Answers
Answer
(a) KNO₃: The oxidation number of the bolded element N is +5
(b) AlH₃: The oxidation number of the bolded element Al is +3
(c) H₂PO₄⁻: The oxidation number of the bolded element P is +5
Explanation
Note: The sum of the oxidation numbers of elements in a neutral compound is zero
(a) KNO₃
Potassium belongs to group 1, and oxygen belongs to group 6 so their oxidation numbers are +1 and -2 respectively.
The oxidation number of the bolded element N is calculated as follows:
KNO₃ = 0
K + N + 3(O) = 0
+1 + N + 3 (-2) = 0
+1 + N - 6 = 0
N = +6 - 1
N = +5
(b) AlH₃
Note: The oxidation state of hydrogen in hydrides is -1.
AlH₃ = 0
Al + 3(H) = 0
Al + 3(-1) = 0
Al - 3 = 0
Al = +3
(c) H₂PO₄⁻
H₂PO₄ = -1
2(H) + P + 4(O) = -1
2(+1) + P + 4(-2) = -1
+2 + P - 8 = -1
P = +8 - 2 - 1
P = +8 - 3
P = +5
Related Questions
In the SOLID state of matter ,particles have enough energy to move freely but not enough energy to overcome their attraction for each other
Answers
In the solid state of matter, particles, such as atoms, ions, or molecules, are closely packed and held together by strong intermolecular forces, such as ionic bonds, metallic bonds, or covalent bonds.
In a solid, particles have enough energy to vibrate around fixed positions but do not have enough energy to overcome the attractive forces between them. These attractive forces, also known as cohesive forces, arise from the electrostatic interactions between particles or the sharing of electrons in covalent bonds.
The energy of the particles in a solid is typically much lower than in the liquid or gaseous states, resulting in a fixed arrangement of particles.
The movement of particles in a solid is characterized by vibrations or oscillations around their equilibrium positions.
These vibrations occur due to the thermal energy present in the solid, but the particles remain relatively fixed in their positions due to the strong attractive forces. The amplitude of the vibrations increases with increasing temperature, as the particles gain more thermal energy.
However, the particles in a solid do not have enough energy to break the intermolecular bonds and move freely throughout the entire solid. Instead, they can only move within their local vicinity or lattice positions.
This restricted movement is what distinguishes the solid state from the liquid or gaseous states, where particles have enough energy to overcome intermolecular forces and move more freely.
For more such questions on intermolecular forces visit:
https://brainly.com/question/12243368
#SPJ8
Which of the following is an example of water in a liquid state?
A. ice
B. Steam
C. Snow
D. Rain
Answers
Explanation/
A. = solid
B.= gas
C.= solid
Evaporation occurs then it sort of bunches up and precipitates and falls from the sky as water droplets
How many obits are there
Answers
What happens when sodium undergoes a chemical reaction with chlorine? A The two substances form a heterogeneous mixture B. The two substances form a homogenous mixture C A new compound is formed – salt D The two substances undergo various physical changes
Answers
Answer:
C.) A new compound is formed - salt
Explanation:
Na is a metal
Cl is a nonmetal
When they are combined, they form ionic bonds as they seek to fill their octets. Salts are ionic compounds.
When sodium undergoes a chemical reaction with chlorine, a new compound is formed known as sodium chloride or common table salt. The correct option is C.
Chemical reaction is a chemical process, which involves the transformation of one or more substances, called reactants, into new substances, called products. These reactions occur when atoms or ions or molecules rearrange their bonds to form different chemical compounds.
The reaction between sodium and chlorine is highly exothermic (releases heat energy) and produces a violent reaction, resulting in the formation of a stable ionic compound.
The balanced chemical equation for this reaction is:
\(2Na + Cl_2\rightarrow 2NaCl\)
In this reaction, the sodium atoms (Na) lose one electron each to achieve a stable electron configuration, forming sodium ions (Na+), while the chlorine molecules \((Cl_2)\) gain one electron each to form chloride ions (Cl-). The resulting sodium ions and chloride ions attract each other due to their opposite charges, forming a three-dimensional crystal lattice of sodium chloride.
Therefore, when sodium undergoes a chemical reaction with chlorine, a new compound is formed that is salt. Option C is the correct answer.
