Answers
Answer:
The answer is B(scale)
Explanation:
Since the question asked about a range, the scale would be the most logical answer since scales are used to measure.
Answer:
the answer is C.
Explanation:
Related Questions
At STP, how many liters of hydrogen are needed to react with 88 g of copper (II) oxide?
Answers
Answer:
So if we need to react with 88 gm. of copper 2 then at least 3-4 liters.
Explanation:
Question 1 (4 points)
What is the molecular weight of Magnesium nitride, Mg3N2 (OR Mg3 N2). Report
your answer to two decimal places.
Do not include units with your answer.
The atomic weight of Mg is 24.31 grams/mole
The atomic weight of N is 14.01 grams/mole
Answers
what leads to the formation of a wind chill factor
Answers
Answer:
What leads to the formation of a wind chill factor? A change in the air temperature creates air pressure differences in the atmosphere
Explanation:
hope this helps :)
Dennie wanted to see if crayfish preferred one kind of food over another. He gave one group of crayfish plants, both living and dead, to eat. He gave the other group insects, living and dead, to eat. What is the independent variable in Dennie's experiment? a)type of food b)weight of crayfish c)typeof water used d)age of crayfish
Answers
Answer:
A) type of food.
Mean, median and mode are best described as which?
A. Qualitative and quantitative data
B. Measures of central tendency
C. Discrete and continuous data
D. Histogram, scatterplot and circle
Answers
Answer:
B. Measures of central tendency
Explanation:
Mean, median and mode are best described as measures of central tendency of a given data set.
Mean is the average of the samples given
Mode is the data point with the most frequent occurrence
Median is the data point that lies in the middle
All these parameters tells us how far a data point is from the middle or how close they are.Here, do the following: (1) Name three professions/careers/jobs/situations, where knowing exactly how much of something that you start with and keeping things in balance is extremely important. (2) Name the importance of balance in that job/situation and finally, (3) name one bad thing that can happen if balance is not maintained
Example Job/Situation
Avery adulting with his digital wallet
Importance of Measurement for Job/ Situation 1:
Not doing this well can lead to an eviction, past due bills, and bad credit
What bad thing could happen if things are not kept in balance for Job/Situation 1?
Credit could decrease, he could get evicted, his heat will not work due to non-payment
.
Your Turn:
Job/Situation 1:
Importance of Measurement for Job/ Situation 1:
What bad thing could happen if things are not kept in balance for Job/Situation 1?
Job/Situation 2:
Importance of Measurement for Job/Situation 2:
What bad thing could happen if things are not kept in balance for Job/Situation 2?
Job/Situation 3:
Importance of Measurement for Job/Situation 3:
What bad thing could happen if things are not kept in balance for Job/Situation 3?
Answers
Job/Situation 1: Chemical laboratory technician, where the importance is ,in a chemical laboratory, precise measurement of chemicals and substances is crucial for conducting experiments, formulating accurate solutions, and bad things are are not accurate or balanced in a chemical laboratory. Another is financial analyst,they deal with managing financial data, analyzing investments, bad thing is inaccuracy. Another is Chef where the cooking skill must be good. Risk is fire etc.
As a chef bad thing is ,it can lead to improperly seasoned dishes, inconsistent flavors, undercooked or overcooked food, and dissatisfied customers. It can harm the reputation of the restaurant, lead to negative reviews, and impact customer satisfaction and loyalty. , misinformed investment decisions, and financial losses are the bad of financial analyst etc.
Learn more about professionals heere
https://brainly.com/question/23186207
#SPJ1
How many moles are in 7.00E24 atoms of Ca?
Answers
Explanation:
In chemistry, a mole is the base unit of the amount of substance in a system containing elementary entities (atoms, molecules, ions, electrons), and one mole of substance corresponds to the Avogadro's constant or \(6.023 \ \times \ 10^{23}\) entities. The stoichiometry equation that relates the amount of substance into the measurement in moles is
\(n \ = \ \displaystyle\frac{\alpha}{N_{A}}\),
where \(n\) is the number of moles, \(\alpha\) is the amount of substance and \(N_{A}\) is the Avogadro's constant.
