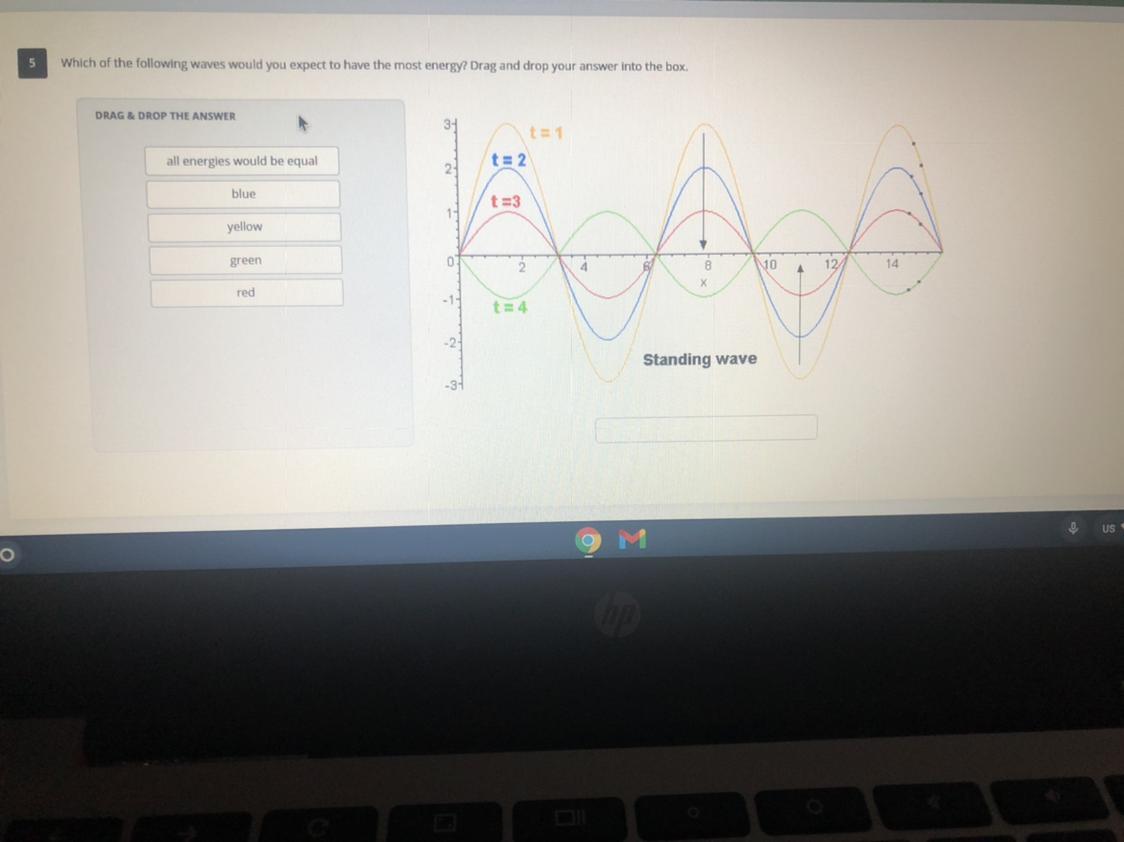
Answers
Answer:
I think it would be Yellow
Related Questions
A student is given an object and is asked to identify its density. The object has a volume of 3 cubic centimeters and a mass of 6 grams. Which of the following equations correctly sets up the formula for density?
Answers
Density =mass/volume
=6/3
=2
Explain why liquid freshwater is considered a limited resource.
Answers
Answer:
Freshwater is such a limited resource because there is such a little amount of freshwater found on Earth. About 77% of fresh water on Earth is frozen in glaciers and polar ice caps. Because of this, there is very little fresh water available for humans to use.
Explanation: Hope this helps.
Freshwater is a scarce resource, making up just around 3% of the total amount of water on Earth. Although freshwater is regarded as a renewable resource, certain areas utilize more freshwater than can be replaced by natural processes.
There is a very small amount of fresh water on Earth, making it a scarce resource. The polar ice caps and glaciers contain around 77% of the fresh water on Earth. This results in a relatively limited supply of fresh water for human use.
Since freshwater only accounts for around 3% of the total amount of water on Earth, it is a limited resource.
Learn more about freshwater, here:
https://brainly.com/question/17443499
#SPJ2
What is the specific heat capacity of diamond if 64.4 J of heat is required to heat 25.0 g of diamond from 10.5°C to 15.6°C?
