Answers
Answer:
c
Explanation:
Related Questions
Calculate the ionization constant for the following acids or bases from the ionization constant of its conjugate base or conjugate acid: Keep your answer to 2 significant figures (CH3)3NH+
Answers
Answer:
7.41 × 10⁻⁵
Explanation:
Let's consider the basic dissociation reaction of trimethylamine (CH₃)N).
(CH₃)N + H₂O = (CH₃)NH⁺ + OH⁻
According to Brönsted-Lowry, in this reaction (CH₃)N is a base and (CH₃)NH⁺ is its conjugate acid. The pKb for (CH₃)N is 9.87. We can calculate the pKa of (CH₃)NH⁺ using the following expression.
pKa + pKb = 14
pKa = 14 - pKb = 14 - 9.87 = 4.13
Then, we can calculate the acid dissociation constant for (CH₃)NH⁺ using the following expression.
pKa = -log Ka
Ka = antilog - pKa = antilog -4.13 = 7.41 × 10⁻⁵
A metal carbonate, XCO3 of mass 2.012 g was heated resulting in the formation of XO, a metal oxide and carbon dioxide with a mass of 0.855 g according to the reaction shown below: XCO3 (s) → XO (s) + CO2 (g) (Atomic mass of O-15.999 g/mol; H-1.008 g/mol; C-12.011 g/mol).
Answers
The metal X has an approximate molar mass of 42.36 g/mol and the metal is most likely calcium.
What is the molar mass of XCO₃?The molar mass of the metal carbonate XCO₃ and identify the metal X, we need to calculate the number of moles of XCO₃ and CO₂ using the given masses and molar masses.
The molar mass of CO₂ (carbon dioxide) is 12.011 g/mol (for carbon) + 2 * 15.999 g/mol (for oxygen) = 44.01 g/mol.
The number of moles of CO₂ can be calculated using the formula:
moles of CO₂ = mass of CO₂ / molar mass of CO₂
moles of CO₂ = 0.855 g / 44.01 g/mol
moles of CO₂ ≈ 0.01944 mol
Since the reaction stoichiometry is 1:1 between XCO₃ and CO₂, the number of moles of XCO₃ is also approximately 0.01944 mol.
molar mass of XCO₃ = mass of XCO₃ / moles of XCO₃
molar mass of XCO₃ = 2.012 g / 0.01944 mol
molar mass of XCO₃ ≈ 103.38 g/mol
The molar mass of XCO₃ is approximately 103.38 g/mol.
To determine the metal X:
molar mass of X = molar mass of XCO3 - molar mass of CO3
molar mass of X = 103.38 g/mol - (12.011 g/mol + 3 * 15.999 g/mol)
molar mass of X ≈ 42.36 g/mol
Metal X is most likely Calcium that has a molar mass of 40 g/mol
Learn more about molar mass at: https://brainly.com/question/837939
#SPJ1
Which of the following is the correct wedge and dash conformation for the following Newman projection?

Answers
We have that the correct wedge and dash conformation for the following Newman projection is
IV
From the Diagrams above
Two CH_3 groups points on opposite sides in plane
Two Br are on same side of plane
Two H also on same side of the plane so the plausible structure is IV
Therefore
The Correct option is IV
For more information on this visit
https://brainly.com/question/17756498?referrer=searchResults
The rabbit in this food web would be considered a
Answers
Answer:primary consumer
Explanation:
i got it right
75 POINTS!!!
Describe the plate movements in a Divergent(Constructive), Convergent (Destructive) and a Transform (Conservative) Plate Margin. (these are also called plate boundaries). Your answer should define these THREE types of margins or boundaries by explaining the type of movement that occurs.
Answers
The type of movement that occurs in the plate movement listed above include the following:
A divergent boundary occurs when two tectonic plates move away from each other.A convergent boundary occurs when lithospheric plates are moving towards one another.Transform boundaries are created when tectonic plates slide past each other horizontally.What is a Tectonic plate?These are gigantic pieces of the Earth's crust and uppermost mantle and are made up of oceanic crust and continental crust.
A convergent boundary as the name implies occurs when lithospheric plates are moving towards one another.
Read more about Tectonic plate here https://brainly.com/question/1162125
#SPJ1
why does the ratio of chloride ions to calcium ions is 2:1 when calcium chloride forms
Answers
hot lead with a mass of 200.0 g of (Specific heat of Pb = 0.129 J/gºC) at 176.4°C was dropped into a calorimeter containing an unknown volume of water. The temperature of the water increased from 21.7°C to 56.4°C. What volume of water is in the calorimeter?