Therefore,
\(n \ = \ \displaystyle\frac{7.00 \ \times \ 10^{24} \ \text{atoms}}{6.023 \ \times \ 10^{23} \ \text{atoms mol}^{-1}} \\ \\ n \ = \ 11.624 \ \text{mol}\).
What is the answer? Please

Answers
Answer:
Leeunwenhoek
Explanation:
Anton Von Leeunwenhoek was the first scientist to observe live cells and in greater details. He described spirogyra in the mid 15th century.
His contribution to the scientist community opened up the world of micro-organisms. He produced several microscopes to observed the world of micro-organisms that we cannot see with our naked eyes. He also studied plants extensively.Brainliest get's +30 Points!

Answers
Answer:
Yesssssss , option two is the correct one click it buddy
Explanation:
A student places 25.0 mL of 0.100 M hydrazoic acid (HN3) in an Erlenmeyer flask and adds indicator. She then adds 5.0 mL of standardized 0.200 M NaOH. What is the pH of the solution
Answers
Answer:
pH = 4.42
Explanation:
HN3, a weak acid reacts with NaOH as follows:
HN3 + NaOH → H2O + NaN3
Where 1mol HN3 reacts with 1mol NaOH
After the reaction of only a part of HN3, you will have in solution HN3 (Weak acid) and NaN3 (Conjugate base). This mixture produce a buffer that follows H-H equation:
pH = pKa + log [Conjugate base] / [Weak acid)
Where pH is the pH of the buffer
The hydrazoic acid is a weak acid with pKa = 4.6
And [] could be taken as the moles of each species
After the reaction, the moles of NaOH added = Moles NaN3 produced
And moles HN3 = Initial moles HN3 - Moles NaOH
Moles NaOH = Moles NaN3:
5x10⁻³L * (0.200mol / L) = 1x10⁻³ mol NaN3
Initial moles HN3:
0.025L * (0.100mol / L) = 2.5x10⁻³ moles HN3
final moles: 2.5x10⁻³ moles HN3 - 1x10⁻³ mol = 1.5x10⁻³ moles HN3
Replacing in H-H equation is:
pH = 4.6 + log [1x10⁻³ mol NaN3] / [1.5x10⁻³ moles HN3]
pH = 4.42Can someone teach me step by step how to find the compound name of the following compound:
V(SO4)2
Answers
Vanadium(II) sulfate
Vanadium(III) sulfate is the inorganic compound with the formula V2(SO4)3
What is the general method/rule/formula for calculating where the ball will land?
Answers
The general method/rule/formula for calculating where a ball will land depends on various factors such as the initial velocity, angle of projection, air resistance, and gravity.
In general, the most common method to calculate the landing position of a projectile is to use the kinematic equations of motion, which relate the position, velocity, and acceleration of an object. These equations can be used to determine the time it takes for the ball to hit the ground and the horizontal distance it travels during that time.
However, the calculation can become more complex when considering factors like air resistance and varying conditions, and may require more advanced mathematical models.
Learn more about Resistance at
brainly.com/question/29427458
#SPJ1
1.40 g H2 is allowed to react with 9.66 g N2, producing 2.24 g NH3
.
What is the theoretical yield in grams for this reaction under the given conditions?
Answers
The theoretical yield of NH₃ produced under the given conditions is 11.75 g.
The balanced equation for the reaction between hydrogen (H₂) and nitrogen (N₂) to form ammonia (NH₃) is;
N₂ + 3H₂ → 2NH₃
To determine the theoretical yield of NH₃ produced from the given amounts of H₂ and N₂, we need to calculate the limiting reactant and then use stoichiometry to find the maximum amount of NH₃ which can be produced.
