Answers
Answer:
Decomposition!
Answer:
ayo sooooo roasie how did u get ur mindreading superpowers cuz u read my mind when i was ab answer
Explanation:
Related Questions
Please answer quickly with a correct answer

Answers
Answer: The answer is D. Plant A has more turgor pressure than plant B
Explanation:
Answer:
plant A has more turgor pressure than B
calculate the concentration of h3o ions present in a solution of hcl that has a measured ph of 3.110 .
Answers
The concentration of H₃O⁺ present in a solution of HCL is 7.76247 × 10⁻⁴.
Since we know,
pH<7, therefore there are only H₃O+ particles in the solution.
[H₃O⁺ ] = \(10^{-pH}\) = \(10^{3.110}\) = 7.76247× 10⁻⁴
Pure water will have fewer hydroxide and hydronium ions. They can combine to form water. There will be a dynamic equilibrium between the concentration of molecules of water and ions. The definition of pH states that negative logarithms of hydronium ion concentration. The self-ionization will take place and increase the H⁺ concentration and side by side reduce the OH⁻ concentration.
To learn more about concentration and pH and solutions,
https://brainly.com/question/4154626
#SPJ4
draw arrows to show the path of the electricity in this series circuit
pls answer quickly as possible
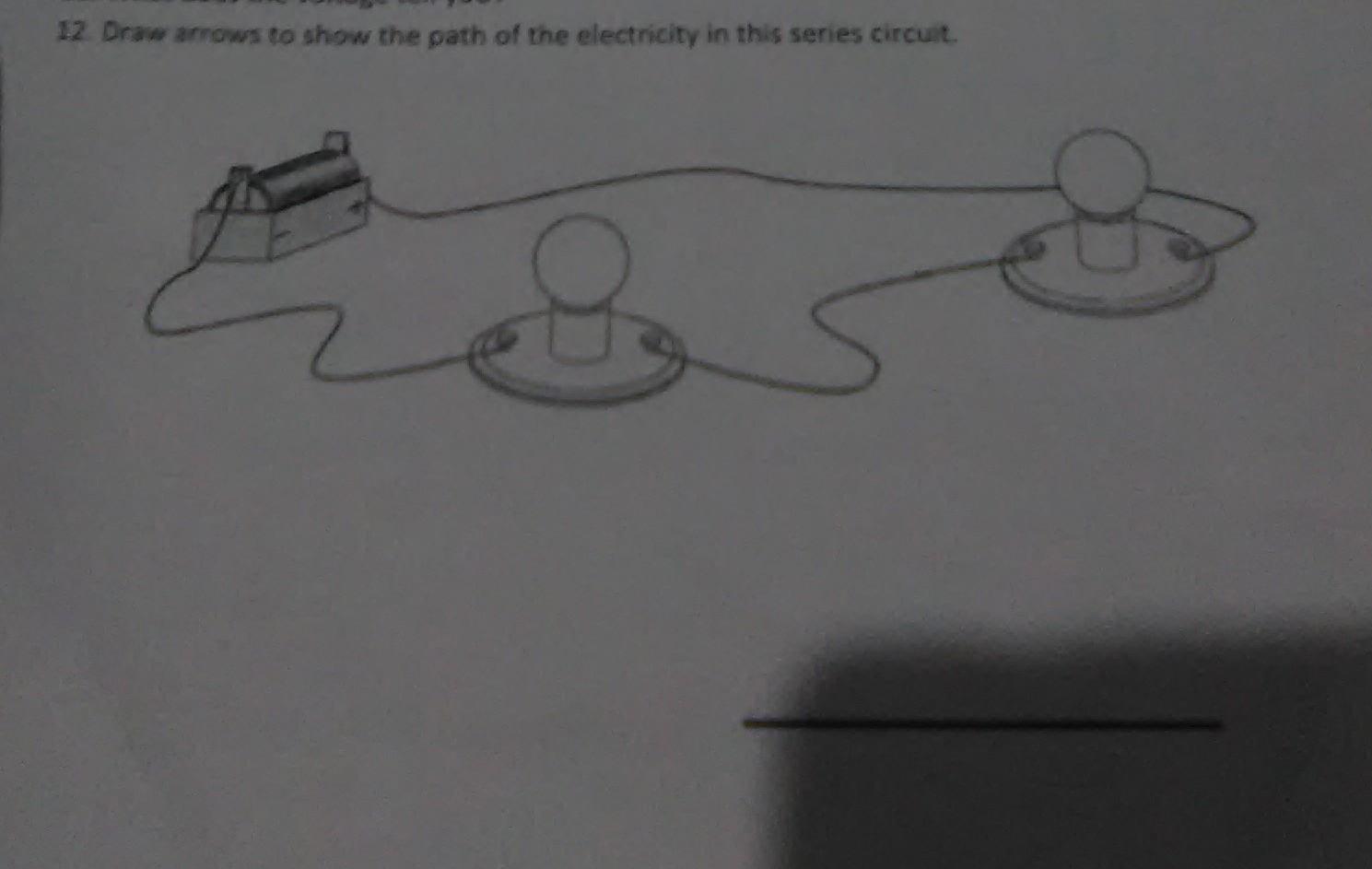
Answers
Explanation:
from positive to negative terminal

There will be a flow of current from the battery through the wires to the bulbs.
What is a circuit?The term circuit speaks of a path that is designed for the flow of current. We know that current often flows from the battery to other circuit components.
It then follows that in the circuit as shown, there will be a flow of current from the battery through the wires to the bulbs.
Learn more about circuit:https://brainly.com/question/21505732
#SPJ6
Write the net ionic equation for the hydrolysis reaction that occurs when sodium acetate, NaCH3CO2, is dissolved in water (hint: the equation should predict whether this salt is acidic, neutral or basic).
Answers