Answers
the Calorimetry relationships you can find the amount of water in the calorimeter is m = 21.3 g
given parameters
Lead mass M = 200.0 gInitial lead temperature T₁ = 176.4ºCSpecific heat of Lead \(c_{e Pb}\) = 0.129 J / g ºCSspecific heat of water \(c_{e H_2O}\) = 4.186 J / g ºCInitial water temperature T₀ = 21.7ºCEquilibrium temperature T_f = 56.4ºCto find
The body of water
Thermal energy is the energy stored in the body that can be transferred as heat when two or more bodies are in contact. Calorimetry is a technique where the energy is transferred between the body only in the form of heat and in this case the thermal energy of the lead is transferred to the calorimeter that reaches the equilibrium that the thematic energy of the two is equal
Q_{ceded} = Q_{absorbed}
Lead, because it is hotter, gives up energy
Q_{ceded} = M c_{e Pb} (T₁ - T_f)
The calorimeter that is colder absorbs the heat
Q_{absrobed} = m c_{e H_2O} (T_f - T₀)
where M and m are the mass of lead and water, respectively, c are the specific heats, T₁ is the temperature of the hot lead, T₀ the temperature of cold water and T_f the equilibrium temperature
M c_{ePb} (T₁ - T_f) = m c_{eH2O} (T_f - T₀)
m = \(\frac{ M\ c_{ePb} \ (T_1 - T_f)}{c_{eH_2O} \ (T_f - T_o)}\)
let's calculate
m = \(\frac{200 \ 0.129 (176.4-56.4)}{ 4.186 \ (56.4 -21.7)}\)
m = 3096 / 145.25
m = 21.3 g
Using the Calorimetry relationships you can find the amount of water in the calorimeter is:
m = 21.3 g
learn more about calorimetry here:
https://brainly.com/question/15073428
Ethylene gas is frequently used for fruit ripening and seed germination in agriculture. A dry, clean and evacuated container weighs 36.1235 and it weighs 142.3415 g when is hilled with water. When it is filled with ethylene gas at 755.3 mmHg and 25.0 °C, it weighs 36.2449 g. Determine the molar mass of ethylene gas. (dH2O: 0.9970 g/mL)
Answers
The general gas equation, commonly referred to as the ideal gas law, represents the state of a fictitious ideal gas through an equation. The molar mass of ethylene gas when the pressure is 755.3 mmHg, the temperature is 25.0 °C and it weighs 36.2449 g is 28.29 g/mol.
According to the ideal gas law, the sum of the absolute temperature of the gas and the universal gas constant is equal to the product of the pressure and volume of one gram of an ideal gas.
The ideal gas equation is:
PV = nRT
n = PV / RT
755.3 mmHg = 0.99 atm
25.0 °C = 298 K
R = 0.082 atm·L·mol⁻¹·K⁻¹
Mass of water = 142.3415 - 36.1235 = 106.218 g
Density = Mass / Volume
V = m / d = 106.218 / 0.9970 = 106.53 mL = 0.106 L
n = 0.99 × 0.106 / 0.082 × 298 = 0.00429 mol
Mass of ethylene gas = 36.2449 - 36.1235 = 0.1214 g
Molar mass = 0.1214 / 0.00429 = 28.29 g/mol
To know more about ideal gas law, visit;
https://brainly.com/question/20217978
#SPJ1
What is the maximum mass, in kilograms, of (NH4)2U2O7 that can be formed from the reaction of 100 kg of water and 100 kg of ammonia with 481 kg of UO2SO4?
Answers
Mass of (NH₄)₂U₂O₇ : 410.05 kg
Further explanationReaction
2UO₂SO₄ + 6NH₃ + 3H₂O → (NH₄)₂U₂O₇ + 2(NH₄)₂SO₄
MW UO₂SO₄ : 366.091
MW (NH₄)₂U₂O₇ : 624.131
MW H₂O : 18.0153
MW NH₃ : 17.0306
mol of 100 kg water :
\(\tt \dfrac{100}{18.0153}=5.55\)
mol of 100 kg ammonia :
\(\tt \dfrac{100}{17.036}=5.87\)
mol of UO₂SO₄ :
\(\tt \dfrac{481}{366.091}=1.314\)
Limiting reactants : smallest mol ratio(mol : coefficient)
\(\tt \dfrac{5.55}{3}\div \dfrac{5.87}{6}\div \dfrac{1.314}{2}=1.85\div 0.98\div 0.657\)
UO₂SO₄ ⇒ Limiting reactants
mol (NH₄)₂U₂O₇ : mol UO₂SO₄
\(\tt \dfrac{1}{2}\times 1.314=0.657\)
mass (NH₄)₂U₂O₇
\(\tt 0.657\times 624.131=410.05\)
You are given a bulb, one metre copper wire, a switch and a battery. How could you use them to distinguish the samples of metals and non-metals? Write the usefulness of this test in distinguishing metals and non-metals.
