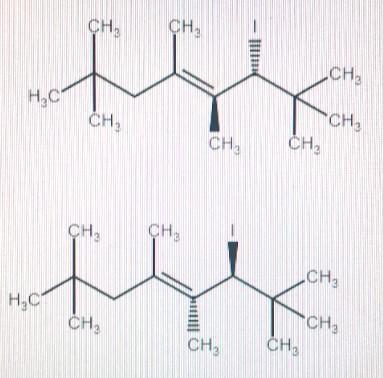
predict the major organic product formed upon treatment of the following diene with hydroiodic acid under cold conditions. be sure your answer accounts for regiochemistry and stereochemistry, where appropriate.
Answers
Two major organic products will be formed which would be enantiomers of each other.
when hydrogen halide is added to an alkene at low temperature then 1,2- addition product is formed and at higher temperature 1,4-addition product is formed.
The hydrogen-halogen link must be broken for the hydrogen halides to react with alkenes. The hydrogen-fluorine bond is especially strong, and the bond strength decreases as you move from HF to HI. The addition of HF is destined to be gradual because it is challenging to dissolve the link between the hydrogen and fluorine.
The given equation proceeds at lower temperature 1,2 addition product is formed.
Learn more about enantiomers:
brainly.com/question/6249935
#SPJ4
Related Questions
What do cells provide?
Answers
Answer:
cells are what make up they body they produce lots of things to keep you alive.
Explanation:
They provide structure for the body, take in nutrients from food, convert those nutrients into energy, and carry out specialized functions. Cells also contain the body's hereditary material and can make copies of themselves
Determine the molar solubility of BaF 2 in pure water. K sp for BaF 2 = 2.45 × 10^ -5.
1.83 × 10-2 M
1.23 × 10-5 M
6.13 × 10-6 M
2.90 × 10-2 M
4.95 × 10-3 M
Answers
The molar solubility of BaF2 in pure water is 6.13 × \(10^{-6}\)M. The correct answer is option C.
What is Solubility?
Solubility is the property of a substance to dissolve in a solvent (usually a liquid) to form a homogeneous solution. The amount of solute that can dissolve in a given amount of solvent at a given temperature and pressure is known as the solubility of the substance.
The solubility product expression for BaF2 is:
Ksp = [Ba2+][F-]2
Let the molar solubility of BaF2 be x. Then at equilibrium, we have:
[Ba2+] = x
[F-] = 2x (because there are two moles of F- ions for each mole of BaF2 that dissolves)
Substituting these values into the Ksp expression gives:
Ksp = x * (2x)2 = 4x3
Solving for x:
x = (Ksp/4)^(1/3) = (2.45 × 10\(10^{-5}\) / 4)^(1/3) = 6.13 × \(10^{-6}\) M
Learn more about Solubility from the given link
https://brainly.com/question/9098308
#SPJ4
Enzymes are able to act on any molecules they come in contact with . True or false
Answers
Answer:
Explanation:
True
which of the followings correctly order the organization in plants and animals from most complex to least complex?
A.cells,tissues,organs,organ systems,organisms
B.tissues,organs,organisms,organ systems,cells
C.organisms,organs,organ systems,tissues,cells
D.organisms,organ systems,tissues,cells
Answers
Answer:
the answer is C
Hope this will help you ❤️
in which compound does oxygen have an oxidation number other than −2? select the correct answer below: co2 h2o h3po4 h2o2
Answers
Out of the compounds listed below, the compound in which oxygen has an oxidation number other than −2 is H₂O₂. The correct option is (d)H₂O₂.
Oxidation number can be described as the number that is given to an atom of an element when it combines with other atoms. It is assigned to an atom of an element in a particular compound, which shows its ability to either donate or accept electrons when it reacts with other atoms.
The oxidation number of an atom in a molecule indicates the electron sharing that occurs in chemical bonds. The general rules for determining the oxidation number of an atom are: In an uncombined or elemental state, atoms have an oxidation number of 0.