The net ionic equation for this reaction is CH3COO- + H2O → CH3COOH + OH-, and the salt is basic. The ionic equation shows only the species that are involved in the reaction. It includes the ions that take part in the reaction, which are the spectator ions excluded from the net ionic equation.
The hydrolysis reaction that occurs when sodium acetate (NaCH3CO2) is dissolved in water predicts whether this salt is neutral, acidic or basic. The net ionic equation for this hydrolysis reaction is:
CH3COO- + H2O → CH3COOH + OH-
Sodium acetate (NaCH3CO2) is a salt composed of a cation (Na+) and an anion (CH3COO-) that does not undergo hydrolysis. However, the CH3COO- ion that is produced upon dissolution in water reacts with water through hydrolysis to produce CH3COOH (acetic acid) and OH-. Thus, the reaction is basic, and its pH will be greater than 7.
Acetic acid (CH3COOH) is a weak acid that will only partially dissociate in water to form H+ and CH3COO-. The CH3COO- ion produced upon dissociation, in turn, reacts with water through hydrolysis to produce CH3COOH and OH-.
Therefore, the net ionic equation for this reaction is CH3COO- + H2O → CH3COOH + OH-, and the salt is basic. The ionic equation shows only the species that are involved in the reaction. It includes the ions that take part in the reaction, which are the spectator ions excluded from the net ionic equation.
In summary, when sodium acetate is dissolved in water, the CH3COO- ion produced undergoes hydrolysis to produce CH3COOH and OH-, making the salt basic. The net ionic equation for this reaction is CH3COO- + H2O → CH3COOH + OH-. This reaction takes place with an increase in the pH value, indicating that the solution is basic.
To know more about ionic equation visit: https://brainly.com/question/29299745
#SPJ11
17. How many joules of heat are absorbed to raise the
temperature of 435 grams of water at 1 atm from
25°C to its boiling point, 100.°C?
A) 4.5 X 10^4 J
C) 2.5 X 10^7 J
B) 1.4 X 10^5 J
D) 7.4 X 10^7 J
Answers
The amount of heat absorbed to raise the temperature of 435 grams of water at 1 atm from 25°C to its boiling point, 100°C, is approximately 1.4 × 10^5 joules
What is specific heat?