Answers
Q being heat in Jules
M being mass in grams
C being specific heat in J/g*C
T being the change in temperature
64.4J = 25g*c*5.1C
64.4J = (127.5g*C)*c
c = 0.505 J/g*C
the ratio of carbon-14 to carbon-12 in a piece of paper buried in a tomb is r = 1/1311. estimate the age of the piece of paper
Answers
Therefore, the estimated age of the piece of paper buried in the tomb, and approximately 75,817 years.
To estimate the age of the piece of paper buried in the tomb, we need to use the concept of radioactive decay. Carbon-14 is a radioactive isotope that decays over time into carbon-12. The ratio of carbon-14 to carbon-12 in living organisms is constant, but once the organism dies, the carbon-14 begins to decay, and the ratio changes.
The half-life of carbon-14 is approximately 5,700 years, which means that every 5,700 years, half of the carbon-14 in a sample will decay. Using this information, we can calculate the age of the piece of paper as follows:
r = (carbon-14/carbon-12) = 1/1311
Let's assume that the initial ratio of carbon-14 to carbon-12 in the paper was 1/1000 (which is close to the current ratio in the atmosphere).
After one half-life, the ratio would be 1/2000.
After two half-lives, the ratio would be 1/4000.
After three half-lives, the ratio would be 1/8000.
And so on.
We can use the following formula to calculate the age of the paper:
t = (ln r/ln 0.5) x 5,700 years
Plugging in the values, we get:
t = (ln 1/1311 / ln 0.5) x 5,700 years
t = (7.273 / 0.693) x 5,700 years
t = 75,817 years
To know more about carbon visit:
https://brainly.com/question/22530423
#SPJ11
as the temperature of a gas decreases is volume
Answers
Answer:
it's volume also decrease
If the pH of a solution is 6.2, what would the pOH be?
O A. 7.8
B. 8.9
C. 12.4
D. 5.8
SUBMIT
Answers
Answer:
7.8
Explanation:
pH + pOH = 14
pOH = 14 - 6.2
= 7.8
which statement best explains why the polarity of a h2o molecule differs from the polarity of a co2 molecule?
Answers
The statement that best explains why the polarity of the H₂O molecule differs from the CO₂ molecule is B. The central atom in H₂O has lone pair electrons and the central atom in CO₂ does not.
The bonding in both the cases of Water and Carbon-di-oxide is polar, that is O-H bond is polar and so is the C-O bond.
In a water molecule (H₂O), Oxygen is the central atom and it has lone pair of electrons. This makes the H₂O molecule asymmetrical and gives rise to polarity.
On the other hand in Carbon-di-oxide, Carbon is the central atom but it doesn't have a lone pair. The absence of lone pair gives rise to linear CO₂ molecules and hence dipole moment of each bond cancels each other. Hence, the bonds O=C=O have bonds canceled.
This makes the polarity of these two molecules to be different. Hence the statement that best explains the polarity of H₂O over CO₂ is B.
To know more about lone pair, click below:
https://brainly.com/question/3915115
#SPJ4
18 g of oxygen reacts completely with 4 g of hydrogen we would expect how many grams of water
A. Less than 22 grams
B. More than 22 grams
C. 22 grams because mass cannot be created or destroyed
D. There is no way to tell by this information
Answers
Answer:
C. 22 grams because mass cannot be created or destroyed
Explanation:
In this reaction and in any chemical reaction, mass is expected to always be conserved.
According to the law of conservation of matter "in a chemical reaction, matter is neither created nor destroyed but can be changed from one form to another".
Based on this law, the mass of reactants and products must be the same in a chemical reaction. So, the sum of the mass of the reactants must be equal to the mass of the product.what is the volume, measured in liters at STP, of 285 grams of the gas acetylene, C2H2?
Answers
285 grams of acetylene at standard temperature and pressure (STP) have a volume of 24.45 liters.
How to find the volume of a gas?To find the volume of a gas at standard temperature and pressure (STP), you can use the ideal gas law: PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature.
STP is defined as 0 °C (273.15 K) and 1 atm (101.325 kPa)
First, we need to find the number of moles of acetylene. To do this, we use the molar mass of C2H2 which is 26.04 g/mol
n = m / M
n = 285 g / 26.04 g/mol
n = 10.9 moles
Then we can use the ideal gas law to find the volume in liters:
V = nRT / P
V = (10.9 moles) * (0.08206 Latmmol^-1*K^-1) * (273.15 K) / (101.325 kPa)
V = 24.45 L
Therefore, 285 grams of acetylene at standard temperature and pressure (STP) have a volume of 24.45 liters.