Answers
With the given materials following experiment can be done
Electrical conductivity test:
Metals are good conductors of electricity, therefore when you join metals with a battery, wire, and bulb, you get a bulb.
Similarly, if nonmetals are poor electrical conductors, they may not ignite the bulb when attached to a wire and a battery.
Generally, the above method can be used to identify metals and non-metals. But there are some exceptions also,. Graphite, an allotrope of non-metal carbon, is a good conductor of electricity.
Learn more about Metals and their properties;
https://brainly.com/question/25090336
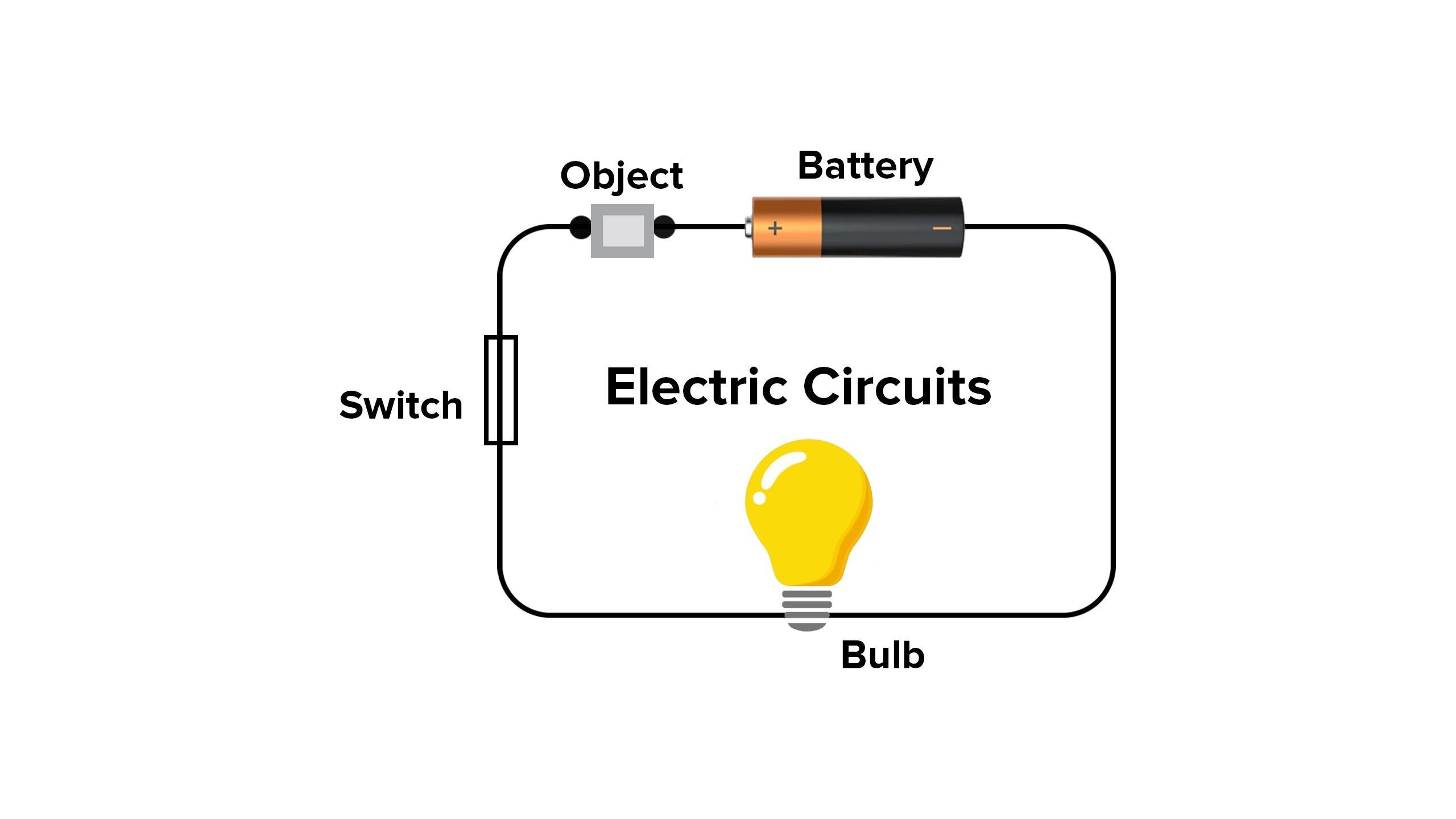
The periodic chart has many different types of elements. Some elements are metals, some are nonmetals, and yet others are metalloids. Therefore, by electrical conductivity test, we can detect metal and non metal.
