Predict the geometry of NO2^- using the VSEPR method.
Answers
Prediction of the geometry of NO2^- using the VSEPR method. Here are the steps:
1. Identify the central atom: In NO2^-, the central atom is nitrogen (N).
2. Count the total number of valence electrons: Nitrogen has 5 valence electrons, each oxygen has 6, and there is an additional electron due to the negative charge. So, the total number of valence electrons is 5 + 2(6) + 1 = 18.
3. Distribute the electrons in the Lewis structure: Place the single bonds between the central atom (N) and the surrounding atoms (O) first. Then, complete the octet for the outer atoms (O). Finally, place any remaining electrons on the central atom.
4. Calculate the electron pair geometry: There are two bonding pairs (N-O) and one lone pair on the central atom (N). This corresponds to a total of three electron groups, which results in a trigonal planar electron pair geometry.
5. Determine the molecular geometry: Since there are two bonding pairs and one lone pair, the molecular geometry is bent (also known as V-shaped or angular).
In conclusion, the geometry of NO2^- using the VSEPR method is bent.
Learn more about VSEPR from : brainly.com/question/31162132
#SPJ11
Related Questions
Which phrases describe the open-ocean zone? Check all that apply.
very little sunlight
few nutrients
low temperatures
low density
located near coasts
Answers
Answer:
- few nutrients
- low temperatures
-very little sunlight
Explanation:
Answer:
I think it's: located near coasts.
Complete the paragraph to describe binary systems.
A binary star system is made of
stars, one of which is brighter than the other. Astronomers are able to detect the dimmer star because its gravity causes the bright star to
. Astronomers can also spot the dimmer star by observing a phenomenon called a(n)
binary. This happens when the dim star passes in front of the bright star.
Answers
Answer: Two, Wobble, Eclipsing.
Explanation:
A binary star system is made of two stars, one of which is brighter than the other. Astronomers are able to detect the dimmer star because its gravity causes the bright star to wobble. Astronomers can also spot the dimmer star by observing a phenomenon called an eclipsing binary.
Answer:
Two, Wobble, Eclipsing.
Explanation:
Analyze the given diagram of the carbon cycle.
An image of carbon cycle is shown. The sun, a cloud, two trees, one towards left and the other towards right, an animal, lake, and a factory are included. Arrow A points from the sun to the left tree. Arrow B points from the air above the clouds to the left tree. Arrow C points from the factory towards the air above the clouds. Arrow D points from the air to the lake labeled Carbonates in Water. Arrow E points from the label Dead Organism under the animal to label Fossils and Fossil Fuels. Arrow F points from the right tree to the air.
Part 1: Which process does arrow A represent?
Part 2: Which type of energy transformation does arrow A represent?
Part 3: Justify why this process is a recycling of carbon in the carbon cycle.
Use complete sentences to explain your answer.
Answers
Answer;
1.Arrow A represents the process of photosynthesis.
2.This shows the process of light energy converting into chemical energy
3.Photosynthesis absorbs carbon dioxide and converts it into energy as biomass, so that its able to be used for something else later on.
Explanation:
Part I
At location G, the process of combustion is taking place where hydrocarbon or organic carbon from the fossil fuel is burnt in the presence of oxygen to produce carbon dioxide and water as the products.
CxHy = CO₂ + H₂O
Part II
At point G there is no transformation of energy since during the process of combustion energy will still be stored in form of chemical energy in the bonds of carbon Iv oxide and the water produced during the reaction.
Part III
The process shows recycling of carbon in the carbon cycle. This is because carbon from living organisms is cycled to non living organisms. When plants and animals die and are buried deep in the ground, they are then slowly converted to fossil fuels which contain organic hydrocarbon compounds including petrol, kerosene and other compounds. Then the fossils are used in the industries and undergoes combustion releasing carbon iv oxide which is then released to the atmosphere and used by the plants and animals. The process starts once again.
it the same:]
Photosynthesis is a process by which plants absorb carbon dioxide from the atmosphere and use energy from the Sun to convert it into glucose.