Learn more about chemical reactions here:
https://brainly.com/question/29762834
#SPJ6
What is the charge of an atom that has 6 protons, 4
neutrons, and 6 electrons?
Answers
Answer:
neutral/no charge
Explanation:
What is the number of protons neutrons and electrons in a family Osito that is used in medical diagnosis atomic number 43 mass number 99 charge of 7+
Answers
Answer:
43 protons
36 electrons
56 neutron
Explanation:
neutron is mass number minus proton number.
in a non neutral atom the electron is determined after minusing the charges or electron lost.
the number of proton obviously is 43
The most powerful laser in the world fires a pulse at 25 fempto seconds producing 30
terawatts of power. In physical sciences, power (watt) = Energy (J or kg•m2•s-2)/time (s).
If the average laser fires 7.5 x 1020 photons in a pulse, then what is the wavelength of the light fired in the world’s most powerful laser?
Answers
The most powerful laser in the world fires a pulse at 25 fempto seconds producing 30 terawatts of power in physical sciences, power (watt) =Energy (J or kg•m²×s⁻²)/time (s)if the average laser fires 7.5 x 10²⁰ photons in a pulse, then wavelength of the light fired in the world’s most powerful laser is 37×10⁻¹⁰ nm
A laser is the device that emit a beam of coherent light through an optical amplification process
Here given data is
Energy = time × power
Energy = 25 × 30
Energy = 750 J
Photons = 7.5 x 10²⁰
We have to calculate wavelength = ?
Then the formula is E =hcλ where E = energy, h = plank constant, c= speed, λ = wavelength
λ = hcE
λ = 6.62×10⁺³⁴×7.5 x 10²⁰ ×750 J
λ = 37×10⁻¹⁰ nm
Know more about wavelength
https://brainly.com/question/17329599
#SPJ1
Convert 0.809 mol AlCl3 to formula units AlCl3.
Answers
Answer:
133.340538.
Explanation:
The answer is 133.340538. We assume you are converting between grams AlCl3 and mole. You can view more details on each measurement unit: molecular weight of AlCl3 or mol This compound is also known as Aluminium Chloride. The SI base unit for amount of substance is the mole.
a. Phenol (C6H5OH) is an aromatic compound and a colourless liquid widely used in household products
and medicine. When it reacts with chlorine liquid (chlorine is a diatomic molecule), in the presence of a
catalyst, one of the aromatic hydrogen atoms attached directly to a C atom is replaced with a chlorine
atom, and liquid chlorophenol is formed. This replacement process is called chlorination
Write a balanced chemical equation for the chlorination reaction and explain how you balanced it. Note
that hydrogen chloride gas (HCI) is also a product of this chemical reaction and you should ignore the
presence of the catalyst in the equation.
Answers
Because there are 2 Cl on the left, we will put a coefficient 2in front of HCl on the right side to balance out the Cl. This would result in an unequal amount of H, with 6 on the right side and 7 in the left, so we have to put a coefficient of 2 in front of C6H5OH and C6H4OH on both sides to balance out the H. By doing this, we would obtain an equal amount of H on both sides. The Carbon is already balanced, and so is the Oxygen.
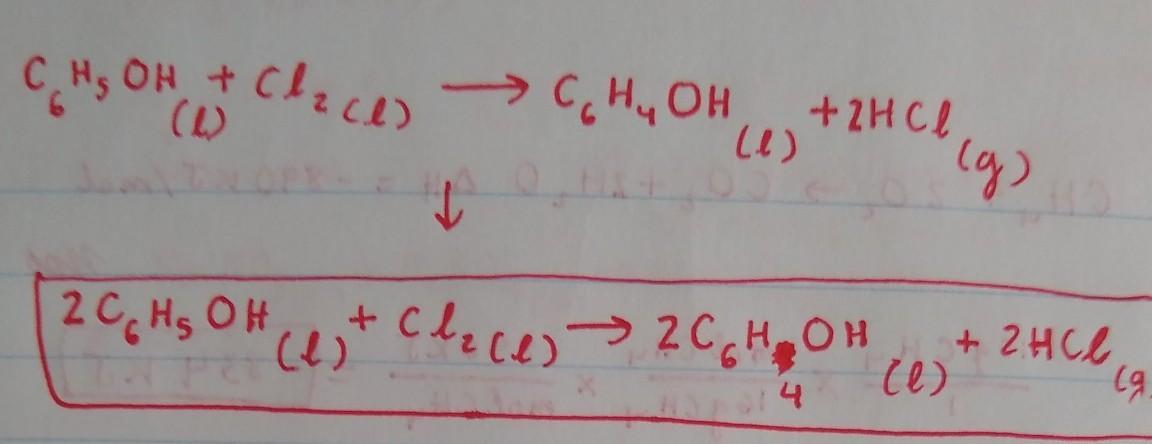
The balanced chemical reaction equation for the reaction between aromatic phenol and chlorine gas in the presence of FeCl3 as catalyst is as follows;
C6H6O + Cl2 -------> C6H5OCl + HCl
An aromatic compound has 4n + 2 number of pi electrons. This condition is satisfied by phenol. Hence, phenol has the stability associated with aromatic compounds.