Specific heat is the amount of heat energy required to raise the temperature of a substance by one unit of temperature per unit of mass. It is a physical property that helps to characterize a substance and is typically measured in units of joules per gram per degree Celsius (J/g°C) or calories per gram per degree Celsius (cal/g°C).
The amount of heat absorbed to raise the temperature of a substance can be calculated using the formula:
Q = mcΔT
where Q is the amount of heat absorbed (in joules), m is the mass of the substance (in kilograms), c is the specific heat capacity of the substance (in joules per kilogram per degree Celsius), and ΔT is the change in temperature (in degrees Celsius).
Substituting the given values, we get:
m = 435 grams = 0.435 kg
ΔT = 100°C - 25°C = 75°C
The specific heat capacity of water is 4.184 J/g°C. We can convert this to joules per kilogram per degree Celsius (J/kg°C) by dividing by 1000:
c = 4.184 J/g°C ÷ 1000 = 4.184 J/kg°C
Substituting these values, we get:
Q = (0.435 kg) × (4.184 J/kg°C) × (75°C)
Q = 140,089.2 J
Therefore, the amount of heat absorbed to raise the temperature of 435 grams of water at 1 atm from 25°C to its boiling point, 100°C, is approximately 1.4 × 10^5 joules. The answer closest to this value is option (B).
To know more about Boiling point visit :-
https://brainly.com/question/40140
#SPJ9
The decay rate, k, for a particular radioactive element is 3.1%, where time is measured in years. Find the half-life of the element. The half-life is years. (Round to one decimal place as needed.)
Answers
The decay rate, k, for a particular radioactive element is 3.1%, where time is measured in years. The half life of elements is 22.3 years.
Thus, The following equation relates a radioactive substance's half-life (T12) to the decay rate constant, k = ln(2) / T½.
The decimal representation of the decay rate constant k, which is 3.1%: k = 0.031.
T½ = ln(2) / k
T1+2=ln(2)/0.031 = 22.3 years
The radioactive element has a half-life of about 22.3 years.
Learn more about Radioactive element, refer to the link:
https://brainly.com/question/31865009
#SPJ12
a car travels 120 kilometers due north, 82 kilometers due west and 120 kilometers south going from town A to town B, what is the cars total displacement from town A to B?
Answers
\(\rm{\pink{\underline{\underline{\blue{GIVEN:-}}}}}\)
A car travels 120km due to North .82km due to west .120km due to south ..At last the car reached on town B from town A .\(\rm{\pink{\underline{\underline{\blue{TO\:FIND:-}}}}}\)
The car's total displacement from town A to B .\(\rm{\pink{\underline{\underline{\blue{SOLUTION:-}}}}}\)
✍️ See the attachment diagram .
✯ Hence, Total displacement from town A to town B is “ 82km ” .

Answer:
displacement is the is shortest distance travelled by an object between the initial and final the position.
So, the displacement between towns A and town B is 82 km.
pls follow me!
Vernier calipers with a zero error of -0.02 gave the diameter of a marble as 1.67cm
Answers
The actual diameter of the marble is 1.69 cm.
Vernier calipers are precision measuring tools used to measure the dimensions of objects with high accuracy. However, they can sometimes have a zero error, which means that the zero mark on the calipers does not align exactly with the true zero position. In this case, the zero error is -0.02, indicating that the calipers consistently measure 0.02 cm less than the actual value.
When the calipers are used to measure the diameter of the marble and the reading is obtained as 1.67 cm, we need to account for the zero error. Since the calipers consistently measure 0.02 cm less, we need to add this value to the reading to obtain the actual diameter.
Therefore, the actual diameter of the marble is obtained by adding the zero error (-0.02 cm) to the reading obtained from the calipers (1.67 cm), resulting in an actual diameter of 1.69 cm.
It is important to consider and correct for zero errors when using measuring instruments to ensure accurate measurements. By understanding the zero error of the calipers and making the necessary adjustments, we can obtain more precise and reliable measurements.