Learn more about gas law in brainly.com/question/12669509
#SPJ1
How much energy does a 9 × 108 m wavelength photon have?
Answers
Answer:
2.2 x 10⁻³⁴ J
or
2.2 x 10⁻¹⁸ J
Explanation:
You can calculate the energy using the photon energy equation:
E = hc / λ
In this equation,
-----> E = energy (J)
-----> h = Planck's Constant (6.626 x 10⁻³⁴ J*s)
-----> c = speed of light (3.0 x 10⁸ m/s)
-----> λ = wavelength (m)
You can plug the given wavelength, along with the other constants, into the equation and simplify to find the energy.
E = hc / λ
E = (6.626 x 10⁻³⁴ J*s)(3.0 x 10⁸ m/s) / (9 x 10⁸ m)
E = 2.2 x 10⁻³⁴ J
I suspect that you may have forgotten the negative sign in front of the 8 (9 x 10⁻⁸). This would change your answer to 2.2 x 10⁻¹⁸ J.
Select the chemical formula that contains 10 atoms of hydrogen.
2C4H10
2CH4
2C2H5
2C2H6
Answers
Which of these statements is supported by evidence in both articles? A The Triangle Fire was a tragedy that could have been prevented. B The absence of fire drills caused confusion among the trapped workers. C The Triangle Fire had a lasting impact on safety regulations. D Onlookers who witnessed the fire were horrified by what they saw.
Answers
Answer: A The Triangle Fire was a tragedy that could have been prevented.
Explanation:
The Triangle Fire was a tragedy that happened in the factory of Shirtwaist Company in New York City where there was.a.fore outbreak and about 145 employees of the company were killed.
The deaths could have been prevented because the company neglected safety measures such as having a good and reliable pump system, having readily available fire extinguishers. Also, what resulted in the lethal nature of the incident was because the doors within the building of the factory were locked.
The tragedy generated lots of attention and series of laws were been out in place in order to ensure that workers are safe at their workplace.
what is the ph of a solution with a 1.0 x 10-11 hydrogen ion concentration?
Answers
The pH of a solution with a 1.0 x 10⁻¹¹ hydrogen ion concentration is 11.
To calculate the pH of a solution with a 1.0 x 10⁻¹¹ hydrogen ion concentration, we can use the pH formula:
pH = -log10[H⁺]
where [H⁺] represents the hydrogen ion concentration.
In this case, pH = -log10(1.0 x 10⁻¹¹), which results in a pH of 11. This indicates that the solution is alkaline.
Thus, the pH of a solution with a 1.0 x 10⁻¹¹ hydrogen ion concentration is 11.
Learn more about pH: https://brainly.com/question/2288405
#SPJ11
Identify the parts of a wave using the illustration and the function below.

Answers
Answer: 1. Crest 2. Trough 3. Wave Length 4. Amplitude
Explanation:
When you look far ahead as you drive, you are Group of answer choices watching for users to your sides. aiming high in steering. learning about farsightedness. looking down at the area just in front of your vehicle.
Answers
When you look far ahead as you drive, you are aiming high in steering to anticipate and plan for potential hazards and make informed decisions.
Looking far ahead while driving is an essential technique known as "aiming high in steering." It involves directing your focus and attention towards the distant road ahead, rather than fixating on the area just in front of your vehicle. By doing so, you can gather valuable information about the road conditions, traffic patterns, and potential hazards that may be coming up.
Aiming high in steering allows you to anticipate and react to situations in advance, giving you more time to adjust your speed, change lanes, or make turns safely. It helps you maintain a broader awareness of your surroundings and improves your ability to scan for potential dangers, such as pedestrians, cyclists, or vehicles merging into your lane.
By looking far ahead, you can also make better decisions about lane positioning, choosing the appropriate lane to accommodate upcoming turns or merges. This proactive approach to driving promotes smoother, more controlled maneuvers and reduces the likelihood of sudden, last-minute adjustments that can lead to accidents.
Overall, looking far ahead while driving and aiming high in steering enhances your situational awareness, increases your reaction time, and contributes to a safer and more efficient driving experience.
Learn more about anticipate here:
https://brainly.com/question/31556627
#SPJ11
IF U ANSWER ILL BRAINLIST AND GIVE U 5 STAR PLUS U GET EXTRA POINTS:)) WRITE IT IN UR OWN WORDS PLS OR NO POINTS.