Which process does arrow A represent?Arrow A represents photosynthesis.Arrow A represents a transformation from light energy from the sun to chemical energy stored in the tree.The process of photosynthesis converts light energy from the sun into chemical energy for the tree, which captures carbon from the air. This carbon is then recycled through the carbon cycle as it is respired out of the tree, released into the air, absorbed by the water, and eventually released back into the atmosphere.Arrow A represents photosynthesis.Arrow A represents a transformation of light energy from the Sun into chemical energy stored in the form of carbohydrates in the tree.This process is a critical part of the carbon cycle because it removes carbon dioxide from the atmosphere and stores it in the form of carbohydrates in the plant.The plant can then use the glucose to produce energy and grow, or it can be consumed by animals and eventually broken down into carbon dioxide and returned to the atmosphere. This cycle of removing carbon dioxide from the atmosphere and then returning it is essential to maintaining the balance of carbon in the environment.To learn more about Photosynthesis refer to:
https://brainly.com/question/19160081
#SPJ1
What is the percent by mass of fluorine in CaF2?
65%
24%
49%
51%
Answers
The percentage of fluorine in the given compound is 49%.
The given compound;
CaF₂
The molecular mass of the given compound is calculated as follows;
CaF₂ = (40) + (19 x 2)
= 40 + 38
= 78 g/mol
The mass of fluorine in the given compound = 38 g/mol
The percentage of fluorine in the given compound is calculated as follows;
\(= \frac{38}{78} \times 100\%\\\\= 48.7 \%\\\\\approx 49\%\)
Thus, the percentage of fluorine in the given compound is 49%.
Learn more about percentage of element in a compound here: https://brainly.com/question/21044245
what type of bond does corbon and hydrogen make
Answers
Answer: covalent bond
Explanation: The carbon-hydrogen bond is a bond between carbon and hydrogen atoms that can be found in many organic compounds.
How many grams of rubber stoppers would be needed to contain the same number of stoppers as there are corks in 1.0 kg of corks?
Answers
The weight of each stopper can vary based on the material, size, and manufacturing process used, so it is not possible to determine the number of rubber stoppers needed without more information.
What is weight and average weight?Weight is a measure of the force of gravity on an object and is typically measured in units of Newtons (N), pounds (lb), or kilograms (kg).
Average weight, on the other hand, refers to the sum of the weights of a group of objects divided by the number of objects in the group. It provides an estimate of the typical or central value of the weights in the group. The units of average weight are the same as the units used to measure weight.
The number of rubber stoppers needed to contain the same number of stoppers as there are corks in 1.0 kg of corks would depend on the average weight of each type of stopper.
The weight of each stopper can vary based on the material, size, and manufacturing process used, so it is not possible to determine the number of rubber stoppers needed without more information.
Learn more on average weight here https://brainly.com/question/26952238
#SPJ1
Nitrogen dioxide, NO2(g) (Delta. Hf = 33. 84 kJ/mol), is decomposed according to the following reaction: 2 upper N upper O subscript 2 (g) right arrow upper N subscript 2 (g) plus 2 upper O subscript 2 (g). What is the enthalpy change when 2. 50 mol of nitrogen dioxide decomposes? Use Delta H r x n equals the sum of delta H f of all the products minus the sum of delta H f of all the reactants. 13. 5 kJ of energy released 13. 5 kJ of energy absorbed 84. 6 kJ of energy released 84. 6 kJ of energy absorbed.
Answers
The heat of Reaction or Enthalpy is defined as the changes in the heat during a chemical reaction. The change in heat is calculated as the sum of all the heat change in products minus the sum of all the changes in the reactants.
How do you calculate the heat change in the reaction?The chemical reaction between nitrogen and oxygen is given as:
\(\rm 2 N O_2 \rightarrow N_2 + 2 O_2\\\\ \rm \Delta\; H_{f} &= 33.84 kJ/mol\)
Now, we know:
1 mol Nitrogen dioxide requires = 33.84 kJ/mol2.50 mol of nitrogen oxide will give = \(33.84 \times 2.50 \)Energy released = 84.6 energy releasedThus, the enthalpy change for the reaction will be 84.6 energy released.