Ions' oxidation numbers are the same as their charges. Oxygen in most of the compounds has an oxidation state of -2 except in peroxides, where it has an oxidation state of -1. In H₂O₂, the oxygen atoms have an oxidation number of -1. Hence, H₂O₂ is the only compound from the given options where oxygen has an oxidation number other than −2. Hence, d is the correct option.
You can learn more about oxidation numbers at: brainly.com/question/29100691
#SPJ11
A cart has a mass of 35kg and a speed of 1.2m/s. What is its kinetic energy
Answers
Answer:
The answer is 25.2 JExplanation:
The kinetic energy of an object can be found by using the formula
\(KE = \frac{1}{2} m {v}^{2} \\ \)
where
m is the mass
v is the velocity
From the question we have
\(KE = \frac{1}{2} \times 35 \times {1.2}^{2} \\ = 17.5 \times 1.44 \)
We have the final answer as
25.2 JHope this helps you
Answer:
25.2 J
Explanation:
Using the kinetic energy formula:
KE = \(\frac{1}{2}\)m v²
Plug in the given values:
KE = \(\frac{1}{2}\) (35 kg) · (1.2 m/s)²
KE = \(\frac{1}{2}\) (35) · 1.44
KE = 17.5 · 1.44
KE = 25.2 J
There is a kinetic energy of 25.2 Joules.
water has density of 1.0 g/L. What volume of water has mass 250 g?
Answers
Answer:
Examine the units: If the density is 1 g/mL, how many mL do you need to make 250 g?
X mL * 1 g/mL = 250 g
Solve for X
x mL = 250 g / 1 g/mL = 250 mL
Hope this helps :)
The volume of water = 250 L
The formula for density:
\(density = \frac{mass}{volume}\)
Given, density = 1.0 g/L = 0.001 kg/L
mass = 250 g = 0.25 kg
Putting the values in the equation
\(0.001 = \frac{0.25}{volume}\)
\(volume = \frac{0.25}{0.001}\)
\(volume = 250 L\)
Hence, the volume of the water is 250 L.
Learn more about density here:
https://brainly.com/question/15164682
#SPJ2
When reacted with elemental chlorine at room temperature, liquid phosphorous trichloride (PCl3) is converted to solid phosphorous pentachloride (PCl5).
Answers
PCl₅ reacts upon contact with water to launch hydrogen chloride and supply phosphorus oxides. the primary hydrolysis product is phosphorus oxychloride PCl₅ + H₂O ========> POCl₃ + 2HCl.
Phosphorus trichloride appears as a colorless or slightly yellow fuming liquid with a pungent and irritating odor resembling that of hydrochloric acid. Causes severe burns to skin, eyes and mucous membranes.
Divide the mass of the material through its molar mass. The molar mass of a substance is the mass in grams of 1 mole of that substance. This mass is given via the atomic weight of the chemical unit that makes up that substance in atomic mass units.
One mole is described as the amount of substance containing as many number one entities atoms, molecules, ions, electrons, radicals, and lots of others. As there are atoms in 12 grams of carbon - 12(6. 023×10²³. The mass of one mole of a substance equals to its relative molecular mass expressed in grams.
Learn more about phosphorous trichloride here:-https://brainly.com/question/2626850
#SPJ4
What is the [H] in a solution with a pH of 9.92
Answers
Answer:
the solution is acidic as its ph has already exceeded the neutral level
\(1.1 X 10^-9 M\) is the [H] in a solution with a pH of 9.92.
What is pH?pH is the quantitative measure of the acidity or basicity of aqueous or other liquid solutions.
The pH of a solution is usually found by the following expression:
pH=-log [\(H^+\)]
Therefore, to find the concentration of \(H^+\) we can rearrange this expression and thus,
= [\(H^+\)] = \(10^-^{pH}\)
= [\(H^+\)] = \(10^-^{9.92}\)
=\(1.1 X 10^-9 M\)
Learn more about pH here:
https://brainly.com/question/27359971
#SPJ2
ANSWER ASAP!
How does a combustion reaction form?
Answers
Answer:
A combustion reaction is when a fuel, usually a hydrocarbon, reacts with excess oxygen to form carbon dioxide gas and water vapor.