The reaction of phenol with chlorine in the presence of a catalyst such as FeCl3 is an aromatic electrophillic substitution reaction.
This reaction yields a chlorinated phenol (one of the aromatic hydrogen atoms attached directly to a C atom is replaced with a chlorine atom and chlorophenol is formed).
A balanced chemical reaction equation is one in which the number of atoms of each element at the reactant side and the product side are equal. This condition is satisfied for the reaction;
C6H6O + Cl2 -------> C6H5OCl + HCl
Learn more; https://brainly.com/question/6170291
129.13 mL of a 112.9 mM solution of NH4l is added to a 105.31 mL solution of 0.87 M Mgl2. What
is the final concentration of I ions in the resulting solution? Express your answer in units of
molarity using at least three significant figures.
Answers
The final concentration of I ions in the resulting solution is approximately 0.0311 M, expressed with three significant figures.
To determine the final concentration of I ions in the resulting solution, we need to consider the stoichiometry and volumes of the solutions being mixed.Given:
Volume of NH4l solution = 129.13 mL
Concentration of NH4l solution = 112.9 mM = 0.1129 M (converting from millimolar to molar)
Volume of Mgl2 solution = 105.31 mL
Concentration of Mgl2 solution = 0.87 M
First, we need to determine the moles of NH4l and Mgl2 in their respective solutions:
Moles of NH4l = Volume of NH4l solution * Concentration of NH4l solution
Moles of NH4l = 0.12913 L * 0.1129 M = 0.01459 moles NH4l
Moles of Mgl2 = Volume of Mgl2 solution * Concentration of Mgl2 solution
Moles of Mgl2 = 0.10531 L * 0.87 M = 0.09157 moles Mgl2
Next, we determine the limiting reagent, which is the reactant that is completely consumed and determines the maximum amount of product formed. In this case, the limiting reagent is NH4l because it has fewer moles than Mgl2.
The balanced chemical equation for the reaction between NH4l and Mgl2 is:
2 NH4l + Mgl2 → 2 NH4+ + MgI2
From the balanced equation, we can see that for every 2 moles of NH4l, we get 1 mole of MgI2.
Since the moles of NH4l is the limiting reagent, it will be completely consumed, and the moles of MgI2 formed will be half of the moles of NH4l.
Moles of MgI2 = 0.01459 moles NH4l * (1 mole MgI2 / 2 moles NH4l) = 0.007295 moles MgI2
Finally, we calculate the final concentration of I ions in the resulting solution:
Volume of resulting solution = Volume of NH4l solution + Volume of Mgl2 solution
Volume of resulting solution = 0.12913 L + 0.10531 L = 0.23444 L
Final concentration of I ions = Moles of MgI2 / Volume of resulting solution
Final concentration of I ions = 0.007295 moles / 0.23444 L = 0.0311 M
Therefore, the final concentration of I ions in the resulting solution is approximately 0.0311 M, expressed with three significant figures.
For more such questions on concentration
https://brainly.com/question/17251833
#SPJ8
Silver has a density of 10.49 g/cm³. If 101.0 g of silver is added to 51.0
mL of water in a graduated cylinder, to what volume reading will the
water level in the cylinder rise?
Answers
Density is the mass of a substance per unit volume. So, adding 9.628 ml of Ag to 51ml of H2O, makes the total volume 60.628ml.