Learn more about enthalpy change here:
https://brainly.com/question/25912483
Answer:
C. 84.6 kJ of energy released
Explanation:
edg 22
Please help me, I greatly appreciate your help :)

Answers
Place the characteristics for each phase of matter into the table where it belongs

Answers
Answer:
Solid- Definite shape
Solid and Liquid- Definite volume, Not easy to compress, if at all
Gas- No Definite Volume
Liquid and Gas- No Definite shape

The characteristics for each phase of matter in the table where it belongs are as follows:
Solids: Definite shape, very little particle motion. Liquids: Fluid motion. Gases: No Definite Volume, easily compressed. Solids and liquids: Definite volume, Not easy to compress, if at all. Liquids and Gases: No Definite shape, constant, fast particle motion. What are the different States of matter?The states of matter may be defined as the three distinct physical forms which matter can take in most environments. They are as follows:
Solid.Liquid.Gas.Each of the states of matter significantly possesses specific chemical and physical properties. For example, solids generally have a definite volume, are relatively rigid, and atoms or molecules are attached to each other very compactly.
Liquids possess a definite volume and have the ability to alter their shape through flowing. The atoms are loosely bonded to one another.
Gas has no definite volume or shape. This state of matter is easily compressed as well.
Therefore, the characteristics for each phase of matter in the table where it belongs are well described.
To learn more about the State of matter, refer to the link:
https://brainly.com/question/16982523
#SPJ2
In a first-order reaction involving the decomposition of hydrogen peroxide for a period of 50 mins, the concentration expressed in volume was found to be 10.6ml from an initial concentration of 72.6ml.a. Calculate k b. calculate the amount of hydrogen peroxide decomposed after 30 min.
Answers
The rate constant (k) of the first-order reaction is 0.0172 min^ -1, and the amount of hydrogen peroxide decomposed after 30 minutes is 29.7 ml.
a. To calculate the rate constant (k) of the first-order reaction, we can use the following formula:
ln (Ct/Co) = -kt
Where:
- Ct is the concentration at time t
- Co is the initial concentration
- k is the rate constant
- t is the time
We can rearrange the formula to isolate k:
k = - (ln (Ct/Co)) / t
Substituting the given values, we get:
k = - (ln (10.6/72.6)) / 50
k = 0.0172 min^-1 (rounded to four significant figures)
Therefore, the rate constant (k) of the first-order reaction is 0.0172 min^-1.
b. To calculate the amount of hydrogen peroxide decomposed after 30 minutes, we can use the first-order integrated rate law:
ln (Co/Ct) = kt
Where:
- Co is the initial concentration
- Ct is the concentration at time t
- k is the rate constant
- t is the time
We can rearrange the formula to isolate Ct:
Ct = Co * e^(-kt)
Substituting the given values, we get:
Ct = 72.6 * e^(-0.0172*30)
Ct = 42.9 ml (rounded to three significant figures)
Therefore, the amount of hydrogen peroxide decomposed after 30 minutes is:
72.6 ml - 42.9 ml = 29.7 ml (rounded to three significant figures)
To know more about rate constant visit:
https://brainly.com/question/20305871
#SPJ11
Determine the phase of the substances at the given state using Thermodynamic Properties Tables (in Appendix B) a) water: 60∘C,60kPa b) water: 100∘C,60kPa− c) water: 100∘C,500kPa d) Water: 25∘C,120kPa
e) Ammonia: −25∘C,120kPa f) Ammonia: 25∘C,120kPa g) R-134a: −25∘C,120kPa h) R−134a:25∘C,120kPa
Answers
a) vapor, b) saturated liquid, c) saturated liquid, d) compressed liquid, e) compressed liquid, f) compressed liquid, g) compressed liquid, h) compressed liquid.
The phase of substances in different states using Thermodynamic Properties Tables (in Appendix B) is given below:
a) The phase of water at 60∘C and 60kPa is vapor.
b) The phase of water at 100∘C and 60kPa is saturated liquid.
c) The phase of water at 100∘C and 500kPa is saturated liquid.
d) The phase of water at 25∘C and 120kPa is compressed liquid.
e) The phase of ammonia at -25∘C and 120kPa is compressed liquid.
f) The phase of ammonia at 25∘C and 120kPa is compressed liquid.
g) The phase of R-134a at -25∘C and 120kPa is compressed liquid.
h) The phase of R-134a at 25∘C and 120kPa is compressed liquid.
Learn more About Thermodynamic Properties Tables from the given link
https://brainly.com/question/13013699
#SPJ11
Chlorophyll molecules in chloroplasts normally only fluoresce a very small amount compared to chlorophyll that has been extracted into a solvent solution. True or false?.
Answers
Chlorophyll molecules in chloroplasts normally only fluoresce a very small amount compared to chlorophyll that has been extracted into a solvent solution, true.