The molar masses of H₂, N₂, as well as NH₃ are;
H₂; 2.02 g/mol
N₂; 28.02 g/mol
NH₃; 17.03 g/mol
The number of the moles of each reactant will be calculated as;
moles of H₂ = mass of H₂ / molar mass of H₂ = 1.40 g / 2.02 g/mol = 0.693 mol
moles of N₂ = mass of N₂ / molar mass of N₂ = 9.66 g / 28.02 g/mol = 0.345 mol
To determine the limiting reactant, we need to compare the mole ratios of H₂ and N₂ in the balanced equation with the actual mole ratios of the reactants. The balanced equation shows that 1 mole of N₂ will reacts with 3 moles of H₂ to produce a 2 moles of NH₃. The actual mole ratio of N₂ to H₂ in the reaction mixture is;
moles of N₂ / moles of H₂ = 0.345 mol / 0.693 mol
= 0.498
This ratio is less than the required ratio of 1/3, which means that N₂ is the limiting reactant. This means that all the N₂ will be consumed in the reaction and the amount of NH₃ produced will depend on the amount of N₂ present.
Using the mole ratio from the balanced equation, we can calculate the theoretical yield of NH₃ that can be produced from the 0.345 mol of N₂;
moles of NH₃ = (0.345 mol N₂) × (2 mol NH3 / 1 mol N₂)
= 0.690 mol NH₃
The mass of this amount of NH₃ can be calculated as;
mass of NH₃ = moles of NH₃ × molar mass of NH₃ = 0.690 mol × 17.03 g/mol = 11.75 g
Therefore, the theoretical yield is 11.75 g.
To know more about theoretical yield here
https://brainly.com/question/14966377
#SPJ1
Someone help me I don’t know

Answers
Answer:
What's the gas given in the question??
Object A is at a higher temperature than Object B. The objects have different masses and are made of different materials. Thermal energy transfers between the objects until
A. all of Object A's heat has transferred to Object B B. the objects have changed temperature by the same amount C. an equilibrium temperature is reached D. their specific heats are equal
Answers
Thermal energy transfers between the objects until an equilibrium temperature is reached.
What is thermal energy transfer?
Thermal energy transfer is the process by which heat transmitted from one body to another body, usually from a hotter body to the colder body.
Heat transfer process can occur in three different ways, and they include;
Heat transfer by conductionHeat transfer by radiationHeat transfer by convectionHeat transfer by conduction involves the actual movement of molecules from a hotter region to a colder region.
Heat transfer by radiation occurs through a space, from a hotter source to a colder region.
Heat transfer by convection occurs in a fluid, by the rising of hot molecules and sinking of the colder molecules.
Based on the law of conservation of energy, the heat transferred from a hotter body to a colder body will continue until both bodies reaches a state known as thermal equilibrium.
Thus, in thermal equilibrium, the final temperature of both bodies will be the same.
Learn more about thermal equilibrium here: https://brainly.com/question/9459470
#SPJ1
Please!!
Look at this Bohr model for oxygen. Describe how the Bohr model of oxygen relates to the electron-dot structure of oxygen.
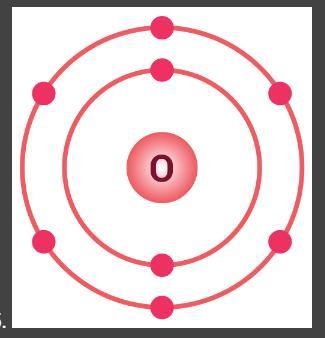
Answers
Bohr model of oxygen relates to the electron-dot structure of oxygen as
in electron dot structure an atom must have 8 electrons in it's surrounding and oxygen also has 8 electrons in total.
What Is Bohr's model of an atom?
The postulates of Bohr's Model of an atom are-
(1) Electrons revolve around the nucleus in a certain definite path called orbit or stationary state of shell.
(2) The shells are having different energy levels denoted as K, L, M, N ...
(3) As long as the electron remains in an orbit, they neither absorb nor emit energy.
(4) The electron can move only in that orbit in which angular momentum is quantized, i.e., the angular momentum of the electron is an integral multiple of h/2π .