What is meant by density ?
Density is the mass of a substance per unit volume. The most commonly used symbol for density is ρ, although the Latin letter D can also be used. Mathematically, density is defined as mass divided by volume: \({\displaystyle \rho ={\frac {m}{V}}}\) where ρ is density, m is mass and V is the volume.
Density is an important concept because it allows us to determine which substances float and which sink when placed in a liquid. In general, substances float until their density is lower than the density of the liquid in which they are placed.
Density = Mass/ water displaced
water displaced = Mass/Density
water displaced = 101 / 10.49
water displaced = 9.628 cm³
Here 1 cm³ = 1ml
so, adding 9.628 ml of Ag to 51ml of H2O, makes the total volume 60.628ml.
To learn more about Density, visit;
https://brainly.com/question/952755
#SPJ9
I'm making a AD for my special ed class room and I am interviewing people. Make 10 unique questions I can ask my fellow classmates about the things they have learned in this room.
Answers
These are 10 unique questions you can ask your fellow classmates about the things they have learned in your special ed classroom:
What is your favorite thing about our classroom?What is one thing you have learned in our classroom that you will never forget?What is one thing you would like to learn more about in our classroom?How has our classroom helped you to succeed?What is one thing you would like to say to your teacher?What is one thing you would like to say to your classmates?What is one thing you would like to say to your parents?What is one thing you would like to say to the world?What is your dream for the future?What is one thing you are grateful for?What are special ed classroom?A special education classroom is a classroom designed to meet the needs of students with disabilities. These classrooms are staffed by specially trained teachers who are able to provide individualized instruction and support to students with a variety of disabilities.
These questions are designed to get your classmates thinking about the things they have learned in your special ed classroom and how those things have impacted them. The answers to these questions can be used to create a powerful and informative ad for your classroom.
Find out more on special ed classroom here: https://brainly.com/question/30029124
#SPJ1
which sector is targeted for the application of hydrogen fuel cells
Answers
The power sector is targeted for the application of hydrogen fuel cells.
What is Hydrogen fuel?This type of fuel produces electricity through the combination of hydrogen and oxygen atoms. The hydrogen reacts with oxygen across an electrochemical cell and produces electricity, water, and heat in a very small quantity.
Hydrogen-powered fuel cell electric vehicles doesn't have any carbon emission or greenhouse gases such as carbon dioxide etc which depletes the ozone layer and causes global warming.
It emit none of these harmful substance and only emits water and warm air which makes it a very clean fuel thereby causing a reduction in pollution and reducing the risk of respiratory diseases.
Read more about Hydrogen fuel here https://brainly.com/question/13603874
#SPJ1
How many valence electrons these elements have
Answers
Answer:
Any element in group 1 has just one valence electron. Examples include hydrogen (H), lithium (Li), and sodium (Na). Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons).
Explanation:
How many valence electrons does each element have?
The Group 1 atoms have 1 valence electron. The Group 2 atoms have 2 valence electrons. The Group 3 atoms have 3 valence electrons. The Group 4 atoms have 4 valence electrons.
I hope it's helpful
a cube of iron pyrite is 0.31 cm on each side and has a mass of 0.040g. what is the density of the sample?
Answers
The density of the iron pyrite cube is 1.343 g/cm³.
Given,
Side of iron pyrite cube = 0.31 cm
Mass of iron pyrite = 0.040 g
The volume of iron pyrite cube = s³ cm³
Or, volume = 0.029791 cm³
We have to find the density of the sample.
Density is defined as the mass per unit volume. Or, it is the ratio of mass to the volume of the substance.
Using the formula for density, we get,
Density = mass/volume
Or, density = 0.40/0.029791
Or, density = 1.343 g/cm³
Hence, the density of the iron pyrite cube is 1.343 g/cm³.
To learn more about density, visit: https://brainly.com/question/15164682
#SPJ9
prop-1-yne + 2HBr/H2O2 = A;
A + 2H2O = B;
B + K2CO3(aq) = C;
C + heat = D;
D + HBr = E.
find the compounds A, B, C, D and E
Answers
Based on the given reactions, the compounds are as follows:
A: The specific product formed from the reaction between prop-1-yne and either 2HBr or H2O2.