What is a chlorophyll?A green pigment found in the mesosomes of cyanobacteria and in the chloroplasts of algae and plants is known as Chlorophyll Chlorophyll is derived from the two Greek words khloros which means "pale green" and phyllon means "leaf". Chlorophyll allow plants to absorb energy from light.Joseph Bienaimé Caventou and Pierre Joseph Pelletier in 1817 first isolated and named Chlorophyll. The presence of magnesium was discovered in 1906 in chlorophyll and that was element's first detection in living tissueChlorophyll a and b are the two types of chlorophyll that exist in the photosystems of green plants.To learn more about chlorophyll: https://brainly.com/question/13500580
#SPJ4
Na2O H2 --> 2Na H2O A student performed the above reaction in the lab, and obtained an actual yield of 3.9 grams of H2O. He calculated a theoretical value of 4.2 grams of H2O. Calculate the percent yield of the reaction.
Answers
Answer:
Percentage yield = 92.86%
Explanation:
We are given;
Actual yield = 3.9 grams
Theoretical yield = 4.2 grams
Formula for percent yield is;
% yield = (actual yield/theoretical yield) × 100%
Thus;
% yield = (3.9/4.2) × 100%
% yield = 92.86%
The d orbital starts in the 4th row, or 4th energy level. However, what energy level (period number) does d actually start with?
Answers
The d-orbital starts in the third energy level (n = 3) of an atom.
Each energy level can contain one or more sublevels, including s, p, d, and f sublevels. The first energy level (n = 1) has one s orbital and can hold a maximum of 2 electrons. The second energy level (n = 2) has one s orbital and three p orbitals, allowing for a maximum of 8 electrons. The third energy level (n = 3) has one s orbital, three p orbitals, and five d orbitals, accommodating a maximum of 18 electrons.
The d-orbitals are found in the third energy level, corresponding to the third period of the periodic table. Therefore, the period number for the energy level where the d-orbital starts is 3.
The filling order of orbitals follows the pattern: 1s, 2s, 2p, 3s, 3p, 4s, 3d, and so on. The d-orbitals start filling after the p-orbitals in the third energy level. The electron configuration for the third energy level is written as 3s^2 3p^6 3d^1-10, depending on the element.
For example, the electron configuration of iron (Fe) is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^6 4s^2. This configuration indicates that the d-orbitals of iron are half-filled with 5 electrons.
Learn more about energy level
https://brainly.com/question/30546209
#SPJ11
What is commonly the starting amino acid for polypeptides?
Answers
Hence, methionine is the common starting amino acid for all polypeptides, however further processing processes may remove some of the original methionine.
While a start codon is necessary for translation to commence, the codon AUG can also come later in an mRNA's coding sequence, where it only designates the amino acid methionine.
646464 distinct codons make up the genetic code, as was already explained. But what are the 444444 additional codons accomplishing if there are only 202020 amino acids? Some are stop codons, as we saw, but the majority are not. Yet, it turns out that the genetic code is degenerate, meaning that some amino acids are defined by more than one codon. Proline, for instance, is encoded by four distinct codons (CCU, CCC, CCA, and CCG). Whenever an mRNA has one of these codons.
Learn more about Amino acid here:
https://brainly.com/question/15687833
#SPJ4
Diffusion and Organelle Retake Activity
(please finish in 5-9 minutes)
Question:
Fill in the blank
Water loves _____ and ____.
This means water will
move ______ the direction of the salt or sugar.
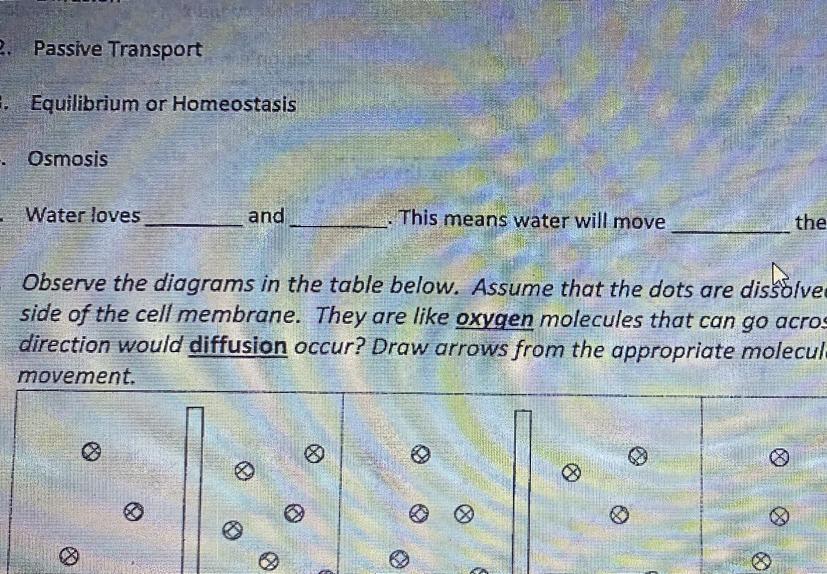
Answers
Answer:
wind and soil? north?