Therefore, Bohr model of oxygen relates to the electron-dot structure of oxygen as in electron dot structure an atom must have 8 electrons in it's surrounding and oxygen also has 8 electrons in total.
Learn more about Bohr's model, here:
https://brainly.com/question/3964366
#SPJ1
How many moles of water would be produced from 3 moles of oxygen in the following reaction? Don’t forget to include units in your answer.
PLZHELP I'LL AWARD BRAINLIEST

Answers
Answer:
6 mol H₂O
General Formulas and Concepts:
Chemistry
StoichiometryExplanation:
Step 1: Define
RxN: 2H₂ + O₂ → 2H₂O
Given: 3 moles O₂
Step 2: Stoichiometry
\(3 \ mol \ O_2(\frac{2 \ mol \ H_2O}{1 \ mol \ O_2} )\) = 6 mol H₂O
Step 3: Check
We are given 1 sig fig.
Our final answer has 1 sig fig, so no need to round.
Use each of the following terms
in a separate sentence: states of
matter and change of state.
Answers
Answer:The states of matter are the physical forms of matter, which include solid, liquid and gas. The Change of Matter is the change of a substance from one physical state another.
Explanation: Hope it helps guys :)
The states of matter are the physical forms of matter. Change of state refers to the chemical or physical changes which change the state of the substance.
What is matter?Matter in chemistry, is defined as any kind of substance that has mass and occupies space that means it has volume .Matter is composed up of atoms which may or not be of same type.
Atoms are further made up of sub atomic particles which are the protons ,neutrons and the electrons .The matter can exist in various states such as solids, liquids and gases depending on the conditions of temperature and pressure.
The states of matter are inter convertible into each other by changing the parameters of temperature and pressure.Matter can be conserved according to law of conservation of matter.
Learn more about matter,here:
https://brainly.com/question/12972782
#SPJ2
A scientist is observing a rare monkey in the jungle, and the monkey begins exhibiting an odd behavior. The scientist thinks this behavior may not have been observed in this species before, but he is not certain. The scientist records video of the behavior and takes many detailed notes.
The scientist becomes very excited about his finding and plans to share it with other scientists. The best way for him to communicate and defend his finding would be to
A.
immediately write a report claiming a newly discovered behavior, and then check later to see if others have observed it before.
B.
confirm that the behavior has not been observed before, and then present his finding in a report.
C.
defend his finding as a new discovery, even if another scientist has already published a report about the behavior.
D.
wait until other scientists ask him about his trip to the jungle, and then tell them about his finding.
Answers
When Mrs. Green describes the physical properties of matter she said that physical properties often concern changes in state, One
physical property that does not describe a change in state is
A)
density
B)
evaporation
C)
freezing
D)
melting
Answers
Answer:
density
Explanation:
Density is an important measurement. It has an unit: g/mL or kg/L, ...
Evaporation, freezing, melting are the processes in which the substances change their states. Other processes are: condensation, sublimation, deposition.
...
Hope this answer can help you. Have a nice day!
A sealed container can hold 6.28 L CO2 at 1.00 atm and 293 K. How many moles of CO2 fill the container?
Answers
The term "molar volume" (Vm) of an ideal gas refers to this amount. (STP) as 293 kelvin (K) of temperature and 1 atmosphere of pressure (P = 1 atm, which is also equal to 760 torr). Any ideal gas has a 22.4 L molar volume at STP.
How can I calculate the molar volume?The volume occupied through one mole of the a chemical element or chemical compound at a standard temperature and pressure (STP) is known as the molar volume (Vm). By dividing the mass density () by the molar mass (M), it can be computed.
Are moles and molar volume the same thing?One mole of any gas has the same volume when it is present at the same temperature and pressure. The volume that one mole of any gas occupies at standard pressure and temperature is known as the molar volume. 24 dm3 is equivalent to the molar volume (24,000 cm 3). In cases where it is necessary, this volume is provided.