B: The product formed when compound A reacts with 2H2O.
C: The product formed when compound B reacts with K2CO3(aq).
D: The product formed from the heat-induced reaction of compound C.
E: The product formed when compound D reacts with HBr.
Based on the given reactions, let's analyze the compounds involved:
Reaction 1: prop-1-yne + 2HBr/H2O2 = A
The reactant prop-1-yne reacts with either 2HBr or H2O2 to form compound A. The specific product formed will depend on the reaction conditions.
Reaction 2: A + 2H2O = B
Compound A reacts with 2H2O (water) to form compound B.
Reaction 3: B + K2CO3(aq) = C
Compound B reacts with K2CO3(aq) (potassium carbonate dissolved in water) to form compound C.
Reaction 4: C + heat = D
Compound C undergoes a heat-induced reaction to form compound D.
Reaction 5: D + HBr = E
Compound D reacts with HBr (hydrobromic acid) to form compound E.
For more such questions on compounds
https://brainly.com/question/704297
#SPJ8
A student is researching how chemical reactions occur and how temperature impacts the rate of the reaction. She
measures how long it takes for 5 grams of calcite to dissolve in a strong solution of hydrochloric acid at different
temperatures. Her data is shown in the graph

Answers
Based on the data shown in the graph, the rate of reaction is directly proportional to the temperature of a reaction.
What is the rate of a reaction?The speed at which a chemical reaction occurs is called the reaction rate or rate of reaction. The rate of a reaction is proportional to the increase in product concentration per unit time and the decrease in reactant concentration per unit time.
The rate of a reaction is affected by the following:
the temperature of the reaction - the rate of reaction is directly proportional to the temperature of a reaction. Hence, the rate of a reaction increases with an increase in temperature.
presence of a catalyst - the rate of a reaction increases with the addition of a catalyst. A catalyst speeds up the rate of a reaction.
the surface area of the reactants - the rate of a reaction increases with an increase in the surface area of the reactants,
Learn more about the rate of reaction at: https://brainly.com/question/25724512
#SPJ1
Answer:
At higher temperatures, chemical reactions occur more quickly.
Explanation:
edmentum
Which treatment(s) will help remove contaminants from minerals or from the pipes carrying water from a source? you can select more than one (Water Contamination Gizmos) **ONLY ANSWER IF YOU ACTUALLY KNOW ❗️❗️**
answer choices:
Sedimentation
Disinfection
Filtration
Coagulation

Answers
Sedimentation, filtration, and coagulation are the treatments that will help remove contaminants from minerals or from the pipes carrying water from a source.
Sedimentation is a process in which suspended particles settle out of water. It is one of the most basic techniques for removing particles from water. As particles settle, they become trapped in the bottom of a container or settle to the ground in an outdoor setting
Filtration is a method of removing particles from a fluid. It is a physical or chemical separation method that separates solids from fluids (liquids or gases) by adding a medium through which only the fluid can pass.
Coagulation is the process of using chemicals to remove contaminants from water. By creating a chemical reaction, coagulation destabilizes particles and causes them to clump together. This helps to remove the contaminants from the water.
Disinfection is the process of eliminating or destroying pathogens that cause infection. Disinfection eliminates harmful microorganisms by destroying or inactivating them. The disinfectant is a chemical or physical agent that is used to destroy or inactivate harmful microorganisms.
Know more about Filtration here:
https://brainly.com/question/29756050
#SPJ8
why is the sun earth and moon system important
Answers
The Sun-Earth-Moon system is important because it sustains life on Earth, regulates Earth's climate, and influences natural phenomena like tides.
The Sun-Earth-Moon system plays a vital role in supporting and sustaining life on Earth. The Sun is the primary source of energy for our planet, providing heat and light necessary for photosynthesis, the process by which plants convert sunlight into food and oxygen. Sunlight is also crucial for maintaining Earth's temperature and driving weather patterns.
The Moon, as Earth's only natural satellite, contributes to several essential functions. Its gravitational pull creates the tides, which influence coastal ecosystems and shape coastal landscapes.
The Moon's orbit also stabilizes Earth's axial tilt, providing a stable climate for life to thrive. Additionally, the Moon's phases have cultural and historical significance, influencing human activities such as agriculture, navigation, and calendar systems.