Explanation:
Be sure to answer all parts. Calculate δg o and kp for the following equilibrium reaction at 25. 00°c: 2h2o(g) ⇌ 2h2(g) o2(g)
Answers
We need the concentrations of hydrogen gas, water vapour, and oxygen gas to proceed further. If these concentrations are provided, we can substitute them into the equations and solve for δG° and Kp.
To calculate δG°, we need to use the equation δG° = -RT ln(Kp), where R is the gas constant and T is the temperature in Kelvin. To calculate Kp, we use the equation Kp = [H2]²/[H2O]²[O2]. By substituting the given values and solving the equations, we can find δG° and Kp.
To calculate δG° for the given equilibrium reaction at 25.00°C, we can use the equation δG° = -RT ln(Kp), where δG° is the standard Gibbs free energy change, R is the gas constant (8.314 J/(mol·K)), and T is the temperature in Kelvin. In this case, we need to convert the temperature from Celsius to Kelvin by adding 273.15 (25.00°C + 273.15 = 298.15 K).
To calculate Kp for the equilibrium reaction 2H2O(g) ⇌ 2H2(g) + O2(g), we can use the equation Kp = [H2]²/[H2O]²[O2]. Here, [H2] represents the concentration of hydrogen gas, [H2O] represents the concentration of water vapour, and [O2] represents the concentration of oxygen gas.
Now, let's substitute the given values into the equations and solve:
δG° = -RT ln(Kp)
= -(8.314 J/(mol·K)) * 298.15 K * ln(Kp)
Kp = [H2]²/[H2O]²[O2]
= ([H2]²) / ([H2O]²[O2])
To know more about Gibbs free refer to this:
https://brainly.com/question/13795204
#SPJ11
at 25°c, a 15.000 g sample of an unknown liquid was determined to have a volume of 19.01 m
Answers
At 25°c, a 15.000 g sample of an unknown liquid was determined to have the volume of 19.01 mL. The density of the liquid is 0.789 g/mL.
The mass of the sample of the unknown liquid = 15 g
The volume of the sample of the unknown liquid = 19.01
The expression for the density is expressed as follows :
The density = mass / volume
The density of the substance is the measurement of the mass per unit the volume.
The density = mass / volume
where,
Mass = 15 g
Volume = 19.01 mL
The density = 15 / 19.01
The density = 0.789 g/ mL
This question is incomplete , the complete question is :
At 25°c, a 15.000 g sample of an unknown liquid was determined to have a volume of 19.01 mL. calculate the density of the liquid.
To learn more about density here
https://brainly.com/question/29775886
#SPJ4
Callie did a lab during which she investigated the difference in cellular respiration rates between two different types of corn: germinating and non-germinating. A germinating seed is one from which a plant has started to grow. A non-germinating seed is usually dry and a new plant has not yet emerged. The data that she gathered are displayed in the graph.
Which of the following statements are true concerning the data that Callie gathered during the lab? Choose the two that apply.
Graph has a horizontal label of time and a vertical label of mL of oxygen consumed. The germinating corn seed line extends from 0-1.6 at the far end of the graph. The non-germinating corn seed line extends from 0-0.2.
A. The germinating corn seed consumed more oxygen than the non-germinating corn seed.
B. The non-germinating corn seed produced more carbon dioxide than the germinating corn seed.
C. The non-germinating corn seed performed more cellular respiration than the germinating corn seed
D. The germinating corn seed produced more energy than the non-germinating corn seed.
E. The non-germinating corn seed performed cellular respiration and the germinating corn seed performed fermentation.
Answers
A germinating seed is one from which a plant has started to grow. A non-germinating seed is usually dry, and a new plant has not yet emerged. The germinating corn seed consumed more oxygen than the non-germinating corn seed. This statement is true. Therefore, option A is correct.