What is metal ?Metals are hard, conduct electricity, are ductile, lustrous, and malleable materials.
Since metals have free electrons that's why they can conduct electricity. Since atoms in metals are very closely packed in a definite crystal solid. It is not brittle that is it can not be broken down easily.
Metals carry electricity well, therefore when you combine metals with a battery, wire, and bulb, you get a light. Similarly, if nonmetals are poor electrical conductors, when connected to a wire and a battery, they may not light the bulb.
Therefore, by electrical conductivity test, we can detect metal and non metal.
Learn more about the metals, here:
https://brainly.com/question/27859211
#SPJ2
Gaseous methane (CH4) will react with gaseous oxygen (O₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). Suppose 5.5 g of methane is mixed with 11.5 g of oxygen. Calculate the minimum mass of methane that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits.
Answers
Answer:
Top answer:
The maximum mass of water (H₂O) produced from the reaction between
Explanation:
Gaseous methane ch4 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gaseous water h2o . suppose 1.44 g of ...
1) The equivalence point in the titration of this weak acid is 50.80 mL. At 25.40 mL, the pH was measured to be 3.86. Calculate the Ka of the unknown acid.
2)The equivalence point in the titration of this weak acid is 50.80 mL. At 25.40 mL, the pH was measured to be 3.86.What is the pKa of the unknown acid.
Answers
1. The pKa of the unknown acid is equal to the pH measured at 25.40 mL, which is 3.86.
2. The Ka of the unknown acid is 1.39 x \(10^{-4}\).
StepsTo calculate the Ka of the unknown acid, we can use the Henderson-Hasselbalch equation:
\(pH = pKa + log([A^-]/[HA])\)
At the equivalence point, \([A^-] = [HA]\), so we can simplify the equation to:
pH = pKa + log(1)
which means that pH = pKa.
Therefore, the pKa of the unknown acid is equal to the pH measured at 25.40 mL, which is 3.86.
To calculate the Ka of the unknown acid, we can use the relationship between Ka and pKa:
Ka = \(10^{-pKa}\)
We already know the pH and pKa values, so we can solve for Ka:
Ka =\(10^{-pKa}\) = \(10^{-3.86}\) = 1.39 x \(10^{-4}\).
Therefore, the Ka of the unknown acid is 1.39 x \(10^{-4}\).
learn more about equivalence point here
https://brainly.com/question/2496608
#SPJ1
Calculate the ratio of the moles of produced to the moles of each of the reactants used. (Write two separate ratios.)
Answers
Ratio of moles of NH₃ produced to moles of N₂ used: 2 moles of NH₃ / 1 mole of N₂
Ratio of moles of NH₃ produced to moles of H₂ used: 2 moles of NH₃ / 3 moles of H₂
What is the mole ratio of the reaction?From the balanced chemical equation:
N₂ + 3 H₂ ⟶ 2 NH₃
We can determine the ratio of moles of products to the moles of each reactant.
Ratio of moles of NH₃ produced to moles of N₂ used:
From the balanced equation, we can see that 1 mole of N₂ reacts to produce 2 moles of NH₃. Therefore, the ratio is:
2 moles of NH₃ / 1 mole of N₂
Ratio of moles of NH₃ produced to moles of H₂ used:
From the balanced equation, we can see that 3 moles of H₂ react to produce 2 moles of NH₃. Therefore, the ratio is:
2 moles of NH₃ / 3 moles of H₂
Learn more about the mole ratio at https://brainly.com/question/19099163
#SPJ1
Given the equation of reaction;
N₂ + 3 H₂ ---> 2 NH₃
Calculate the ratio of the moles of produced to the moles of each of the reactants used. (Write two separate ratios.)
Identify the type of reaction and predict the product: Calcium + water -->
Answers
Answer:
Exothermic Reaction
Product = Calcium hydroxide + hydrogen
Explanation:
Calculate the pH of a 0.307 M solution of NaNO2. The ionization constant, a, for the acid, HNO2, is 4.60×10−4.
Answers
8.253 is the pH of a 0.307 M solution of NaNO\(_2\). The ionization constant, a, for the acid, HNO\(_2\), is 4.60×10⁻⁴.