Learn more about diameter
brainly.com/question/32968193
#SPJ11
Calculate the standard reaction enthalpy for the reaction below:
3Fe2O3(s) → 2Fe3O4(s) + ½O2(g)
Answers
The standard reaction enthalpy for the given reaction is +235.8 kJ/mol.
What is the standard reaction enthalpy of reaction?The standard reaction enthalpy (ΔH°) for the given reaction is determined as follows:
Equation of reaction: 3 Fe₂O₃ (s) → 2 Fe₃O₄ (s) + ½ O₂ (g)
The standard enthalpy of formation values for Fe₂O₃ (s), Fe₃O₄(s), and O₂(g) is used to calculate the standard reaction enthalpy.
ΔH° = [2 × ΔH°f(Fe₂O₃)] + [½ × ΔH°f(O₂)] - [3 × ΔH°f(Fe₃O₄)]
where;
ΔH°f(Fe₂O₃) = -824.2 kJ/mol
ΔH°f(Fe₃O₄) = -1118.4 kJ/mol
ΔH°f(O₂) = 0 kJ/mol
ΔH° = [2 × (-1118.4 kJ/mol)] + [½ × 0 kJ/mol] - [3 × (-824.2 kJ/mol)]
ΔH° = -2236.8 kJ/mol + 0 kJ/mol + 2472.6 kJ/mol
ΔH° = 235.8 kJ/mol
Learn more about standard reaction enthalpy at: https://brainly.com/question/15174388
#SPJ1
Define [Fluid compressibility, Solution-gas/liquid ratio, Fluid FVF, Fluid densities, and Fluid viscosities], write their equations, symbols, units \& correlations. (25-points)
Answers
1. Fluid compressibility (C): Fluid compressibility refers to the measure of how much a fluid's volume changes in response to a change in pressure.
2. Solution-gas/liquid ratio (SGLR): The solution-gas/liquid ratio represents the volume of gas dissolved in a given volume of liquid at a specific pressure and temperature.
3. Fluid formation volume factor (FVF): The fluid formation volume factor represents the ratio of the volume of a fluid at reservoir conditions (pressure and temperature) to its volume at surface conditions.
4. Fluid densities (ρ): Fluid densities refer to the mass per unit volume of a fluid.
5. Fluid viscosities (μ): Fluid viscosities represent the measure of a fluid's resistance to flow.
1. Equation: C = -1/V * dV/dP
Symbol: C
Unit: 1/Pascal (Pa^-1)
Correlation: The compressibility of fluids can vary depending on the fluid type. For ideal gases, the compressibility is inversely proportional to pressure.
2.Equation: SGLR = V_gas / V_liquid
Symbol: SGLR
Unit: Volumetric ratio (e.g., scf/bbl)
Correlation: The solution-gas/liquid ratio is influenced by the pressure and temperature conditions, as well as the composition of the fluid.
3. Equation: FVF = V_reservoir / V_surface
Symbol: FVF
Unit: Volumetric ratio (e.g., bbl/STB)
Correlation: The fluid formation volume factor depends on the composition and properties of the fluid, as well as the reservoir conditions.
4. Equation: ρ = m / V
Symbol: ρ
Unit: Mass per unit volume (e.g., kg/m^3)
Correlation: Fluid densities can vary depending on the type and composition of the fluid. For example, water has a density of approximately 1000 kg/m^3.
5. Equation: No single equation; viscosity is measured experimentally using viscometers.
Symbol: μ
Unit: Pascal-second (Pa·s) or centipoise (cP)
Correlation: The viscosity of a fluid is influenced by temperature and pressure. Different fluids exhibit different viscosities, ranging from low-viscosity fluids like water to high-viscosity fluids like heavy oil.
To know more about Fluid formation volume factor (FVF)
https://brainly.com/question/31458735
#SPJ11
what is the binding energy in kj/mol nucleons for silver-109? kj/mol nucleons 47 62 the required masses (g/mol) are:
Answers
The binding energy of silver-109 (Ag-109) in kJ/mol nucleons is not a well-defined concept, as binding energy is typically calculated for atomic nuclei rather than individual isotopes.