What is germinating seed ?A dry seed starts to absorb water through its seed coat when it comes into touch with damp soil or growing medium. The seed swells and the seed coat splits open as it absorbs more water. Small shoots and roots make up the embryo inside the seed. First to appear from the seed is the root.
The prerequisites for seed germination include oxygen, water, temperature, and, only for particular seeds, light. The sprouting of seeds is impacted when one or more of these are missing. Similar internal elements that impact this process include seed viability, dormancy, and embryonic maturity.
Thus, option A is correct.
To learn more about germinating seed, follow the link;
https://brainly.com/question/15976369
#SPJ1
(image attached)
what mistake did carl make?
A) He did not multiply with calcium atoms by the subscript 2.
B) He did not add the coefficient 4 to the chlorine and oxygen atoms.
C) He did not add the subscript 2 to the calcium atoms.
D) He did not multiply the chlorine and oxygen atoms by the coefficient 4.
(pls answer question with letter choice as well as explaination)

Answers
Answer:
D) He did not multiply the chlorine and oxygen atoms by the coefficient 4.
Explanation:
The coefficient 4 at the beginning of the chemical formula indicates that there are four Ca(ClO3)2 molecules. Think of this as Ca(ClO3)2 × 4. This means that he had to multiply the number of atoms for each element by 4 as well, so he should've ended up with 4 total calcium atoms (which is correct), 8 total chlorine atoms, and and 24 total oxygen atoms. He did not get all these answers because he didn't multiply the chlorine and oxygen atoms by the coefficient 4.
Answer:
D) He did not multiply the chlorine and oxygen atoms by the coefficient 4.
Explanation:
Determine how many electrons are either produced or consumed by completing and balancing the half-reaction of chromium (iii) forming from dichromate.
Cr2o2^−7⟶Cr3 (aq)
a. Three electrons are consumed
b. Three dectrons are produced
c. Six electrons are consumed
d. Sixelectrons are produced
Answers
On completing and balancing the half-reaction of chromium (iii) forming from dichromate the number of electrons are either produced or consumed are option (b): Three electrons are produced.
To determine the number of electrons produced or consumed in the half-reaction of chromium(III) forming from dichromate, let's first write the balanced half-reaction. The dichromate ion (Cr2O7^2-) is reduced to chromium(III) ion (Cr^3+) in this reaction. We can represent the reduction half-reaction as follows:
Cr2O7^2- ⟶ Cr^3+
To balance the half-reaction, we need to equalize the number of chromium atoms and oxygen atoms on both sides. Since there are two chromium atoms on the left side and only one on the right side, we add a coefficient of 2 in front of the chromium ion:
2Cr2O7^2- ⟶ 2Cr^3+
Now, let's examine the changes in oxidation state for chromium in this reaction. In dichromate (Cr2O7^2-), chromium has an oxidation state of +6, while in chromium(III) (Cr^3+), it has an oxidation state of +3. Therefore, the oxidation state of chromium decreases by 3 in this reduction half-reaction.
Reduction involves a gain of electrons. Since the oxidation state of chromium decreases by 3, it means that three electrons are gained by each chromium ion. Therefore, the correct answer is option (b): Three electrons are produced.
for more questions on electrons
https://brainly.com/question/26084288
#SPJ8
Answer:
c. Six electrons are consumed
Explanation:
The first step is to ensure that the chromium atoms are balanced by adjusting the coefficients if needed. Do so by giving the Cr3+ ion a coefficient of 2.
Cr2O2−7 ⟶ 2Cr3+ (aq)
Next, balance the oxygen atoms by adding H2O molecules. The half-reaction has seven O atoms on the left and none on the right, so add 7H2O (l) to the right side.
Cr2O2−7 ⟶ 2Cr3+ (aq) + 7H2O (l)
Next, balance the hydrogen atoms by adding H+ ions. The half-reaction has 14 H atoms on the right side and none on the left, so add 14H+(aq) to the left side.
Cr2O2−7 + 14H+ (aq) ⟶ 2Cr3+ (aq) + 7H2O (l)
At this point, the charge can be balanced by adding electrons (e−). To do so, find the total charge on each side of the reaction. The left side has a total charge of (−2) + 14 × (+1) = 12+. The right side has a total charge of 2 × (+3) = 6+. Adding 6e− to the left side brings the charge down to 6+ to match the right side.