Explanation:
-intervención de los neurotransmisores de la felicidad y de la tristeza en los trastornos de la bipolaridad. Como estimular los neurotransmisores de la felicidad en nuestro cuerpo.
doy 5 estrellas
Answers
Answer: Desarrollar y ceñirse a un horario diario puede ayudar a estabilizar los cambios de humor del trastorno bipolar. Incluya horarios establecidos para dormir, comer, socializar, hacer ejercicio, trabajar y relajarse. Trate de mantener un patrón regular de actividad incluso durante los altibajos emocionales.
Explanation:
6. A sealed flask filled with an ideal gas is moved from an ice bath into a hot water bath. The initial temperature is 273K and
the final temperature is 350 K. The initial pressure is 100kPa. The volume does not change. What is the final pressure of the flask? Name the gas law.
Answers
Answer:
Explanation:
Since the volume of the gas does not change, we can use the Gay-Lussac's law (also known as Pressure-Temperature law), which states that the pressure of an ideal gas is directly proportional to its absolute temperature when the volume is kept constant. Mathematically, this can be expressed as:
P1/T1 = P2/T2
where P1 and T1 are the initial pressure and temperature, respectively, and P2 and T2 are the final pressure and temperature, respectively.
Substituting the given values in the above equation, we get:
P2 = (P1/T1) × T2
= (100 kPa/273 K) × 350 K
= 128.83 kPa (approx.)
Therefore, the final pressure of the flask is approximately 128.83 kPa.
What is the molar mass (g/mol) of Thorium (V) Nitrate?
Answers
Answer:
480.06 g/mol, Thorium nitrate.
Explanation:
If 9000. J of heat are absorbed by 800. g of water at 25.0oC, what will be its final temperature?
Answers
Answer:
22 626 000 J. = 22 626 kJ. 4. If 9000 J of heat are absorbed by 800 g of water at 5.0 o. C, what maximum temperature will the water attain? Q = mc ∆T.
Explanation:
The final temperature can be calculated using the calorimetric equation. Here, the final temperature of water is 27.71 °C.
What is calorimetric equation ?The calorimetric equation connecting the heat energy absorbed or released q by a system with the mass m, temperature difference ΔT and the specific heat capacity c as follows:
q = m c ΔT
The specific heat capacity of water = 4.15 J/ °C g.
given that heat energy absorbed q = 9000 J
mass = 800 g
initial temperature = 25 °C.
Then,
9000 J = 4.15 J/ °C g. × 800 g (25 °C + T)
then T = [ 9000 J/ 4.15 J/ °C g. × 800 g ] + 25 °C = 27.71 °C.
Therefore, the final temperature of water is 27.71 °C.
Find more on calorimetry:
https://brainly.com/question/30884598
#SPJ2
When the ph of a solution decreases from 4. 0 to 2. 0, how does the concentration of h3o+ change?.
Answers
Hydronium ion concentration increases by 100 fold on decreasing the pH of solution from 4 to 2.
In order to compute pH, the log of the hydronium ion concentration was used.
pH = -log [Hydronium ion concentration]
So, at pH 2.0 number of hydronium ions are 10^-2
And at pH 4.0 number of hydronium ions are 10^-4
By decreasing pH there will be 100 times more hydronium ions.
To know more about hydronium ions, click here
brainly.com/question/12047300
#SPJ4
A metal cube is 2.76g/cm3 and 43.7g.
What is the volume of the cube?
Answers
The volume of the metal cube that has a mass of 43.7g and a density of 2.76g/cm³ is 15.83cm³.
How to calculate volume?The volume of a substance can be calculated by dividing the mass of the substance by its density. That is;
Volume = mass ÷ density
According to this question, a metal cube has a mass of 43.7g and a density of 2.76g/cm³. The volume cab be calculated as follows:
Volume = 43.7g ÷ 2.76g/cm³
Volume = 15.83cm³
Therefore, the volume of the metal cube that has a mass of 43.7g and a density of 2.76g/cm³ is 15.83cm³.