What is pH?In chemistry, the pH scale is used to define the level of basicity or acidity of an aqueous. pH has historically stood for " Hydrogen potential" (or "power of hydrogen").
Lower pH values are recorded for acid solution (solutions with greater H ion concentrations) than for basic or alkaline solutions.
NO\(_2\)⁻ + H\(_2\)O → HNO\(_2\) + OH⁻
Kb = [HNO\(_2\) ][OH⁻]/[NO\(_2\)⁻]
Kb = 1.00X10⁻¹⁴
Kb = 1.00X10⁻¹⁴/ 4.60X10⁻⁴= 2.17X10⁻¹¹
[HNO\(_2\) ] = [OH⁻] = x
[NO\(_2\)⁻ ] = 0.148-x
Kb = 2.17X10⁻¹¹= x² / 0.148
x = [OH⁻] = 1.79X10⁻⁶
pOH = 5.747
pH = 14.000 - pOH = 8.253
Therefore, 8.253 is the pH of a 0.307 M solution of NaNO\(_2\). The ionization constant, a, for the acid, HNO\(_2\), is 4.60×10⁻⁴.
To know more about pH, here:
https://brainly.com/question/27945512
#SPJ1
Calculate to the correct number of significant figure (1.7x10^6)/(2.63x10^2) + 7.33 =
Answers
Answer: 64.7 \(*10^2\)
Explanation:
Significant figures are the number of digits in a value that correlate with the accuracy of that value.
First lets solve the equation:
(1.7x10^6)/(2.63x10^2) + 7.33 = ?
(1,700,000)/(263) + 7.33
(6,463.8783) + 7.33 = 6,471.2083
We have to simplify the answer to the correct amount of significant digits.
6,471.2083 -> 64.7 \(*10^2\)
17. Which of the following represent the correct net ionic equations from the double replacement reaction of
calcium nitrate and potassium phosphate?
a. Caaq) + PO43- (aq) → CaPO4(s)
b. Q Ca2+ (aq) + 3 PO43- (aq) → Caz(PO4)3(S)
c. 3 Ca2+ (aq) + 2 PO.3(aq) → Caz(PO4)2(S)
d. 2 NO3(aq) + Ca2+
+ Ca(NO3)2(S)
Answers
Answer:
D. 2 NO3(aq)+Ca2+
Explanation:
Reaction of calcium nitrate and potassium phosphate produce calcium phosphate and potassium nitrate. The net ionic equation for this is written as : \(3 Ca^{2+} (aq) +2PO_{4} ^{3-} (aq) \rightarrow Ca_{3}(PO_{4} )_{2} (s)\). Thus option C is correct.
What is double displacement reaction?
In a double displacement reaction, two species or groups in reactant side are displaced in the products.
In a balanced chemical equation, all the reactants have to be in proper stochiometric ratio and the states of all species are mentioned in brackets.
In an ionic equation all the ions and charges are balanced both side and the number of each elements on both side too. The net ionic equation represents the formation of a solid product from its ions.
The balanced molecular equation and complete ionic equation are written for the given reaction below:
\(3 Ca (NO_{3})_{2} (aq)+ 2K_{3} PO_{4} (aq) \rightarrow Ca_{3}(PO_{4} )_{2} (s) + 6 KNO_{3}(aq)\)
\(3 Ca^{2+} (aq) +6 NO_{3}^{-} + (aq)+ 6K^{+} + (aq) 2PO_{4} ^{3-} (aq) \rightarrow Ca_{3}(PO_{4} )_{2} (s) + 6 NO_{3}^{-} (aq)+ 6K^{+} (aq)\)
Now when the same species on both side is cancelled, then the net ionic equation is written as:
\(3 Ca^{2+} (aq) +2PO_{4} ^{3-} (aq) \rightarrow Ca_{3}(PO_{4} )_{2} (s)\)
Therefore, the correct et ionic equation is option c.
To learn more about ionic equations, refer the link below:
https://brainly.com/question/15467511
#SPJ2
Question 5 of 10
Platinum has a density of 21 g/cm³. A platinum ring is placed in a graduated
cylinder that contains water. The water level rises from 4.0 mL to 4.2 mL
when the ring is added. What is the mass of the ring?