Cr2O2−7 + 14H+ (aq) +6e− ⟶ 2Cr3+ (aq) + 7H2O (l)
This gives us the answer to the question, which is that six electrons are consumed, since they are on the left side of the equation. Note that it does not make a difference whether the reaction is assumed to be in an acidic or a basic solution. The extra step taken for a basic solution is to add the same number of OH− ions to both sides of the reaction, which does not affect the number of electrons needed to balance the charges.
Which of the following compounds are secondary (29) amines? 1. (CH3)3CNHCH3 2. CH3CH(NH2)CH2CH3 3. CH3CH2CH2NHCH3 4. CH3CH2CH2CH2CH2NH2
Answers
The compound that is a secondary amine among the following is CH3CH2CH2NHCH3.
Amines are organic compounds that contain nitrogen. They are classified according to the number of alkyl or aryl groups that are bonded to nitrogen. Primary, secondary, and tertiary amines are the three classifications. 1. (CH3)3CNHCH3 is a tertiary amine and not a secondary amine. 2. CH3CH(NH2)CH2CH3 is a primary amine, not a secondary amine. 3. CH3CH2CH2NHCH3 is a secondary amine and falls under the classification of a secondary amine. 4. CH3CH2CH2CH2CH2NH2 is a primary amine, not a secondary amine.Therefore, the compound that is a secondary amine among the following is CH3CH2CH2NHCH3.
Learn more about compounds here,
https://brainly.com/question/30828953
#SPJ11
What happens to your hypotheses as new information is gained
Answers
why do molecules change speed
Answers
Answer:
Molecules change speed based on temperature and state of matter. The warmer they are, the faster they move and vice versa. Solids are at a lower temperature than gases and liquids, which means the molecules are moving slower, and hold together better, also explaining why solids aren't malleable.
The particles gather kinetic energy and accelerate as the temperature rises.
What is kinetic energy?Kinetic energy is defined as an object is the energy it has since it is in motion. It is explained as the amount of effort required to move a mass-based body from rest to the indicated velocity. The body keeps its kinetic energy, which it acquired during acceleration, unless its speed changes.
The actual average speed of the particles is influenced by both their mass and temperature; at a given temperature, larger particles travel more slowly than lighter ones. Temperature and the physical state of the materials affect the speed of molecules. They move more quickly in warmer temperatures and vice versa. Since solids have lower temperatures than gases and liquids, their molecules move more slowly and adhere to one another more tightly, which also explains why solids aren't bendable.
Thus, the particles gather kinetic energy and accelerate as the temperature rises.
To learn more about kinetic energy, refer to the link below:
https://brainly.com/question/15764612
#SPJ2
As a Formulation chemist, you're required to do a diet (dark) chocolate D optimal (experimental design) Table with variables and response factors ( viscosity, polyphenol content, fat content). How would you do the D optimal design table? (Note!! You can use other literature papers or other online papers to check how it's done. Also you don't have to have the results for the response factors but you need values on how you would set up the variables).
Answers
By following these steps, you can create a D optimal design table for a diet (dark) chocolate formulation, which will help optimize the variables and response factors for your experiment.To create a D optimal design table for a diet (dark) chocolate formulation, follow these steps:
1. Identify the variables: Start by listing the variables that may affect the desired response factors. In this case, the variables could include cocoa percentage, sugar content, emulsifier type, and temperature during processing.
2. Determine the response factors: Identify the response factors that you want to measure and optimize. In this case, the response factors could be viscosity, polyphenol content, and fat content.
3. Use a statistical software or online tool: Utilize statistical software or online tools specifically designed for experimental design, such as Design-Expert or JMP. These tools can help generate a D optimal design table based on the identified variables and response factors.
4. Set up the design table: Enter the identified variables and their corresponding levels in the software/tool. For example, cocoa percentage can be set at levels of 60%, 70%, and 80%, while sugar content can be set at levels of 20%, 30%, and 40%.
5. Specify the number of experimental runs: Decide on the number of experimental runs you want to conduct. A D optimal design table will suggest the most efficient and informative number of runs based on the specified variables and desired level of accuracy.
6. Run the experiments: Follow the experimental plan provided by the D optimal design table and conduct the experiments accordingly. Make sure to record the values of the response factors for each run.