Learn more about volume at: https://brainly.com/question/1578538
#SPJ1
what are the trends of group 1 metals on the periodic table as the metals go down?
Answers
Answer:
The reactivity of group 1 elements increases as you go down the group because: the atoms become larger. the outer electron becomes further from the nucleus. the force of attraction between the nucleus and the outer electron decreases.
Explanation:
plz if this helped name me brainliest
-
PLEASE HELP ME ASAPPP PLS
31. Which compound contains an alkaline earth metal and a halogen?
Cas
RbC1
b. Rhys
d. CaCl,
a.
c.
Answers
The compound which contains an alkaline earth metal and a halogen is CaCl₂.
What is a compound ?Compound is defined as a chemical substance made up of identical molecules containing atoms from more than one type of chemical element.
Molecule consisting atoms of only one element is not called compound.It is transformed into new substances during chemical reactions. There are four major types of compounds depending on chemical bonding present in them.They are:
1)Molecular compounds where in atoms are joined by covalent bonds.
2) ionic compounds where atoms are joined by ionic bond.
3)Inter-metallic compounds where atoms are held by metallic bonds
4) co-ordination complexes where atoms are held by co-ordinate bonds.
They have a unique chemical structure held together by chemical bonds Compounds have different properties as those of elements because when a compound is formed the properties of the substance are totally altered.
Learn more about compound,here:
https://brainly.com/question/13516179
#SPJ6
For the equation SnO2 + 2H2 Sn + 2H2O, tin (IV) oxide reacts with excess hydrogen to produce tin and water. What is the limiting reactant?
A) SnO2
B) H2
C) Sn
D) H2O
Answers
Answer:
SnO2
Explanation:
The limiting reactant is the reactant that runs out first during a chemical reaction.
Reactants are on the left side of the reaction, so SnO2 and H2. They tell us in the question that there is "excess" hydrogen (H2), so we know there is plenty of it, as "excess" means "more than enough." If we have more than enough hydrogen, then we know that we will run out of SnO2 first.
:)
Answer:SnO2
Explanation:
True or False. A producer is a heterotrophic organism that consumes other organisms for nutrients and energy.
Answers
Answer:
True.
Explanation:
a phase change that occurs at the normal melting point by the addition of very small amounts of heat at constant temperature and pressure will have
Answers
The phase change that occurs at the normal melting point by the addition of very small amounts of heat at constant temperature and pressure is called a "fusion" or "melting" transition.
Fusion is the transition of a solid into a liquid state. It is usually accompanied by an increase in temperature and an increase in pressure.
Fusion occurs when energy is added to a solid and the intermolecular forces are overcome, allowing the solid particles to move freely. The temperature of the solid at which fusion occurs is called the melting point. It is the same as the normal freezing point, but with the addition of heat.
When fusion occurs, the solid particles begin to move more freely and the solid take on a liquid form. The solid particles remain in contact with each other, but they can now move freely. The solid particles are now able to move past each other, allowing the solid to take the shape of the container in which it is held.
In summary,
Fusion is the phase change that occurs at the normal melting point by the addition of very small amounts of heat at constant temperature and pressure. The energy added causes the intermolecular forces to be overcome, allowing the solid particles to move freely and form a liquid.
To know more about Fusion transition, refer here:
https://brainly.com/question/29853732#
#SPJ11
What happens in stage one of photosynthesis
Answers
Answer:
Photosynthesis occurs in two stages. During the first stage, the energy from sunlight is absorbed by the chloroplast. Water is used, and oxygen is produced during this part of the process.
Explanation:
Answer:
Chloroplast absorbs the energy emitted by the Sun.
_____________________________________________________
Context:
Photosynthesis:
The complex process by which carbon dioxide, water, and certain inorganic salts are converted into carbohydrates by green plants, algae, and certain bacteria, using energy from the sun and chlorophyll.
Chloroplast:
A plastid containing chlorophyll.
Chlorophyll:
The green coloring matter of leaves and plants, essentially to the production of carbohydrates by photosynthesis, and occurring in a blush-black form, C55J72MgN4O5 (chlorophyll a), and a dark-green form, C55H70MgtN4O6 (chlorophyll b).