The binding energy of an atomic nucleus is the energy required to completely separate all of its constituent protons and neutrons into individual particles. It is usually expressed in units of energy per nucleon, which is the energy required to separate one proton or neutron from the nucleus.
The average binding energy per nucleon for an atomic nucleus is typically highest for medium-mass nuclei, such as those found in the region of the so-called "valley of stability" on the nuclear chart.
The binding energy per nucleon for silver-109 is not likely to be particularly high, as silver is a relatively heavy element and the binding energy per nucleon tends to decrease with increasing atomic number (Z).
Without more information about the specific calculation being used to determine the binding energy of Ag-109, it is not possible to accurately provide a value for the binding energy in kJ/mol nucleons.
The required masses (g/mol) are also not specified in the question, so it is not clear what context these values might be used in.
Learn more about binding energy:
https://brainly.com/question/29756225
#SPJ4
In a fractionating column, what process is caused by heating?
Answers
Answer:
During the fractional distillation of crude oil: heated crude oil enters a tall fractionating column , which is hot at the bottom and gets cooler towards the top. vapours from the oil rise through the column. vapours condense when they become cool enough.
Hope it helps you :)
The fractionating column's crystal clear glass beads provide such a vast surface area for heated vapors to cool and condensed continuously.
The fractionating column is inserted into the mouth of the distillation flask, which contains the saturated solution to be separated.
Heated crude oil is pumped into a tall fractionating column that is heated at the bottom and cools as it rises.
The oil fumes ascend through the column. When vapors cool down sufficiently, they condense. Various heights are used to lead liquids out of the columns.
Learn more:
https://brainly.com/question/25671371?referrer=searchResults
Which of the following could be the rate equation for a first order reaction? please help this is timed

Answers
Rate = k[A] = first order reaction
Further explanationGiven
Rate law
Required
A first-order reaction
Solution
The rate law : equation for the rate of chemical reaction
For reaction
aA + bB ⇒ C
The rate : r = k[A]ᵃ[B]ᵇ
The sum of exponents(a+b) is the reaction order
From the choice :
a. a+b = 2, second order reaction
b. a+b = 3, third order reaction
c. a+b = 4, fourth order reaction
d a+0 = 1, first order reaction
Select the single best answer. Classify silicon as a macromineral, micromineral, or trace mineral. macromineral micromineral trace mineral ces
Answers
Silicon is classified as a. a micromineral.
Microminerals, also known as trace minerals, are minerals that are required in very small amounts in the body, typically less than 100mg/day. Silicon is a component of connective tissue and bone, and plays a role in the health of skin, hair, nails, and cartilage. It also has been shown to improve bone density and strength, and may have a protective effect against Alzheimer's disease. While it is not considered an essential nutrient, studies have shown that adequate intake of silicon may be beneficial for overall health.
Foods that are high in silicon include whole grains, beans, nuts, and some fruits and vegetables. It is important to note that there is currently no recommended daily intake for silicon, but a balanced and varied diet can help ensure adequate intake. Silicon is classified as a. a micromineral.
Learn more about micromineral at:
https://brainly.com/question/24030423
#SPJ11
Calculate the pH at 25^oC of a 0.19 M solution of potassium butanoate (KC, H,CO). Note that butanoic acid (HC, H,Co,) is a weak acid with apk, of 4.82.
Answers
The pH at 25^oC of a 0.19 M solution of potassium butanoate (KC, H,CO approximately 2.96.
Given that potassium butanoate, KC, H, CO, is a weak acid with pKa of 4.82 and a solution of 0.19 M concentration is provided, we can calculate the pH at 25°C as follows:
\(Kw = Ka × Kb\)
Kb = Kw/Ka
Where, Kw = 10^-14 (at 25°C)
Ka = 10^-pKa
We have the pKa value of potassium butanoate as 4.82.