To know more about molar volume visit:
https://brainly.com/question/29884686 visit:
#SPJ1
The conversion of methyl isonitrile to acetonitrile in the gas phase at 250 °C CH3CN(g) is first order in CH3NC with a rate constant of 3.00×10^-3 s-1. If the initial concentration of CH3NC is 5.45×10^-2 M, the concentration of CH3NC will be 1.56×10^-2 M after_____________ s have passed.
Answers
Answer: The concentration of \(CH_3NC\) will be \(1.56\times 10^{-2}M\) after 416 seconds have passed.
Explanation:
Expression for rate law for first order kinetics is given by:
\(t=\frac{2.303}{k}\log\frac{a}{a-x}\)
where,
k = rate constant = \(3.00\times 10^{-3}s^{-1}\)
t = age of sample = ?
a = let initial amount of the reactant = \(5.45\times 10^{-2}M\)
a - x = amount left after decay process = \(1.56\times 10^{-2}M\)
\(t=\frac{2.303}{3.00\times 10^{-3}}\log\frac{5.45\times 10^{-2}}{1.56\times 10^{-2}}\)
\(t=416s\)
The concentration of \(CH_3NC\) will be \(1.56\times 10^{-2}M\) after 416 seconds have passed.
For each of the following compounds, indicate the pH at which 50% of the compound will be in a form that possesses a charge and at which pH more than 99% of the compound will be in a form that possesses a charge.
ClCH2COOH (pKa = 2.86)
CH3CH2NH+3 (pKa = 10.7)
Express your answer using two decimal places
a. Determine a pH at which 50% of ClCH2COOH will be in a form that possesses a charge.
b. Determine a pH at which pH more than 99% of ClCH2COOH will be in a form that possesses a charge.
c. Determine a pH at which 50% of CH3CH2NH+3 will be in a form that possesses a charge.
d. Determine a pH at which pH more than 99% of CH3CH2NH+3 will be in a form that possesses a charge.
Answers
Answer:
a. 2..86 b. 4.86 c. 10.7 d. 8.7
Explanation:
a. Determine a pH at which 50% of ClCH2COOH will be in a form that possesses a charge.
Using the Henderson-Hasselbalch equation,
pH = pKa + log[A⁻]/[HA]
where [A⁻] = concentration of conjugate base (or charged form) and [HA] = concentration of acid.
At 50% concentration, [A⁻] = [HA] ⇒ [A⁻]/[HA] = 1
So, pH = pKa + log[A⁻]/[HA]
pH = pKa + log1
pH = pKa = 2.86
b. Determine a pH at which pH more than 99% of ClCH2COOH will be in a form that possesses a charge.
Let x be the concentration of the acid. Since 99% of it should possess a charge, the basic concentration is 0.99x while the acidic concentration is remaining 1 % (1 - 0.99)x = 0.01x
Using the Henderson-Hasselbalch equation,
pH = pKa + log[A⁻]/[HA] where [A⁻] = concentration of conjugate base (or charged form) = 0.99x and [HA] = concentration of acid = 0.01x.
pH = pKa + log0.99x/0.01x
pH = pKa + log0.99/0.01
pH = 2.86 + log99
pH = 2.86 + 1.996
pH = 4.856
pH ≅ 4.86
c. Determine a pH at which 50% of CH3CH2NH+3 will be in a form that possesses a charge.
Using the Henderson-Hasselbalch equation,
pH = pKa + log[A⁻]/[HA]
where [A⁻] = concentration of conjugate base and [HA] = concentration of acid.
At 50% concentration, [A⁻] = [HA] ⇒ [A⁻]/[HA] = 1
So, pH = pKa + log[A⁻]/[HA]
pH = pKa + log1
pH = pKa = 10.7
d. Determine a pH at which pH more than 99% of CH3CH2NH+3 will be in a form that possesses a charge.
Let x be the concentration of the acid. Since 99% of it should possess a charge, the basic concentration is 0.01x while the acidic concentration is remaining 99 % (1 - 0.01)x = 0.99x (which possesses the charge).