Sun:
The star that is the central body of the solar system, around which the planets revolve and from which they receive light and heat: its mean distance form earth is about 93 million miles (150 million km), its diameter about 864,000 miles (1.4 million km), and it's mass about 330,000 times that of earth; its period of surface rotation is about 26 days at its equator but longer at higher latitudes.
Explanation:
There are two main phases in photosynthesis:
depending on light stage
Clause Cycle (AKA the dark stage or the light-independent stage)
In the light-dependent stage, ATP and NADPH are produced using photons, or light energy. Oxygen molecules start to form during this stage as a byproduct.
Utilizing the materials produced during the light-dependent stage, the Calvin cycle fixes CO2 molecules to produce carbohydrates (sugars). This stage can repeat itself indefinitely since RuBP, the initial chemical required for the stage to go further, also serves as its byproduct.
if 6 moles of a a compound produce 84 J of energy, what is the h reaction in j/mol
Answers
The enthalpy of the reaction is 14 J/mol.
The enthalpy of a reaction (ΔH) is the amount of energy transferred between a system and its surroundings during a chemical reaction at constant pressure, measured in joules per mole (J/mol). This value is important because it can tell us whether a reaction is exothermic or endothermic, as well as give us information about the strength of chemical bonds within the reactants and products.To calculate the enthalpy of a reaction, we need to know the amount of energy released or absorbed (Q) and the number of moles of the compound involved in the reaction (n). We can use the equation:
ΔH = Q/n
Given that 6 moles of a compound produce 84 J of energy, we can calculate the enthalpy of the reaction as follows:
ΔH = Q/n
ΔH = 84 J / 6 mol
ΔH = 14 J/mol
This means that for every mole of the compound involved in the reaction, 14 J of energy is transferred between the system and the surroundings. Since the value is positive, we can conclude that the reaction is endothermic, meaning that it requires an input of energy to occur.It is worth noting that the enthalpy of a reaction can depend on a number of factors, such as temperature, pressure, and the specific conditions under which the reaction occurs. As such, it is important to take these factors into account when calculating or predicting enthalpy values.
for such more questions on enthalpy
https://brainly.com/question/14047927
#SPJ8
a sealed flask has 0.541 atm of so3 at 1000k. the following equilibrium is established: 2so3 (g) -> 2so2 (g) o2 (g). at equilibrium, the partial pressure of oxygen is measured to be 0.216 atm. calculate k for the decomposition of so3 at 1000k
Answers
The equilibrium constant (K) for the decomposition of SO₃ at 1000 K is 0.00943.
Use the partial pressures of the gases involved.
The balanced equation for the reaction is:
2SO₃(g) ⇌ 2SO₂(g) + O₂(g)
According to the information given, the initial pressure of SO₃ is 0.541 atm, and the partial pressure of O₂ at equilibrium is 0.216 atm.
Use the equation for Kp (equilibrium constant in terms of partial pressures) to calculate K:
Kp = (P(SO₂)² × P(O₂)) / (P(SO₃)²)
Here, P(SO₂) is the partial pressure of SO₂, P(O₂) is the partial pressure of O₂, and P(SO₃) is the initial partial pressure of SO₃.
Since the stoichiometric coefficient of SO₂ is 2, divide the partial pressure of SO₂ by 2.
Let's plug in the values:
Kp = ((P(SO₂) / 2)² × P(O₂)) / (P(SO₃)²)
Kp = ((0.216 / 2)² × 0.216) / (0.541²)
Kp = (0.108² × 0.216) / (0.541²)
Kp = 0.002764112 / 0.293281
Kp ≈ 0.00943
Therefore, the equilibrium constant (K) for the decomposition of SO₃ at 1000 K is 0.00943.