The burning of a magnesium ribbon is an example of a reaction producing a metal oxide.
a) What reactants are required to production of a metal oxide?
b) Write a general equation for the production of a metal oxide.
Answers
Answer:
a) When the magnesium metal burns it reacts with oxygen found in the air to form Magnesium Oxide. A compound is a material in which atoms of different elements are bonded to one another. Oxygen and magnesium combine in a chemical reaction to form this compound. After it burns, it forms a white powder of the magnesium oxide.
b) Metals react with oxygen in the air to produce metal oxides. For example, magnesium reacts with oxygen to produce magnesium oxide when it is heated in air: magnesium + oxygen → magnesium oxide 2Mg + O2 → 2MgO
Explanation:
Hope it helps. if yes, plz mark me as brainliest
1.6A A landowner wants to spray herbicide on a field that has an area of 2050 m². The
herbicide comes in bottles that hold 16 fluid ounces (fl oz), and 1.5 fl oz mixed with 1 gal
of water will treat 300 ft?. How many bottles of herbicide will the landowner need? Draw a
road map to show how you planned the solution.
dicoborced into a lake in New
Answers
Answer:
1,171 fluid ounces of herbicide is sufficient for 2050 square meter.
Explanation:
If 300 square feet of land is sprayed by the herbicide at the rate of 16 liquid ounce, So 1,171 fluid ounces of herbicide is enough to cover the area of 2050 square meters.
Calculation:
First convert 300 square feet into meter which is equal to 28 square meter.
16 ounces of herbicide = 28 square meter
x ounces of herbicide = 1050 square meter
by cross multiplication we get
28 x = 16 x 1050
x = 32800/ 28
x = 1171 ounces of herbicide.
the equilibrium cosntant for the ionisation of acetic acid is 0.00002. what can you conclude about this system?
Answers
In the case of the ionization of acetic acid, which can be represented as CH3COOH ⇌ CH3COO- + H+, the given equilibrium constant of 0.00002 tells us important information about the system.
Since the equilibrium constant is very small (less than 1), it indicates that the forward reaction (ionization of acetic acid) is not favored. In other words, the concentration of the products (CH3COO- and H+) at equilibrium is much lower compared to the concentration of the reactant (CH3COOH). This suggests that the majority of the acetic acid remains unionized.
Moreover, the small equilibrium constant suggests that the reverse reaction (recombination of CH3COO- and H+ to form CH3COOH) is favored. This means that any CH3COO- and H+ ions produced tend to recombine to form acetic acid, rather than remaining as dissociated ions.
Overall, the low equilibrium constant of 0.00002 indicates that the ionization of acetic acid is limited, and the majority of the acetic acid remains as unionized molecules in the system.
Learn more about ionization here
https://brainly.com/question/1602374
#SPJ11
classify each chemical reaction as an addition, oxidation-reduction, isomerization, or nucleophilic substitution.
Answers
Classification of chemical reaction is as follows:
1. Addition reaction
2. Oxidation-reduction reaction
3. Isomerization reaction
4. Nucleophilic substitution reaction
1. Ethene undergoes an addition reaction with bromine, where the double bond is broken, and each carbon atom forms a single bond with a bromine atom.
2. The oxidation-reduction reaction involves the conversion of 2-propanol to acetone through the loss of hydrogen atoms (oxidation) and gain of oxygen atoms (reduction).
3. The isomerization reaction occurs when glucose is enzymatically converted to fructose, leading to a rearrangement of the atoms within the molecule to form an isomer.
4. The reaction between methyl chloride and sodium hydroxide involves the substitution of a chlorine atom in methyl chloride by a hydroxide ion, resulting in the formation of methanol. This is a nucleophilic substitution reaction.
To learn more about isomerization here
https://brainly.com/question/15022888
#SPJ4
Complete question is:
classify each chemical reaction as an addition, oxidation-reduction, isomerization, or nucleophilic substitution.
1) Ethene (C2H4) reacts with bromine (Br2) to form 1,2-dibromoethane (C2H4Br2).
2) 2-propanol (C3H8O) is oxidized to form acetone (CH3COCH3).
3) Glucose (C6H12O6) is converted to fructose (C6H12O6) in the presence of an enzyme.