Using the Henderson-Hasselbalch equation,
pH = pKa + log[A⁻]/[HA] where [A⁻] = concentration of conjugate base = 0.01x and [HA] = concentration of acid = 0.99x.
pH = pKa + log0.01x/0.99x
pH = pKa + log1/99
pH = 10.7 - log99
pH = 10.7 - 1.996
pH = 8.704
pH ≅ 8.7
Just need to know the one with the Hexagon

Answers
Answer:2,4-dichlorohexane
Explanation:
Hope it helps
What happens as elevation increases?
Answers
Answer:
As altitude increases, the amount of gas molecules in the air decreases the air becomes less dense than air nearer to sea level.
Explanation:
Which of the following addresses the economic question of how to produce?
a growing corn instead of potatoes
b. requiring individuals to complete specific types of work
c. producing more capital goods and fewer consumer products
d. selling natural resources to other countries
Answers
Answer:
b
Explanation:
The correct answer would be requiring individuals to complete specific types of work.
A question of how to produce deals with the processes of production itself. There are several small processes that eventually aggregate together to lead to the production of goods or services. Some of these small processes will definitely require that individuals within the production unit complete specific types of work.
The option of growing corn instead of potatoes and producing more capital goods and fewer consumer products address what to produce while the option of selling natural resources to other countries has to do with for whom to produce.
Correct option: b
where is water stored after it has infiltrated deep into the ground?
A. river
B. lake
C. aquifer
D. aquaduct
Answers
Answer:
C. aquifer
Explanation:
I just did a lesson on this
:)
Does anyone know Chemistry
Answers
Answer:
so so
Explanation:
this your question?? <_>
Which element has chemical properties most similar to sodium? a. magnesium b. oxygen c. phosphorus d. rubidium
Answers
The element that has chemical properties most similar to sodium is d. rubidium.
What is rubidium?
Rubidium is in the same group (group 1) as sodium in the periodic table and has similar chemical properties, such as reactivity with water and the tendency to form ionic compounds with halogens. Magnesium, oxygen, and phosphorus are not in the same group as sodium and have different chemical properties.
What is periodic table?
The periodic table is a tabular display of all known chemical elements, arranged according to their atomic structure and properties. It is arranged in rows and columns, with elements placed in order of increasing atomic number, which is the number of protons in an atom's nucleus. The periodic table is a powerful tool for predicting the chemical behavior of elements and for understanding the relationships between different elements. It is used extensively in chemistry, physics, and other sciences to help understand the properties and behavior of different elements, and to guide research and development in many different fields.
To know more about rubidium, visit:
https://brainly.com/question/28838867
#SPJ1
A. Direction: Identify the word or phrase being described in the sentence. Write your
answers in your notebook.
1. It is an electronic measuring instrument that combines several
measurement functions in one unit.
2. In diodes, what do the silver stripe represents?
3. This is an Electronic/Electrical component that stores energy in
the form of Electric Charge.
4. This allows you to change the function between volts, ohms, and
amps, and to change the scale of the meter.
5. In testing capacitor, if the multimeter shows very low resistance,
it means that the capacitor is .
6. These are components used to resist the flow of electric current.
7. It is a system used to determine the value of a resistor without
using a multimeter.
8. What defective capacitor that shows very low Resistance?
9. A type of transformer that is used to increase the output voltage.
10. A type of transformer that is used to decrease the output voltage.
Answers
Answer:
1. Multimeter
2. Cathode
3. Capacitor
4. Selector switch
5. Short or Shorted
6. Resistors
7. Resistor color coding
8. Open Capacitor
9. Step-up transformer
10. Step-down transformer
Explanation:
The above-described elements are electronic components. Resistors for instance are designed to resist the flow of electric current. They are also standardized such that a deviation from the set resistance level will indicate a problem. The capacitor is another electrical component that stores energy as an electrical charge. Knowledge of these electrical components and the ways they are tested will make a person proficient in electrical electronics.