To learn more about equilibrium constant, follow the link:
https://brainly.com/question/29809185
#SPJ4
Help please!!! need done fast! (with pic this time)

Answers
Considering the definition of pH, the pH values obtained are:
pH= - log (1×10⁻¹³ M)= 13 → BasepH= - log (1×10⁻⁹ M)= 9 → BasepH= - log (1×10⁻² M)= 2 → AcidpH= - log (1×10⁻⁷ M)= 7 → NeutralpH= - log (1×10⁻⁵ M)= 5 → AcidDefinition of pHpH is the Hydrogen Potential and indicates the amount of hydrogen ions present in a solution or substance.
Mathematically, pH is calculated as the negative base 10 logarithm of the activity of hydrogen ions:
pH= - log [H⁺]
The numerical scale that measures the pH of substances includes the numbers from 0 to 14. The pH value 7 corresponds to neutral substances. Acidic substances are those with a pH lower than 7, while basic substances have a pH higher than 7.
pH in this caseIn this case, you know:
[H⁺]= 1×10⁻¹³ M[H⁺]= 1×10⁻⁹ M[H⁺]= 1×10⁻² M[H⁺]= 1×10⁻⁷ M[H⁺]= 1×10⁻⁵ MReplacing in the definition of pH and taking into account its numerical scale:
pH= - log (1×10⁻¹³ M)= 13 → BasepH= - log (1×10⁻⁹ M)= 9 → BasepH= - log (1×10⁻² M)= 2 → AcidpH= - log (1×10⁻⁷ M)= 7 → NeutralpH= - log (1×10⁻⁵ M)= 5 → AcidLearn more about pH:
brainly.com/question/3992824
#SPJ1
Which chemical property of alkanes is responsible for generating electricity in diesel
power stations or for moving motor vehicles?
Answers
Alkanes' comparatively low reactivity, which is a chemical property, is what allows diesel to produce energy. Strong acids, bases, oxidising, or reducing agents do not react with alkanes (reductants). Alkanes can be used as fuels because they burn quickly when exposed to oxygen and release energy.
What is Chemical property?
Any quality that can only be formed by altering a substance's chemical identity is referred to as a chemical property, and it can occur during or after a chemical reaction. Simply put, it is impossible to detect a substance's chemical properties by looking at it or feeling it; instead, a substance's internal structure needs to be significantly altered before its chemical qualities can be examined. A substance's properties will alter significantly as a result of a chemical reaction, leading to chemical change. A catalytic property, on the other hand, would also be a chemical property.
To learn more about Chemical Property
https://brainly.com/question/28308645
#SPJ9
At 400K, Kc = 7.0. If 0.30 mol of Br2 and 0.30 mol Cl2 are introduced into a 1.0 L at 400 K, what will the equilibrium concentrations be for Br2, Cl2, and BrCl?
Answers
At 400K, Kc = 7.0. If 0.30 mol of Br2 and 0.30 mol Cl2 are introduced into a 1.0 L at 400 K, the equilibrium concentrations will be approximately:
[Br₂] = 0 mol
[Cl₂] = 0 mol
[BrCl] = 0.794 mol
The balanced chemical equation for the reaction is: Br₂ + Cl₂ ⇌ 2BrCl
Let's denote the initial concentration of Br₂ as [Br₂]₀, Cl₂ as [Cl₂]₀, and BrCl as [BrCl]₀.
The change in concentration for each species will be denoted as Δx.
At equilibrium, the concentrations will be given by:[Br₂] = [Br₂]₀ - Δx
[Cl₂] = [Cl₂]₀ - Δx
[BrCl] = 2Δx
Using the given information and the equilibrium constant expression, we can set up an equation:
Kc = [BrCl]² / ([Br₂] * [Cl])
Substituting the values into the equation:
7.0 = (2Δx)² / (([Br₂]₀ - Δx) * ([Cl₂]₀ - Δx))
Now, we can solve this equation to find the value of Δx.
However, since Δx is small compared to the initial concentrations, we can approximate ([Br₂]₀ - Δx) and ([Cl₂]₀ - Δx) to their initial concentrations.