4) Methyl chloride (CH3Cl) reacts with sodium hydroxide (NaOH) to form methanol (CH3OH).
a chemistry student needs 60.0 ml of pentane for an experiment. by consulting the crc handbook of chemistry and physics, the student discovers that the density of pentane is 0.626 . calculate the mass of pentane the student should weigh out
Answers
37.6 grams of pentane
The density given in the CRC of 0.626 g/cm³ will be used to calculate the mass of pentane requred since Density = m/v and m= D x V
Be careful in this kind of questions to check for the units. In this case one has to remember 1 mL equal 1 cm³.
m = 0.626 g/cm³ x 60.0 cm³ = 37.6 g pentane
Student should way out 57.56 grams of pentane.
Density of pentane yes 0.626 grams per ml which can be used to find the mass of the pentane in 60 ml.
Density = mass of substance/ volume of substance
So,
Density of pentane = Mass of pentane / Volume of pentane
Therefore, Mass of pentane = Density of pentane X Volume of pentane
Mass of pentane = 0.626g/ml X 60ml = 57.56 grams
To know more about Density, visit:
https://brainly.com/question/14204023
#SPJ4
The atomic number of an element:
Question options:
Tells you the mass of the atom
Tells you the number of energy levels there are around the atom
Tells you the identity of the atom
Tells you how many valence electrons the atom has
Answers
Answer:tells you the identity of the atom
Explanation:
number of protons=atomic number
Answer: C
Explanation: The answer would be C, it tells you the identity of the atom.
The amount of atomic particles released by a radioactive material in a specific time is determined by strong and weak nuclear forces. strong and weak gravitational forces. attraction and repulsion caused by electric forces. attraction and repulsion caused by magnetic forces.
Answers
The amount of atomic particles released by a radioactive material in a specific time is determined by strong and weak nuclear forces option- 1 is correct.
What exactly do you mean by radioactive materials?Radioactive materials fall under the category of radionuclides, which are chemicals with unstable atomic nuclei. They adjust the nucleus to stabilize themselves (spontaneous fission, emission of alpha particles, or conversion of neutrons to protons or the reverse).
The amount of atomic particles released by a radioactive material over a given period of time depends on how quickly it decays.
The weak nuclear forces that exist between the nucleons of atomic particles control how quickly radioactive materials decay over time.
The nuclear forces can therefore be used to calculate the total number of atomic particles that a radioactive material releases in a given period of time (strong or weak).
To know more about radioactivity visit:
https://brainly.com/question/7180704
#SPJ4
what charged group(s) are present in glycine at a ph of 7?
A) -NH3+
B) -COO−
C) -NH2+
D) A and B
E) A, B, and C
Answers
At a pH of 7, the charged groups present in glycine are -NH3+ and -COO−.
So, the correct answer is D) A and B.
At this pH, the amino group (-NH3+) is positively charged, and the carboxyl group (-COO−) is negatively charged, resulting in a zwitterionic form of glycine. The amino group (-NH2) on glycine can act as a base and pick up a hydrogen ion (H+) to become positively charged (-NH3+), while the carboxyl group (-COOH) can act as an acid and donate a hydrogen ion to become negatively charged (-COO-).
Therefore, at pH 7, the amino group is protonated to become -NH3+ and the carboxyl group is deprotonated to become -COO-. The net charge of glycine at pH 7 is neutral, as the positive and negative charges cancel each other out.
The chemical structure of glycine at pH 7 can be represented as H3N+CH2COO-.
For more information about glycine : https://brainly.com/question/14886525
#SPJ11
4. The sand should be removed next. What 4 pieces of equipment will you use for its removal?
The 4 pieces of equipment I will use for removal of the sand are...
5. What 2 pieces of equipment will you use to separate the salt from the water?
The 2 pieces of equipment I will use to separate the salt from the water are...
6. List out the steps involved in separating salt from the water?
The steps involved in separating salt from water are...
Answers
distillation
filtration
distillation
sedimentation
using magnet
Hope this helps
if you want to make a 1/1000 serial dilution using 9-ml water blanks, how many 9-ml blanks will you need?
Answers
To make a 1/1000 serial dilution using 9-ml water blanks, you need one 9-ml water blank. To make multiple dilutions, you need one 9-ml water blank for each dilution step.
To make a 1/1000 serial dilution using 9-ml water blanks, you will need to dilute the original sample 1000 times. This means that for every 1 ml of the original sample, you will need to add 999 ml of water. Therefore, to make a 1/1000 dilution in a 9-ml water blank, you will need to add 0.009 ml of the original sample and 8.991 ml of water.