∴ Ka = 10^-4.82
= 1.35 × 10^-5mol/L
Now, Kb = Kw/Ka
= 10^-14/1.35 × 10^-5
= 7.41 × 10^-10M
At 25°C, we can calculate the concentration of H+ ions by using the expression given below:
Ka = [H+] × [A-] / [HA]
[H+] = Ka × [HA] / [A-]
= (1.35 × 10^-5) × √0.19 / 0.19
= 1.1 × 10^-3M
Thus, pH = -log[H+]= -log(1.1 × 10^-3)≈ 2.96
Hence, the pH of 0.19 M potassium butanoate solution at 25°C is approximately 2.96.
To learn more about pH visit;
https://brainly.com/question/2288405
#SPJ11
All atoms are positively charged, with the number of protons exceeding the number of electrons. negatively charged, with the number of electrons exceeding the number of protons. neutral, with the number of protons equaling the number of electrons.
Answers
Answer:
Yah, it's true.
because protons are represented as helium (²4He) which are positive.
electrons are represented as 0-1e which is negative
Boiling is a bulk phenomenon . True or false
Answers
Answer:
true
boiling is a bulk phenomena
Parrotfish live in the ocean and eat plantlike organisms called algae. Sunlight is shining on the
fish and the algae. Is carbon moving into the living things, moving out of the living things, or
both?
Answers
Answer:
Carbon is moving into living things (Parrotfish eating), and (I assume) Carbon is moving out of their body after they finish digesting.
This is what happens in nature, but I suspect the answer is moving into living things
Explanation:
Which of the following pairs of organisms excrete nitrogenous wastes in the form of uric acid? a) Mice and birds. b) Insects and birds. c) Lions and horses. d) Humans and frogs.
Answers
Mice and birds excrete nitrogenous wastes in the form of uric acid. The correct option is a).
Uric acid is a nitrogenous waste product that is excreted by some animals, including birds, reptiles, and insects. It is a relatively non-toxic compound that can be excreted in a semi-solid form, which helps conserve water.
Mammals, on the other hand, typically excrete nitrogenous wastes in the form of urea or ammonia, which requires more water to eliminate from the body. Therefore, among the given options, only mice and birds excrete nitrogenous wastes in the form of uric acid.
Mice are mammals that produce urea as their primary nitrogenous waste product. However, birds excrete nitrogenous wastes in the form of uric acid, which is produced in the liver and excreted from the cloaca. Therefore, option a) is the correct answer.
To know more about Uric acid refer here:
https://brainly.com/question/28583464#
#SPJ11
1. The theory of
traits of a population change over time.
Answers
The theory of traits of a population change over time explains how people can change with respect to the strength and intensity of basic trait dimensions.
What is theory of traits?Trait theory in psychology serves as the thorry that focus on the idea that people differ whichg can be attributed to their strength as well as intensity of basic trait dimensions.
It shouuld be noted that the criteria that characterize personality traits involves the act of consistency as well as stability, along with individual differences. Natural selection give us the underswtandng of how genetic traits of a species undergo change over time.
Learn more about theory of traits at:
https://brainly.com/question/4443909
#SPJ1
How do we reuse material like plastic container and footwear
Answers
Answer:
Explanation:
-Soda Bottle Sprinkler
-DIY Kitchen Storage Containers
-Piggy Bottle Bank
- pencil case
- shoe pot for plants
- turn shoe into sandals
write the net-ionic reaction that would occur between nitric acid and any carbonate ions in solution.
Answers
The net ionic equation for the reaction between nitric acid (HNO3) and a carbonate ion (CO32-) in solution is:
2H+ + CO32- → CO2 + H2O
In this reaction, the H+ ions from the nitric acid react with the CO32- ions from the carbonate, producing carbon dioxide (CO2) and water (H2O). The nitrate (NO3-) ion does not participate in the reaction and is therefore not included in the net ionic equation.