The Sun-Earth-Moon system's interactions are responsible for natural phenomena like eclipses, both solar and lunar, which have fascinated humans throughout history and continue to be important for scientific study and exploration.
Understanding these celestial events enhances our knowledge of astrophysics and helps us comprehend the vastness and complexity of the universe.
Furthermore, the study of the Sun-Earth-Moon system provides insights into celestial mechanics, orbital dynamics, and the broader field of planetary science. By examining the interplay between these celestial bodies, scientists can gain a deeper understanding of Earth's place in the universe and explore potential habitable conditions on other celestial bodies.
Overall, the Sun-Earth-Moon system is of immense importance as it sustains life, regulates climate, influences natural phenomena, and provides a platform for scientific exploration and discovery.
For more question on climate visit:
https://brainly.com/question/12801279
#SPJ8
What are the noble gas configurations for
-Li
-Na
-K
Answers
The noble gas configurations for
Li= [He] 2s¹, Na=[Ne] 3s¹ and K =[Ar] 4s¹.
Noble gas configurations are the term for the electronic configurations of the atoms for which the electronic configuration becomes very larger i.e, for the higher atomic numbers.
For the noble gas electronic configurations, the noble gas, which is before that atom is written.
So, the Lithium can be represented as [He] 2s¹.
So, the Sodium can be represented as [Ne] 3s¹.
So, the Potassium can be represented as [Ar] 4s¹.
Hence, the noble gas configurations for Lithium= [He] 2s¹, Sodium=[Ne] 3s¹ and potassium =[Ar] 4s¹.
Learn more about electronic configurations here:
https://brainly.com/question/14267765
#SPJ10
what is stem distillation?how is it done?
Answers
Answer:
Steam distillation is a separation technique that harnesses the low boiling point property of immiscible mixtures.
Steam distillation is a separation technique that harnesses the low boiling point property of immiscible mixtures.it is done by passing dry steam through the plant material whereby the steam volatile compounds are volatilized, condensed and collected in receivers.
Write and balance the following equation:
zinc (II) nitrate + copper
→ copper (1) nitrate + zinc
Answers
Answer:
This reaction will not occur since copper is less reactive than zinc.
Explanation:
Since copper is located at a lower position in the reactivity series than zinc, there will be no displacement reaction if copper is put into zinc (II) nitrate solution.
what happens to a circuits resistance (R), voltage (V), and current (I) when you increase the diameter of the wire in the circuit?
a. R increases, V is constant, I increases
b. R decreases, V is constant, I increases
c. R decreases, V increases, I increases
d. R increases, V decreases, I decreases
Answers
Answer:
b. R decreases, V is constant, I increases
Explanation:
when we increase the diameter of wire increases ,resistance decreases and current increases.Therefore, the option is b.
Resistance is an electrical quantity which opposes the electric current in the circuit. Resistance is directly proportional to the length of the wire and inversely proportional to the diameter of the wire. If length of wire increases resistance will increases and if the diameter of the wire increase resistance will decreases.
R = ρ L/A
Here ρ is the resistivity
L is the length of the wire
A is the cross-sectional area of wire/diameter of the wire
Voltage is the potential difference between two terminals of voltage source. Current is the flow of electrons in the circuit. Voltage is the product of current and resistance.
V=IR
Rewrite the above equation interms of current
I=V/R
From the above equation we can say that current is inversly proportional to the resistance.Therefore,the correct option is b.
Learn more about,resistance
https://brainly.com/question/4289257
Rank the following compounds in order of decreasing acid strength using periodic trends.Rank the acids from strongest to weakest. To rank items as equivalent, overlap them.
HI, HBr, MgH2, H2Se.
Answers
Explanation:
H2Se, HBr, MgH2, Hl
Because H2Se is stronger than Hl..
Which is NOT correct for when the silver and vanadium half-cells are connected via a salt bridge and a potentiometer
Answers
The question is incomplete, the complete question is
Which is NOT correct for when the silver and vanadium half-cells are connected via a salt bridge and a potentiometer? Ag^+ + 1 e^- rightarrow Ag Edegree = 0.7993 V V^2+ + 2e^- right arrow V E degree =-1.125 V Ag+ is reduced V is oxidized 1.924 V V2^+ is reduced Ag is oxidized I and II III, IV, and V I, II, and III III only IV and V
Answer:
only IV and V
Explanation:
If we look at the values of reduction potential for the two species, we will discover that vanadium has a negative reduction potential indicating its tendency towards oxidation.