To know more about software, visit at:
https://brainly.com/question/32393976
#SPJ11
what is the electrons, valence electrons, and electron shells number please answer all 3 questions (look at the picture)

Answers
In picture one, there are four electrons, one valance electrons and two shells, in image two there are three electrons, one valance electrons and two shells whereas there are 13 electrons, three valance electrons and three shells.
What is Electrons, Valence Electrons, and Electron Shells Numbers?Electrons:
An electron is a negatively charged particle which can be either bounded with to an atom. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure.
Valence Electrons:
Valence electrons are those electrons which are present in outermost shell or in valence shell or energy level of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell.
Electron Shells Numbers:
Each shell can contain only a fixed number of electrons: the first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on.
So we can conclude that In picture one, there are four electrons, one valance electrons and two shells, in image two there are three electrons, one valance electrons and two shells whereas there are 13 electrons, three valance electrons and three shells.
Learn more about Valence Electrons here: brainly.com/question/371590
#SPJ1
How many Liters of space will a 70.0g sample of CO2 occupy? You must show all work to receive full credit.
Answers
Answer: the answer is 35.6
Explanation:
Look up the answer but take out you must show all your work and resources should pop up I’m on the same question now for chemistry so I’m putting 35.6L
Q4 This question relates the combustion reactions of acetylene, hydrogen and ethane. (a) Express the stoichiometric ecpigtions for the combustion reactions of acetylene, hydrogen and ethane with their respective standard heats of combustion obtained from physical property table. (b) Verify the standard heat of combustion of acetylene in Q4(a) by using heat of formation method. (c) The equation below shows the acerylene hydrogenation reaction: C2H2(g)+2H2(g)→C2H6(g) (i) Compute the standard heat of acetylcne hydrogenation reaction using tabulated heats of formation and heats of combustion. (ii) Verify the answer in Q4(e)(1) by using Hess's Law.
Answers
Stoichiometric equations for the combustion reactions ΔHf° (C2H2) = (2 x (-393.5)) + (-285.8) - (-1299.5) = +226.7 kJ mol-1(c) Acetylene hydrogenation reaction
Acetylene combustion reaction:C2H2 (g) + (5/2) O2 (g) → 2 CO2 (g) + H2O (l) ΔHc° = -1299.5 kJ mol-1 Hydrogen combustion reaction:2H2 (g) + O2 (g) → 2 H2O (l) ΔHc° = - 483.7 kJ mol-1Ethane combustion reaction:C2H6 (g) + (7/2) O2 (g) → 2 CO2 (g) + 3 H2O (l) ΔHc° = - 1560 kJ mol-1(b) Heat of formation method for verifying the standard heat of combustion of acetylene: The standard heat of combustion of acetylene from the heat of formation method is:ΔHc° (C2H2) = 2 ΔHf° (CO2) + ΔHf° (H2O) - 2 ΔHf° (C2H2) = -1299.5 kJ mol-1ΔHf° (CO2) = -393.5 kJ mol-1ΔHf° (H2O) = -285.8 kJ mol-1.
For verifying the answer in Q4(e)(1) using Hess's Law, we need to convert acetylene hydrogenation reaction into a combination of other reactions:Reaction 1:C2H2 (g) + (2.5) O2 (g) → 2 CO2 (g) + H2O (l) ΔH1 = -1299.5 kJ mol-1Reaction 2:2 CO2 (g) + 2.5 H2 (g) → C2H6 (g) + 5 O2 (g) ΔH2 = +1560 kJ mol-1After multiplying and adding the above equations, we get the required reaction as:C2H2 (g) + 2 H2 (g) → C2H6 (g) ΔH = -396.1 kJ mol-1.
To know more about reactions visit:
https://brainly.com/question/16737295
#SPJ11
What accurately describes the reactants of a reaction
Answers
Answer:
Substance that are used in the reaction
Explanation:
I hope this will help you
Answer:
Substances that are used in the reaction. Hope my answer helps you!
Suppose a student completes an experiment with an average value of 2.6 mL and a calculated standard deviation of 0.80 mL. What is the minimum value within a 1 SD range of the average
Answers
Answer:
Explanation:
1 standard deviation range will look like: [average - SD, average + SD]
Here the miximum value will be: average - SD = 2.6 - 0.80 = 1.80 ml
and the maximum value will be: average + SD = 2.6 + 0.80 = 3.40ml