To know more about nitric acid refer here:
https://brainly.com/question/29769012
#SPJ11
Which of the following statements is TRUE o On a given day, the temperature value of west facing wall reaches a peak before east facing wall o On a given day, the temperature value of north facing wall reaches a peak before south facing wall o From thermal comfort point of view, thick walled structures are beneficial in hot and humid climates o From thermal comfort point of view, thick walled structures are beneficial in hot and dry climates
Clear my choice
Answers
From thermal comfort point of view, thick walled structures are beneficial in hot and humid climates is the statement that is true.The correct option is C.
Thick walled structures are beneficial in hot and humid climates from the thermal comfort point of view.How do thick walled structures help in hot and humid climates - In hot and humid climates, thick walls tend to absorb the heat present in the environment. This absorption helps to keep the interior of the structure cool.
Additionally, thick walls take more time to heat up and more time to cool down. Thus, in hot and humid climates, thick walls are a better choice than thin walls because they provide thermal comfort.
To know more about thermal visit :
brainly.com/question/13147561
#SPJ11
Which of the following reactions corresponds to the thermochemical equation for the standard molar enthalpy of formation of solid zinc nitrate? Zn? "(aq) 2NO; (aq) Zn(NO3)z(s) b) Zn(s) 2N(g) 6O(g) Zn(NO3)z(s) Zn(OH)z(s) 2HNO3(aq) Zn(NO3)z(s) 2Hz0(€) Zn(s) Nz(g) 30-(g) Zn(NO3)z(s) Zn(s) 2HNO;(aq) Zn(NOg)z(s) Hz(g)
Answers
The correct answer for the thermochemical equation for the standard molar enthalpy of formation of solid zinc nitrate is option b) Zn(s) + 2N(g) + 6O(g) → Zn(NO3)2(s).
This equation represents the formation of one mole of solid zinc nitrate from its constituent elements in their standard states, with all reactants and products in their standard states and under standard conditions (25°C and 1 atm pressure).
To determine the standard molar enthalpy of formation of a compound, we need to measure the enthalpy change that occurs when one mole of the compound is formed from its elements in their standard states. In this case, we need to measure the enthalpy change for the reaction Zn(s) + 2N(g) + 6O(g) → Zn(NO3)2(s), which corresponds to the formation of one mole of solid zinc nitrate from its elements in their standard states.
This reaction can be measured experimentally using calorimetry, which involves measuring the heat released or absorbed during the reaction. The enthalpy change for this reaction is then divided by the number of moles of zinc nitrate formed to obtain the standard molar enthalpy of formation of solid zinc nitrate.
learn more about equation
https://brainly.com/question/29545122
#SPJ11
Which property of a substance determines whether the substance is a liquid or a solid at room temperature?
a) melting point
b) flexibility
c) solubility
d) conductivity
Answers
Answer:
a) melting point
classify the type of microscope based on a radiation they use
Answers
Answer:
Light vs electron
Explanation:
Light Microscope vs Electron Microscope. Light microscopes and electron microscopes both use radiation - in the form of either light or electron beams, to form larger and more detailed images of objects (e.g. biological specimens, materials, crystal structures, etc.) than the human eye can produce unaided.
If a 100-mL vial containing calcium chloride dihydrate (MW 147) is added to a 1-L container of water for injection, calculate the concentration of calcium chloride, in mEq/mL in the original vial, if the resultant dilution is 0.56% in strength.
Answers
The concentration of calcium chloride in the original vial is approximately 5.39 mEq/mL.
To calculate the concentration of calcium chloride in mEq/mL in the original vial, we need to consider the dilution that occurs when the 100-mL vial is added to a 1-L container of water for injection.
Given information:
The molecular weight (MW) of calcium chloride dihydrate is 147 g/mol.
The dilution results in a 0.56% strength solution.
First, let's calculate the mass of calcium chloride in the 1-L container:
Mass of calcium chloride = (0.56% / 100%) * 1,000 mL * 1 g/mL = 5.6 g
Next, we need to convert the mass of calcium chloride to moles:
Moles of calcium chloride = 5.6 g / 147 g/mol = 0.038 moles
Since each mole of calcium chloride generates two moles of calcium ions (Ca2+) during dissociation, we have:
Moles of calcium ions = 0.038 moles * 2 = 0.076 moles
Finally, we calculate the concentration of calcium chloride in mEq/mL:
Concentration of calcium chloride = (0.076 moles / 1,000 mL) * (1,000 mEq / 1 mole) = 0.076 mEq/mL
Therefore, the concentration of calcium chloride in the original vial is approximately 5.39 mEq/mL.