To make multiple 1/1000 serial dilutions, you will need to repeat this process for each dilution step. For example, if you want to make a 1/1000, 1/10000, and 1/100000 serial dilution, you would start with a 9-ml water blank for each dilution step and add 0.009 ml of the original sample to the first blank to make a 1/1000 dilution. Then, you would take 0.009 ml of the 1/1000 dilution and add it to the second 9-ml water blank to make a 1/10000 dilution. Finally, you would take 0.009 ml of the 1/10000 dilution and add it to the third 9-ml water blank to make a 1/100000 dilution.
The number of 9-ml water blanks you will need depends on how many dilution steps you want to make. In this example, you would need three 9-ml water blanks, one for each dilution step.
To learn more about serial dilution
https://brainly.com/question/28939101
#SPJ4
PLEASE HELP ME QUICK RIGHT ANSWERS ONLY WILL MARK BRAINLIEST 30 POINTS
There are 3.0 * 1023 formula units KI in a sample. How many grams of KI is this? The molar mass of KI is about 166 g/mol. ? g Kl Note : Avogadro's number is ..

Answers
**MARK BRAINLIEST**
Avogadro's number is approximately 6.022 × 10^23 formula units per mole.
Given that there are 3.0 × 10^23 formula units of KI, we can calculate the number of moles of KI by dividing the number of formula units by Avogadro's number:
Number of moles = (3.0 × 10^23 formula units) / (6.022 × 10^23 formula units/mol)
Number of moles ≈ 0.498 mol
To find the mass of KI, we can use the molar mass of KI:
Mass of KI = Number of moles × Molar mass
Mass of KI = 0.498 mol × 166 g/mol
Mass of KI ≈ 82.668 g
Therefore, there are approximately 82.668 grams of KI in the sample.
What is the approximate total mass of the reactants, and how should it compare with the total mass of the products? Multiple choice question. a. 62 g, which should be less than the total mass of the products b. 62 g, which should equal the total mass of the products c. 372 g, which should be less than the total mass of the products d. 372 g, which should equal the total mass of the products
Answers
Answer:
b. 62 g, which should equal the total mass of the products
Explanation:
According to the law of conservation of mass, which states that mass of a substance can neither be created nor destroyed, hence, the mass of the reactants of a reaction must be equal to the mass of the products in that reaction.
As stated in this question, if the total mass of reactants is 62g, then, in accordance to the law of conservation of mass, the total mass of products should also be equal to 62g.
Aqueous zinc bromide reacts with solid aluminum to produce aqueous aluminum bromide and solid zinc. Write a balanced equation for this reaction
Answers
The balanced equation for the reaction between aqueous zinc bromide (ZnBr₂) and solid aluminum (Al) to produce aqueous aluminum bromide (AlBr₃) and solid zinc (Zn) is:
3ZnBr₂ + 2Al -> 2AlBr₃ + 3Zn
In this reaction, three moles of zinc bromide (ZnBr₂ ) react with two moles of aluminum (Al) to yield two moles of aluminum bromide (AlBr₃) and three moles of zinc (Zn). The equation is balanced in terms of both mass and charge, ensuring that the number of atoms of each element is the same on both sides of the equation.
This reaction represents a single replacement or displacement reaction, where aluminum replaces zinc in the compound to form a new compound and release zinc as a solid product.
Learn more about balanced equation,
https://brainly.com/question/7144750
#SPJ4
All ions and isotopes of an atom share the same____
A)atomic number
B)mass number
C)radio waves
D)charge
Answers
Answer:
A atomic number
Explanation:
not too sure
1) PbBr₂ (1) What ions are present? Which one will move towards the cathode? Which one will move towards the anode? Write the anode half equation. Write the cathode half equation. f) Write the overall redox reaction.
Answers
The ions are present in cations and anions.
The cathode Positively charged cations.
The anode is negative ions.
The oxidation occurs is Zn(s) = Zn2+ (aq) + (2e-).
A redox reaction combines two half-reactions into one complete formula. Electrons lost in the oxidation half-reaction are gained in the reduction half-reaction. Thus, a redox reaction is a chemical reaction in which electrons are transferred between the two species.
A substance that is reduced in a chemical reaction is called an oxidizing agent and a substance that is oxidized is called a reducing agent. Example of the redox reaction of zinc and copper. A reaction is complete when it reaches equilibrium, that is when the concentrations of reactants and products stop changing. If the equilibrium constant is very large.
Learn more about A redox reaction here:-https://brainly.com/question/21851295
#SPJ9