On the other hand, solve has a positive reduction potential indicating a tendency towards reduction.
This implies that vanadium must be oxidized and silver reduced and not the not her way ground? Hence the answer above.
PLEASE HELP!!
ATTACHMENT BELOW

Answers
Answer:
read the story
Explanation:
read it and then you will find the answer
If a car has an EPA mileage rating of 30 miles per gallon, what is this rating in
kilometers per liter? (1 L = 1.06 qt)
Answers
The rating in kilometers per liter is 12.75 kilometer per liter
How to convert 30 miles per gallon to kilometer per gallon1 mile per gallon = 1.60934 Kilometer per gallon
Therefore,
30 miles per gallon = 30 × 1.60934
30 miles per gallon = 48.2802 kilometer per gallon
How to convert 48.2802 kilometer per gallon to kilometer per liter1 kilometer per gallon = 0.264172 kilometer per liter
Therefore,
48.2802 kilometer per gallon = 48.2802 × 0.264172
48.2802 kilometer per gallon = 12.75 kilometer per liter
Thus, the rating in kilometer per liter is 12.75 kilometer per liter
Learn more about conversion:
https://brainly.com/question/1624880
#SPJ1
A 5.4 g sample of a metal is heated to 100.0 °C and is placed in a beaker containing 142 g of water at 24.2 °C. The final temperature of the water is 25.1 °C. What is the specific heat of the metal?
Answers
Answer:
We can use the principle of conservation of energy to solve this problem. The heat lost by the metal is equal to the heat gained by the water:
Q metal = -Q water
where Q metal is the heat lost by the metal, and Q water is the heat gained by the water.
The heat lost by the metal can be calculated using the formula:
Q metal = m metal * c metal * ΔT metal
where m metal is the mass of the metal, c metal is its specific heat, and ΔT metal is the change in temperature of the metal.
The heat gained by the water can be calculated using the formula:
Q water = m water * c water * ΔT water
where m water is the mass of the water, c water is its specific heat, and ΔT water is the change in temperature of the water.
We know the values of all the variables except c metal, so we can solve for it. We can start by calculating the values of Q metal and Q water:
Q metal = -Q water
m metal * c metal * ΔT metal = -m water * c water * ΔT water
Substituting the given values, we get:
5.4 g * c metal * (100.0 °C - T) = -142 g * 4.18 J/(g*°C) * (T - 24.2 °C)
Simplifying and solving for c metal, we get:
c metal = [142 g * 4.18 J/(g*°C) * (T - 24.2 °C)] / [5.4 g * (100.0 °C - T)]
Multiplying out, we get:
c metal = [593.56 J/(°C) * (T - 24.2 °C)] / [5.4 g * (100.0 °C - T)]
To solve for c metal, we need to find the value of T that satisfies the equation. We can do this by substituting the given value of ΔT water = 0.9 °C into the equation and solving for T:
c metal = [593.56 J/(°C) * (T - 24.2 °C)] / [5.4 g * (100.0 °C - T)]
c metal = [593.56 J/(°C) * (T - 24.2 °C)] / [540 g - 5.4 g * T]
0.9 g * [593.56 J/(°C) * (T - 24.2 °C)] = [540 g - 5.4 g * T] * c metal
535.2044 J/(°C) * (T - 24.2 °C) = 540 g * c metal - 5.4 g * T * c metal
535.2044 J/(°C) * T - 12931.7808 J = 540 g * c metal - 5.4 g * c metal * T
5.4 g * c metal * T + 535.2044 J/(°C) * T = 540 g * c metal + 12931.7808 J
T * (5.4 g * c metal + 535.2044 J/(°C)) = 540 g * c metal + 12931.7808 J
T = [540 g * c metal + 12931.7808 J] / [5.4 g * c metal + 535.2044 J/(°C)]
Substituting the given values, we get:
T = [540 g * c metal + 12931.7808 J] / [5.4 g * c metal + 535.2044 J/(°C)]
T = [540 g * c metal + 12931.7808 J] / [5.4 g * c metal + 535.2044 J/(°C)]
T ≈ 23.3 °C
Therefore, the specific heat of the metal is:
c metal = [142 g * 4.18 J/(g°C) * (T - 24.2 °C)] / [5.4 g * (100.0 °C - T)]
c metal ≈ 0.39 J/(g°C)
So the specific heat of the metal is approximately 0.39 J/(g*°C).
