47.5*100=736.84g
Hence, 736.84g is the mass of a sample of "impure" ore if it is 47.5 %by mass CuCO3 and produces 350.0 grams of Cu.
To know more about ore visit
https://brainly.com/question/89259
#SPJ1
A silicon atom has an atomic number of 14. What information does the atomic number tell you? (Choose all possible answers)
Group of answer choices
Silicon atoms have 14 protons.
Silicon atoms will react with other atoms in order to gain stability.
Silicon atoms are stable in their elemental form
Silicon atoms have 14 electrons.
Answers
Answer:
Silicon atoms have 14 protons.
Silicon atoms will react with other atoms in order to gain stability.
Silicon atoms have 14 electrons.
How would covering the sandy banks of the pond affect the turtles’ life cycle?
Answers
Answer:
my answer is i dont know to be honest this is hard and i have the same question to thanks
Explanation:
Explain what is meant by a half-life of 150 years. Then, write a function that models the amount, A, of a 100-mg sample remaining after T years for a substance with a half-life of 150 years. Use graphing software to graph the equation. Answer in complete sentences.
Answers
Answer:
Half-life of 150 years are those year that a radioactive element take to disintegrate to a half of it's original value.
I don't have graphical software
Determine whether or not the mixing of each of the two solutions indicated below will result in a buffer.
Drag the appropriate items to their respective bins.

Answers
Sinve CH3NH2 is weak base and its salt is CH3NH3Cl, mixing 125.0 mL of CH3NH2 and 120.0 mL of CH3NH3Cl so produce a buffer.
What is a buffer?A buffer is a solution which resists changes to its pH when a small quantity of strong acid or base is added to it..
A buffer is made from a weak acid and its salt or a weak base and its salt.
From the given options, CH3NH2 is weak base and its salt is CH3NH3Cl.
Therefore, mixing 125.0 mL of CH3NH2 and 120.0 mL of CH3NH3Cl so produce a buffer.
Learn more about buffers at: https://brainly.com/question/1385846
A sealed container can hold 6.28 L CO2 at 1.00 atm and 293 K. How many moles of CO2 fill the container?
Answers
The number of moles of carbondioxide that can fill the sealed container is 0.261moles.
How to calculate number of moles?The number of moles of an ideal gas can be calculated using the ideal gas law equation as follows:
PV = nRT
Where;
P = pressureV = volumeT = temperaturen = number of molesR = gas law constantAccording to this question, a sealed container can hold 6.28L carbondioxide at 1.00 atm and 293 K. The number of moles of the gas can be calculated as follows:
1 × 6.28 = n × 0.0821 × 293
6.28 = 24.0553n
n = 6.28 ÷ 24.0553
n = 0.261moles
Therefore, 0.261moles is the number of moles of the gas that can fill the container.
Learn more about number of moles at: https://brainly.com/question/29175560
#SPJ1
The basic unit of structure and function of living things is the .
nucleus
cell
tissue
membrane
Answers
Answer &
Explanation:
eukaryotic: Having complex cells in which the genetic material is contained within membrane-bound nuclei. cell: The basic unit of a living organism, consisting of a quantity of protoplasm surrounded by a cell membrane, which is able to synthesize proteins and replicate itself.
therefore it is cell
Method A: Dilute 10.00 mL up to 100 mL with a transfer pipet and volumetric flask. Then take 10.00 mL of the dilute solution and dilute it again to 100 mL.
Answers
Based on dilution methods, the dilution given is a serial dilution with dilution factor of 1/10.
What is serial dilution?A serial dilution is the stepwise dilution of a substance in solution usually using a constant dilution factor at each step of dilution.
The above dilution step is a serial dilution.
Dilution factor = original volume/final volume.Therefire, the dilution factor of the dilution above is:
initial volume = 10 mL
final volume = 100 mL
Dilution factor = 10/100
Dilution factor = 1/10
Therefore, the dilution given is a serial dilution with dilution factor of 1/10.
Learn more about serial dilution at: https://brainly.com/question/2167827
a question was asked by a teacher to a student. She gave the student a jumbled word and told him to make words out of it. The jumbled word is gzeysktqix. Now you know what to do. see ya!
Answers
The jumbled word "gzeysktqix" can be unscrambled to form the word "skyzigtext."
Here are possible words that can be made from this jumbled word:
Sky: Referring to the atmosphere above the Earth.
Zig: Describing a series of sharp turns or angles.
Text: Referring to written or printed words.
Six: The number following five and preceding seven.