A. 2.6 g
OB. 3.8 g
OC. 4.2 g
OD. 5.2 g
B
Answers
Answer:
4.2 g
Explanation:
The VOLUME of the ring is 4.2 - 4.0 = .2 ml = .2 cm^3
the MASS of the ring is this times the density
.2 cm^3 * 21 g/cm^3 = 4.2 g
Answer:
the answer is c
Explanation:
density is mass/volume
so mass=density × volume
but we take the change is volume that is v2-v2=4.2-4=0.2ml
but the density is in gm/cm^3 so we should convert ml into cm^3. eventually they are equal so mass=21×0.2=4.2
Scientists launch a rocket, and they monitor its acceleration and the force exerted by its engines. As the rocket gets higher, the monitors show that the acceleration of the rocket is increasing but the force exerted stays the same. How do Newton’s laws explain why the scientists could expect this to happen?
Answers
The force applied to the rocket by its engines remains constant as it moves up, while its mass decreases, resulting in an increase in acceleration.
Newton's laws of motion provide an explanation for the acceleration of a rocket as it moves away from the ground. According to Newton's second law, the force exerted on an object is directly proportional to its acceleration, and the force required to move an object increases as its mass increases.
In the case of a rocket, its mass decreases as it consumes fuel, which means that less force is required to move it as it climbs higher into the atmosphere.
As the rocket moves up, its acceleration increases while the force exerted on it remains constant. Newton's second law of motion explains that the acceleration of an object is directly proportional to the force applied to it. According to the second law of motion, an object's acceleration is equal to the force exerted on it divided by its mass.
This means that as the rocket climbs higher and its mass decreases due to the consumption of fuel, less force is required to accelerate it, and so its acceleration increases. In other words, the rocket's acceleration is increasing because the force required to move it is decreasing due to the decreasing mass of the rocket.
This phenomenon is also related to Newton's third law of motion, which states that every action has an equal and opposite reaction. The force exerted by the rocket's engines is balanced by an equal and opposite force exerted on the rocket by the exhaust gases, according to this law.
For more such questions on acceleration visit;
https://brainly.com/question/26590057
#SPJ8
Based on a Kc value of 0.250 and the given data table, what are the equilibrium concentrations of XY, X, and Y , respectively?
Answers
From the solution that we have in the question;
The concentration of X and Y is 0.28 MThe concentration of XY is 0.32 MWhat is the equilibrium constant?The equilibrium constant, denoted as K, is a value that quantitatively represents the ratio of the concentrations of products to reactants at equilibrium in a chemical reaction.
It is a fundamental concept in chemical equilibrium.
The value of the equilibrium constant provides valuable information about the position of equilibrium and the relative concentrations of species involved in a chemical reaction.
Kc = [X] [Y]/[XY]
\(0.25 = (0.1 + x)^2/(0.5 - x)\)
\(0.25(0.5 - x) = (0.1 + x)^2\)
\(0.125 - 0.25x =0.01 + 0.2x + x^2\\ x^2 + 0.45x - 0.115 = 0\)
x = 0.18 M
The equilibrium amount of X and Y= 0.28 M and the equilibrium concentration of XY = 0.32 M
Learn more about equilibrium constant:
https://brainly.com/question/29253884
#SPJ1
Based on the answer to the question that we have;
A 0.28 M concentration of X and Y exists at equilibriumXY's concentration at equilibrium is 0.32 M.The equilibrium constantThe ratio of the product to reactant concentrations in a chemical reaction at equilibrium is represented quantitatively by the equilibrium constant, abbreviated as K.
It is a cornerstone of the theory of chemical equilibrium.
A chemical reaction's equilibrium position and the relative concentrations of the species involved can both be learned from the equilibrium constant's value.
Kc = [X][Y]/[XY]
\(0.25 = (0.1 + x)^2/(0.5 - x)\\0.25(0.5 - x) = (0.1 +x)^2\\0.125 - 0.25x = 0.01 +0.2x +x^2\\= 0.18 M\)
The equilibrium concentration of;
XY =0.5 - 0.18
=0.32 M
Then the equilibrium amount of
X and Y is
0.1 + 0.18= 0.28 M.
Learn more about equilibrium:brainly.com/question/29253884
#SPJ1

HELP PLEASE PLEASE PLEASE. Can anyone tell me how to separate the following mixture
A) ethanol in water
B) boiling the mixture of chloride crystals with water
C) pure water from muddy water
D) sodium chloride in water
E) sodium carbonate in water
F) chlorophyll from leaves
G) mixture of acetic acid and alcohol
H) serum from blood sample
I) kerosene from water
J) ammonium chloride in sand
I NEED CORRECT ANSWERS ONLY.
HURRY UP PLEASE. I WILL MARK AS BRAINLIEST
Answers
A) Ethanol in water: Distillation.
B) Boiling the mixture of chloride crystals with water: Evaporation.
C) Pure water from muddy water: Filtration.