7.0 ≈ (2Δx)² / ([Br₂]₀ * [Cl₂]₀)
Rearranging the equation: (2Δx)² ≈ 7.0 * ([Br₂]₀ * [Cl₂]₀)
4Δx² ≈ 7.0 * ([Br₂]₀ * [Cl₂]₀)
Δx² ≈ (7.0 * ([Br₂]₀ * [Cl₂]₀)) / 4
Δx ≈ √((7.0 * ([Br₂]₀ * [Cl₂]₀)) / 4)
Now, let's substitute the given values into the equation: [Br2]₀ = 0.30 mol
[Cl2]₀ = 0.30 mol
Δx ≈ √((7.0 * (0.30 mol * 0.30 mol)) / 4)
Δx ≈ √((7.0 * 0.09 mol²) / 4)
Δx ≈ √(0.63 mol² / 4)
Δx ≈ √(0.1575 mol²)
Δx ≈ 0.397 mol
Now, we can calculate the equilibrium concentrations:
[Br₂] = [Br₂]₀ - Δx = 0.30 mol - 0.397 mol ≈ -0.097 mol (approximately 0 mol, as the concentration cannot be negative)[Cl₂] = [Cl₂]₀ - Δx = 0.30 mol - 0.397 mol ≈ -0.097 mol (approximately 0 mol, as the concentration cannot be negative)[BrCl] = 2Δx = 2 * 0.397 mol ≈ 0.794 mol
Therefore, the equilibrium concentrations will be approximately:
[Br₂] = 0 mol
[Cl₂] = 0 mol
[BrCl] = 0.794 mol
For more questions on equilibrium concentrations: https://brainly.com/question/14696053
#SPJ11
The chemical isoamyl acetate C7H14O2 gives the odor of pears. What is the percent oxygen in isoamyl acetate?
Answers
Answer:
\(\% O=24.6\%\)
Explanation:
Hello!
In this case, since the by-mass percent of an element in a compound is computed by taking into account its atomic mass, the number of atoms in the molecules and the molar mass of the compound, we can write:
\(\% O=\frac{2*16.00}{130.18 } *100\%\\\\\% O=24.6\%\)
Best regards!
Why is Newton's first law of motion is sometimes called the law of inertia
Answers
Answer:
The reason why Newtons first law of motion is sometimes called the law of inertia is because it states that if the object is in motion, it will not rest unless an unbalanced force acts on the object.
1. what type of polymer would you obtain if sorbital (a sugar alcohol found in sugar free gum) was used as a plasticizer addictive?
2a. Starch-borate and starch-glycerol polymers have been used for encapsulation of pharmaceutical drugs or pesticides. Explain what effect ths might have and why it would be beneficial.
2b. Are these polymers considered to be biodegradable? why or why not?
Answers
1. The type of polymer that would you obtain if sorbitol is a polyol.
2a. The use of starch-borate and starch-glycerol polymers for the encapsulation of pharmaceutical drugs or pesticides would have a number of beneficial effects.
2b. Yes, these polymers are considered to be biodegradable.
1. The type of polymer that would you obtain if sorbitol (a sugar alcohol found in sugar-free gum) was used as a plasticizer additive is a polyol. This is because sorbitol is a polyol, which is a substance used to modify the properties of polymers. The process of polymer modification involves adding polyols to the polymer matrix, which helps to reduce the glass transition temperature of the polymer. Sorbitol can be used as a plasticizer addictive because it is a natural and non-toxic compound that is biodegradable.
2a. The use of starch-borate and starch-glycerol polymers for the encapsulation of pharmaceutical drugs or pesticides would have a number of beneficial effects. These polymers are natural and non-toxic, and they are biodegradable, which means that they do not pose a risk to the environment. Additionally, they can be used to modify the properties of the drugs or pesticides, making them more effective and reducing their toxicity.
2b. Yes, these polymers are considered to be biodegradable. This is because they are made from natural materials that can be broken down by biological processes. Starch-borate and starch-glycerol polymers are particularly attractive for use in biodegradable materials because they are non-toxic and biocompatible. They can be used in a variety of applications, including packaging materials, agricultural films, and medical devices, where their biodegradability is an important factor.
To know more about polyol visit:
https://brainly.com/question/32067095
#SPJ11