The concentration of calcium chloride in the original 100-mL vial, when added to a 1-L container of water for injection resulting in a 0.56% strength solution, is approximately 5.39 mEq/mL.
To learn more about concentration, visit
https://brainly.com/question/30639206
#SPJ11
We need to prepare 50 ml of a solution, containing 5 grams/liter of salt. how many grams of salt do we need?
Answers
To prepare 50 ml of a solution, containing 5 grams/liter of salt, the amount in grams of salt we need is 0.25 grams.
To prepare 50 ml of a solution containing 5 grams/liter of salt, we need to use the following formula:
Mass of salt = volume of solution x concentration of solution
Mass of salt = 50 ml x 5 g/L
Convert the volume to liters:
50 ml ÷ 1000 ml/L = 0.05 L
Substitute the values into the formula:
Mass of salt = 0.05 L x 5 g/L
Mass of salt = 0.25 g
Therefore, we need 0.25 grams of salt to prepare 50 ml of a solution containing 5 grams/liter of salt.
Learn more about concentration here; https://brainly.com/question/26255204
#SPJ11
At a certain temperature, the equilibrium constant K for the following reaction is 819.: Use this information to complete the following table. Suppose a 38. L reaction vessel is filled with 0.51 mole of CO_2 and 0.51 mole of H_2. What can you say about the composition of the mixture in the vessel at equilibrium? What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. There will be very little CO and H_2O. There will be very little CO_2 and H_2. Neither of the above is true.
Answers
At equilibrium, the composition of the reaction vessel with 0.51 moles of CO₂ and 0.51 moles of H₂ is given by the equilibrium constant K, which is 819.
The reaction given is:
H₂(g) + CO₂(g) ⇌ CO(g) + H₂O(g)
So, we know the formula for Kp and Kc in terms of partial pressure and concentration are as follows:
Kc = (CO(g) × H₂O (g)) / (CO₂(g) × H₂(g))
Kp = (PCO × PH₂O) / (PCO₂ × PH₂)
At equilibrium, the equilibrium constant expression can be written as:
Kc = [CO][H₂O] / [H₂][CO₂] = x²/ [(0.51-x)(0.51-x)]
Substituting the given value of Kc into the above equation and solving for x gives:
819 = x²/[(0.51-x)(0.51-x)]
x = 0.302
From the results, we can say that "There will be very little CO₂ and H₂" at equilibrium.
The equilibrium constant for the given reaction is 819.
Learn more about equilibrium constants here:
https://brainly.com/question/3159758
#SPJ11
Sarah, Leah, Haley, Lindsay, and Kim bought 4 bottles of water to share among themselves. They divided each bottle of water into 5 equal portions. Sarah took 1 portion from each bottle of water.
Answers
Answer:
4/5 of a bottle
Explanation:
Sarah, Leah, Haley, Lindsay, and Kim bought 4 bottles of water to share among themselves. They divided each bottle of water into 5 equal portions. Sarah took 1 portion from each bottle of water. Which equation represents how much of a bottle of water Sarah took?
Sarah must have taken 4/5 of a bottle
First, the 4 bottles of water were divided into 5 equal portions;
4 x 5 = 20 portions.
Sara too 1 portion from each bottle;
1 portion x 4 bottles = 4 portions
Which equation represents how much of a bottle water Sarah took?
Since each bottle water was divided into 5 equal proportions and assuming that the bottle waters are of the same volumes, then Sarah has taken 4 out of 5 portions of a bottle water.
Answer: 4 ÷ 5 ﹦4/5 ♡♡♡
Explanation: When you see eqautions like these, always remember that the first number goes on the top and the second one goes on the bottom. Hope this helps! (~﹃~)~Hi☆