D) Sodium chloride in water: Evaporation or Crystallization.
E) Sodium carbonate in water: Filtration or Evaporation.
F) Chlorophyll from leaves: Extraction using a suitable solvent like ethanol.
G) Mixture of acetic acid and alcohol: Distillation.
H) Serum from blood sample: Centrifugation.
I) Kerosene from water: Separatory funnel or Decantation.
J) Ammonium chloride in sand: Sublimation or Dissolving in water and Filtration.
A) Ethanol in water: Distillation can be used to separate ethanol from water based on their different boiling points.
B) Boiling the mixture of chloride crystals with water: By heating the mixture, the water will evaporate, leaving behind the chloride crystals.
C) Pure water from muddy water: Filtration can be used to separate the solid particles (mud) from the water.
D) Sodium chloride in water: Evaporation can be used to separate sodium chloride from water by heating the mixture until the water evaporates, leaving behind the salt.
E) Sodium carbonate in water: Filtration can be used to separate solid sodium carbonate from water, similar to muddy water.
F) Chlorophyll from leaves: Extraction using a suitable solvent like ethanol or acetone can be used to separate chlorophyll from leaves.
G) Mixture of acetic acid and alcohol: Distillation can be used to separate the mixture based on their different boiling points.
H) Serum from blood sample: Centrifugation can be used to separate the serum, which is the liquid part of blood, from the solid components like cells.
I) Kerosene from water: Separatory funnel or decantation can be used to separate the immiscible liquids by pouring off the top layer (kerosene) from the bottom layer (water).
J) Ammonium chloride in sand: Sublimation can be used to separate ammonium chloride by heating the mixture, causing the ammonium chloride to vaporize and then condense back into solid form in a cooler region, leaving the sand behind.
Know more about Sublimation here:
https://brainly.com/question/16789108
#SPJ8
0.40 L of HNO3 is titrated to equivalence using 0.20 L of 0.2 MNaOH . What is the concentration of the HNO3
Answers
the absorbance of the solution at 427 nm is 0.15 . if the molar absorptivity of yellow dye at 427 nm is 27400 m–1cm–1, what is the concentration of the solution in m?
Answers
The concentration of the solution if the absorbance of the solution at 427 nm is 0.15 and the molar absorptivity of yellow dye at 427 nm is 5.47 × 10-⁶M.
How to calculate concentration?Absorbance in physics is a logarithmic measure of the amount of light that is absorbed when passing through a substance.
It is the capacity of a substance to absorb light of a given wavelength. According to this question, the absorbance of the solution at 427 nm is 0.15.
A = εmCl
Where;
A = absorbanceεm = molar extinction coefficientC = concentrationl = path length of 1 cmC = 0.15 ÷ (27400 × 1)
C = 5.47 × 10-⁶M
Therefore, 5.47 × 10-⁶M is the concentration of the solution.
Learn more about absorbance at: https://brainly.com/question/28812538
#SPJ1
how many atoms are in water?
Answers
Which of these waves has the greatest wavelength? (3 points) Wave shown with 2 wavelengths. Wave shown with 3 wavelengths. Wave shown with 1 wavelength stretch over a short distance. Wavelength shown with 1 wavelength stretched over a long distance.
Answers
The waves that has the greatest wavelength is Wavelength shown with 1 wavelength stretched over a long distance.
Waves explained.A wave could be a disturbance or variety that voyages through a medium or space, carrying vitality without transporting matter. Waves can take different shapes and happen totally different sorts of waves, counting mechanical waves and electromagnetic waves.
Mechanical waves require a medium to propagate, meaning they require a substance like water, discuss, or a strong fabric to transmit the wave. Illustrations of mechanical waves incorporate water waves, sound waves, and seismic waves. In these waves, particles of the medium sway or vibrate in a design, exchanging energy from one molecule to another.
Electromagnetic waves, on the other hand, don't require a medium and can travel through vacuum, such as in space. Electromagnetic waves comprise of electric and attractive areas swaying opposite to each other and to the heading of wave engendering. Illustrations of electromagnetic waves incorporate obvious light, radio waves, microwaves, infrared waves, bright waves, X-rays, and gamma beams.
Learn more about waves below.
https://brainly.com/question/26116832
#SPJ1
A compound decomposes with a half-life of 8.0 s and the half-life is independent of the concentration. How long does it take for the concentration to decrease to one-ninth of its initial value
Answers
Answer:
The concentration takes 25.360 seconds to decrease to one-ninth of its initial value.