A 5.4 g sample of the metal is heated to the 100.0 °C and is placed in the beaker containing 142 g of the water at 24.2 °C. The specific heat of the metal is 1.322 J/ g °C.
The mass of the metal = 5.4 g
The final temperature = 25.1 °C
The initial temperature = 100 °C
The specific heat capacity of metal = x
The mass of the water = 142 g
The final temperature = 25.1 °C
The initial temperature = 24.2 °C
The specific heat capacity of water = 4.184 J/ g °C
Loss of Heat of Metal = Gain of Heat by Water
-q metal = + q metal
- 5.4 × x × ( 25.1 - 100 ) = 142 × 4.184 ( 25.1 - 24.2 )
404.46 x = 534.71
x = 1.322 J/ g °C
The specific heat capacity of metal is 1.322 J/ g °C.
To learn more about heat capacity here
https://brainly.com/question/21115514
#SPJ1
The type of reactions that alkanes undergo is A) electrophilic substitution reactions. B) electrophilic addition reactions. C) free radical substitution reactions. D) free radical addition reactions. E) nucleophilic substitution reactions.
Answers
Answer:
FREE RADICAL SUBSTITUTION REACTIONS
Explanation:
Alkanes undergo substitution reactions with halogens in the presence of ultra-violent light. In substitution reaction, an atom is exchanged with another atom. For exmaple, methane, an alkane reacts with bromine or chlorine( halogens) to form methylbromine or methylchlorine and hydrogen bromide or hydrogen chloride.
This type of substitution reaction is a free radical substitution reaction. In free radical substitution reaction, free radicals are the reactive intermediates and involves two or more steps as the case may be.
The initiation step where the free radicals are produced by homolysis either by heat or ultra-violent ray of light. The free radicals produced are exchanged at the termination step as the produced free radicals recombines with another radical species of the reacting substances.
Methane + Bromine --------> Methylbromine + Hydrogen Bromide
CH4 + Br2 → CH3Br + HBr
You can see there is an exchange of a bromine atom with one hydrogen of methane that is one hydrogen atom in methane is replaced by a bromine atom.
How many milliliters of 0.050 M EDTA are required to react with 50.0 mL of 0.010 M Ca2+? With 50.0 mL 0.010 M AI3*?
Answers
10 milliliters of 0.050 M EDTA are required to react with both 50.0 mL of 0.010 M Ca2+ and 50.0 mL of 0.010 M Al3+.
The balanced equation for the reaction between EDTA and metal ions is as follows:
Ca2+ (aq) + EDTA (aq) → CaEDTA (complex)
Al3+ (aq) + EDTA (aq) → AlEDTA (complex)
Moles of Ca2+:
moles of Ca2+ = concentration of Ca2+ x volume of Ca2+ solution
moles of Ca2+ = 0.010 M x (50.0 mL / 1000 mL)
moles of Ca2+ = 0.010 M x 0.050 L
moles of Ca2+ = 0.0005 moles
Moles of Al3+:
moles of Al3+ = concentration of Al3+ x volume of Al3+ solution
moles of Al3+ = 0.010 M x (50.0 mL / 1000 mL)
moles of Al3+ = 0.010 M x 0.050 L
moles of Al3+ = 0.0005 moles
The stoichiometry of the reaction tells us that 1 mole of Ca2+ or Al3+ reacts with 1 mole of EDTA. Therefore, the moles of EDTA required are also 0.0005 moles.
Volume of 0.050 M EDTA:
moles of EDTA = concentration of EDTA x volume of EDTA solution
0.0005 moles = 0.050 M x volume of EDTA solution
volume of EDTA solution = 0.0005 moles / 0.050 M
volume of EDTA solution = 0.01 L = 10 mL
For more such questions on milliliters
https://brainly.com/question/19755302
#SPJ11