It seems that the jumbled word has provided a mix of letters that can be rearranged to form these words. This exercise is likely intended to enhance the student's vocabulary skills, spelling ability, and problem-solving skills. By unscrambling the letters, the student is encouraged to explore different word possibilities and apply their knowledge of language. It also promotes critical thinking and creativity as they find valid words from the given set of letters.
for such more questions on unscrambled
https://brainly.com/question/23994485
#SPJ8
4. How many grams is 3 moles of H₂O?
Answers
Answer:
1.67
Explanation:
Mass÷mr=moles
3÷18=1.67
Use your understanding of the ideal gas law to
identify the correct relationships among the
variables.
Pressure is
Temperature is
Volume is
Moles are
Answers
The ideal gas relationships among the variables are PV = nRT
An ideal gas is a theoretical gas composed of many randomly transferring factor particles that aren't difficult to interparticle interactions. the best gasoline idea is beneficial because it obeys the precise gas law, a simplified equation of country, and is amenable to evaluation under statistical mechanics.
Volume is a degree of occupied three-dimensional space. it's far more frequently quantified numerically the usage of SI-derived gadgets or by way of diverse imperial gadgets. The definition of length is interrelated with the extent.
An ideal gas is described as one for which both the extent of molecules and forces between the molecules are so small that they have got no effect on the behavior of the gas. The real gas that acts almost like a really perfect gasoline is helium. that is due to the fact helium, in contrast to maximum gases, exists as an unmarried atom, which makes the van der Waals dispersion forces as low as viable
relation
PV = nRT
Learn more about ideal gas here:-https://brainly.com/question/20348074
#SPJ1
11. How many grams of water are produced from the decomposition of 100 g of H2O2?
2 H2O2 ->2H2O+O2
Answers
Answer:
hence the correct answer is 52.94 or 53g H2O approx.

What is an example of equilibrium?
O the release of a gas product from an open system
O the partial dissolution of salt crystals in saturated water
O the combustion of gasoline in a car engine
O the mixing of oil and water in a closed container
Answers
A good example of equilibrium would be the mixing of oil and water in a closed container.
What is chemical equilibrium?Chemical equilibrium is a condition in which the concentrations of components of a chemical reaction remain unchanged and have no tendency to change.
Of all the options, the only one where the concentrations of the component reactants cannot change is a mixture containing oil and water in a closed container.
Oil and water are immiscible and thus, their concentrations remain constant.
More on chemical equilibrium can be found here: https://brainly.com/question/4289021
#SPJ1
ing The
Ionic bonds are made by electrons.
Answers
How many molecules are there in 2.3 grams of NH4SO2?
Answers
Answer:
Hence, there are approximately $1.686\times {{10}^{22}}$ molecules in 2.3 grams of $N{{H}_{4}}S{{O}_{2}}$.
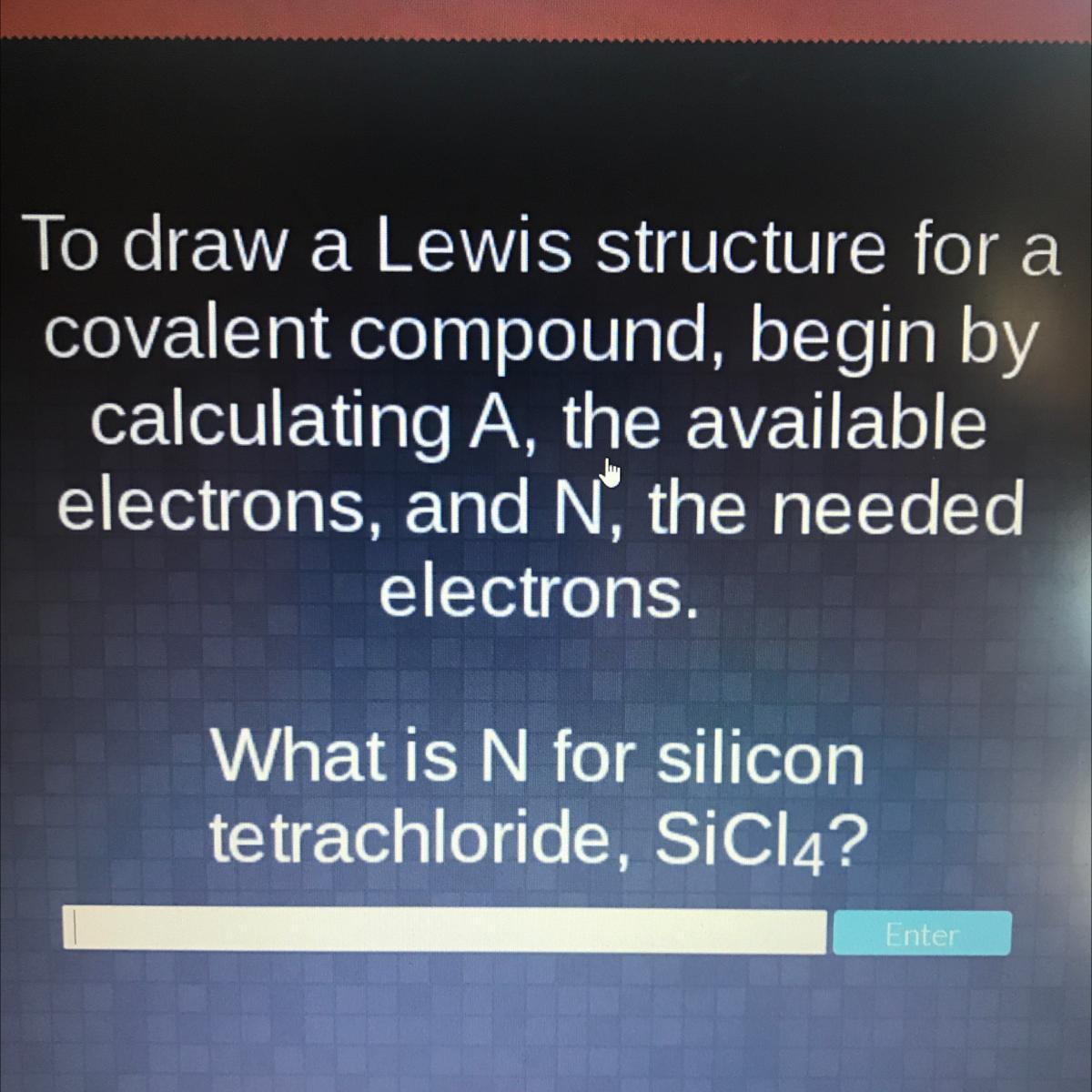
What is N for silicon tetrachloride, SiCl4?
Answers
Similar to For Practice 14.8) Determine the freezing point of an aqueous solution that contains 0.867 m glycerin (CHgOz).
Ki(water) = 1.86°C/m and Kg(water) - 0.512°C/m. Freezing point of water = 0.0 °C.
Similar to For Practice 14.3) Find the mass (in grams) of glucose (CH1206) in 505 mL of 10.5% glucose solution by mass. Assume the density of the solution is 1.04g/mL

Answers
The freezing point of the solution containing 0.867 m glycerin is -1.61442 °C. Option C is correct
The mass of glucose in 505 mL of 10.5% glucose solution is 53.01 g or 5.30 x 10^2 g.
Option C is correct
To find the freezing point depression of the solution containing 0.867 m glycerin:
ΔTf = Kf * molality
ΔTf = (1.86°C/m) * 0.867 m
ΔTf = 1.61442 °C
The freezing point depression is 1.61442 °C.
The freezing point of the solution is:
Freezing point = 0.0 °C - ΔTf
Freezing point = 0.0 °C - 1.61442 °C
Freezing point = -1.61442 °C
To find the mass of glucose in 505 mL of 10.5% glucose solution:
Mass of glucose = Volume of solution * Density of solution * % mass
Mass of glucose = 505 mL * 1.04 g/mL * 10.5%
Mass of glucose = 53.01 g
For more question on freezing point click on
https://brainly.com/question/30948897
#SPJ11
Sea salt is formed by natural evaporation of ocean water and contains 98% sodium chloride. The remaining 2% are natural minerals such as iron and sulfur. Consider a 53.9−g sample of sea salt. What is the mass of chlorine (from sodium chloride) in the sample?
Answers
The mass of chlorine (from sodium chloride) in the sample, given that the sample contains 98% sodium chloride is 32.05 grams
How do i determine the mass of chlorine in the sample?First, we shall determine the mass of sodium chloride, NaCl in the sample. Details below:
Percentage of sodium chloride, NaCl = 98%Mass of sample of sea salt = 53.9 gramsMass of NaCl =?Mass of NaCl = Percentage of NaCl × Mass of sea water
Mass of NaCl = 98% × 53.9
Mass of NaCl = (98/100) × 53.9
Mass of NaCl = 52.822 g
Finally, we shall determine the mass of chlorine. Details below:
Mass of sodium chloride, NaCl = 52.822 gramsMolar mass of NaCl = 58.5 g/molMolar mass of Cl = 35.5 g/molMass of chlorine, Cl = ?Mass of chlorine, Cl = (Molar mass of Cl / molar mass of NaCl) × Mass of NaCl
Mass of chlorine, Cl = (35.5 / 58.5) × 52.822
Mass of chlorine, Cl = 32.05 grams
Thus, the mass of chlorine in the sample is 32.05 grams
Learn more about mass composition:
https://brainly.com/question/28759819
#SPJ1
In making a phase transition from a liquid state to a gas state, what must happen to the energy and particle motion?
a
Energy must be added, particles must slow down
b
Energy must be added, particles must move faster
c
Energy must be removed, particles must slow down
d
Energy must be removed, particles must move faster
Answers
I would have to go with option
A. Energy must be added, particles must slow down
Hope this helps