Explanation:
The decomposition of the compound has an exponential behavior and process can be represented by this linear first-order differential equation:
\(\frac{dc}{dt} = -\frac{1}{\tau}\cdot c(t)\)
Where:
\(\tau\) - Time constant, measured in seconds.
\(c(t)\) - Concentration of the compound as a function of time.
The solution of the differential equation is:
\(c(t) = c_{o} \cdot e^{-\frac{t}{\tau} }\)
Where \(c_{o}\) is the initial concentration of the compound.
The time is now cleared in the result obtained previously:
\(\ln \frac{c(t)}{c_{o}} = -\frac{t}{\tau}\)
\(t = -\tau \cdot \ln \frac{c(t)}{c_{o}}\)
Time constant as a function of half-life is:
\(\tau = \frac{t_{1/2}}{\ln 2}\)
Where \(t_{1/2}\) is the half-life of the composite decomposition, measured in seconds.
If \(t_{1/2} = 8\,s\), then:
\(\tau = \frac{8\,s}{\ln 2}\)
\(\tau \approx 11.542\,s\)
And lastly, given that \(\frac{c(t)}{c_{o}} = \frac{1}{9}\) and \(\tau \approx 11.542\,s\), the time taken for the concentration to decrease to one-ninth of its initial value is:
\(t = -(11.542\,s)\cdot \ln\frac{1}{9}\)
\(t \approx 25.360\,s\)
The concentration takes 25.360 seconds to decrease to one-ninth of its initial value.
The irreversible isomerization A
B was carried out in a batch reactor and the following concentration time data were obtained:
Time vs Concentration data in a Batch reactor
t 0 3 5 8 10 12 15 7.5
mol/h 4 2.89 2.25 1.45 1.0 0.65 0.25 0.07
Determine the reaction order,
, and the specific reaction a rate constant, k, using any method of your choice.

Answers
The reaction order and specific reaction rate constant can be determined by performing the kinetics experiment on irreversible polymerization A. Kinetic experiments can be used to investigate the rate and mechanism of chemical reactions. Chemical kinetics is the study of chemical reactions' speed and pathway.
The term "kinetics" refers to the study of reaction rates, which are determined by measuring the concentration of reactants and products as a function of time.Kinetics experiments can be used to determine the reaction rate and order of reaction. A chemical reaction's rate is defined as the change in the concentration of a reactant or product per unit time. The order of a reaction refers to the number of molecules that must react to produce a product. The order of reaction can be determined by measuring the initial rate of the reaction as a function of concentration.Methods for determining the reaction rate order include the initial rate method, the half-life method, and the integrated rate method. The initial rate method determines the reaction order by measuring the initial rate of the reaction at different reactant concentrations. The half-life method determines the reaction order by measuring the time it takes for the reactant concentration to decrease by half.The integrated rate method determines the reaction order by measuring the concentration of the reactant or product at different times.The specific rate constant can be determined by using the Arrhenius equation, which relates the rate constant to the activation energy, temperature, and frequency factor. The frequency factor can be determined by measuring the rate constant at different temperatures.For such more question on polymerization
https://brainly.com/question/1602388
#SPJ8
What is the formula for iron (II) sulfate hexahydrate?
Answers
The formula for iron (II) sulfate hexahydrate
\(is\)
FeH12O10S

10. Which substance does not take the shape of its container?
A. water
B. air
C. gasoline
D. ice
Answers
Answer:
B. Air
Explanation:
what is the PH scale of 0.02m of hydrochloric acid
Answers
Answer:
Explanation:
The pH of 0.02 M hydrochloric acid is approximately 1.7.
THANKS
IF THE ANSWER IS CORRECT , THEN MARK ME AS BRAINLIST
To determine the pH of a hydrochloric acid solution, we need to know its concentration. You mentioned a concentration of 0.02 M (molar), which refers to 0.02 moles of hydrochloric acid dissolved in 1 liter of solution.
Hydrochloric acid (HCl) is a strong acid that dissociates completely in water, meaning all HCl molecules release their hydrogen ions (H+) into the solution. Since the concentration is given as 0.02 M, it means there are 0.02 moles of H+ ions in 1 liter of the solution.
To calculate the pH, we can use the formula:
pH = -log[H+]
In this case, [H+] represents the concentration of hydrogen ions in moles per liter. Since hydrochloric acid is a strong acid and it dissociates completely, the concentration of hydrogen ions is equal to the concentration of HCl, which is 0.02 M.
pH = -log(0.02) ≈ 1.70
Therefore, a hydrochloric acid solution with a concentration of 0.02 M would have a pH of approximately 1.70, indicating it is strongly acidic.