Please help with 3 b and 4 a , b , c and e! Irrelevant answers will be reported!* ASAP!T_T

Answers
Answer:
3.Test for unsaturation is carried out by either Baeyer's test or bromine water test.
(a) What is Baeyer's reagent?
Ans:
Baeyer's reagent is an alkaline solution of Potassium Permanganate \(\bold{KMnO_4}\) .
(b) How does it detect presence of double bond in organic compound? Write the reaction involved.
Ans:
When a dilute alkaline solution of \(\bold{KMnO_4}\) is added to the given unsaturated organic compound, if the purple color of permanganate solution disappears, the presence of double bond is indicated.
For example:
2\(\bold{KMnO_4}\) + 2KOH \(\longrightarrow\)\(\bold{2K_2MnO_4 + H_2O+[O]}\)
\(\bold{CH_2=CH_2(ethene) +H_2O+[O]\longrightarrow CH_2OH-CH_2OH (ethylene \:glycol :Colourless)}\)
(c) Write the product when ethyne is treated with Baeyer's reagent.
Ans:
Product will not produce as ethyne doesn't contain double bond.
(d) Benzene (an aromatic hydrocarbon) does not give Baeyer's test though it has double bonds. Why?
Ans:
As Benzene is an unsaturated compound. The structure of benzene contains 3 alternate single double bonds.
(e) Write the reaction for bromine water test for ethyne.
Ans:
Reaction:
\(\bold{CH_2CH_2(ethene)+Br_2( red \: color) \longrightarrow CH_2Br-CH_2Br}\)
(1,2-dibromoethane (colorless}
Kolbe electrolytic method can be used to prepare alkane, alkene and alkyne.
(a) At which electrode, alkane, alkene or alkyne is obtained?
Ans:
alkane, alkene or alkyne is obtained at anode.
(b) Which electrolyte should be chosen for alkane, alkene or alkyne for the method? Suggest an example of electrolyte for each.
Ans:
alkane: Potassium or sodium salt of Carboxylic acid
alkene: Potassium or sodium salt of dicarboxylic acid
alkyne: Potassium or sodium maleate
(c) If potassium salt of dicarboxylic acid is taken for the method, which hydrocarbon is prepared?
Ans: If potassium salt of dicarboxylic acid is taken for the method, Alkene hydrocarbon is prepared.
(d) Write the detail process for the electrolysis of aqueous potassium maleate.
Ans: Attachment
(e) Suggest a demerit of the method.
Ans:
Demerits:
Kolbe electrolysis can produce a mixture of different products, including both desired and undesired ones.It requires a significant amount of energy to produce the desired products. This can make the process expensive and inefficient.It is limited to the electrolysis of alkanes with low molecular weight, and it may not be suitable for the electrolysis of more complex organic molecules.This process generates significant amounts of waste and can produce pollutants that can harm the environment.
Related Questions
A wave with a small wavelength has a high frequency.
options:
True
False
Answers
The reactants in a chemical reaction are shown below.
Li₂CO3 + H2SO4
Answers
Answer:
Li2SO4+H2CO3
Explanation:
put d equation this way
negative ions to positive ions
the complete reaction catalyzed by complex i can be summarized as ________.
Answers
NADH + Q + 5H+ QH2 + NAD+ + 4H+ is a shorthand for the entire process that complex I catalyzed. Usually, specialized chemistry is progressed at a faster rate using a catalyzed reaction.
Typically, a catalyst's job is to offer a different, low-energy pathway for a reaction. This is accomplished through the catalyst's interaction with a reactant and formation of an intermediary molecule. To create products, all reactants must first overcome a specific energy, more commonly referred to as activation energy. This activation energy is the difference between the reactant species' and the transition state's energies. While some reactant molecules have sufficient kinetic energy to pass through this energy barrier, others do not.
Learn more about catalyst's here
https://brainly.com/question/28813725
#SPJ4
Which of the following happens when a reaction reaches dynamic equilibrium in a closed system?
A) The concentrations of the reactants and products increase.
B) The concentrations of the reactants and products remain constant
C) The rate of the forward reaction is faster than the rate of the reverse reaction.
D) The rate of the forward reaction is slower than the rate of the reverse reaction
Answers
Answer: B
Explanation:
At equilibrium, the rate of the forward reaction is equal to the rate of the reverse reaction, and thus the concentrations of the reactants and products must be constant.
what happens acetic acid boiling point in c when pressure is 446 torr?
Answers
The boiling point of acetic acid will decrease when the pressure is reduced to 446 torrs.
This is because the boiling point of a substance is directly related to the pressure it is under. When the pressure is decreased, the boiling point will also decrease. At normal atmospheric pressure (760 torr), the boiling point of acetic acid is 118.1°C. However, at a pressure of 446 torr, the boiling point will be lower. The exact boiling point at this pressure will depend on the specific conditions and the purity of the acetic acid.
You can learn more about acetic acid at
https://brainly.com/question/15231908
#SPJ11
In a 0.100M solution of a weak polyprotic acid what is the equilibrium concentration of A-2?
H2A+H20-H30+A-2
Ka1=7.5x10^-5
Ka2=6.2x10^-8
answer= ?x10^?
Answers
Can someone please help with this chemistry question
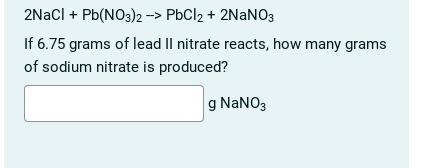
Answers
The mole ratio of NaNO₃ to Pb(NO₃)2 is 2:1. Therefore, 6.75 g of Pb(NO₃)₂ will produce 2 x 6.75 g = 13.5 g of NaNO₃.
What is mole?Mole is a unit of measurement used in chemistry to measure the amount of a substance, usually expressed in terms of the number of atoms, molecules, or other units in a given mass of that substance. The mole, also known as Avogadro's number, is a very large number equal to 6.022 x 10^23. This means that a mole of a substance contains 6.022 x 10^23 atoms or molecules, depending on the substance. The mole is used to measure the amount of a substance in a given sample, and is essential to many chemical calculations.
To learn more about mole
https://brainly.com/question/24191825
#SPJ1
10.0g of fe and 7.00g of 02 react how many grams of fe203 are produced
Answers
10g of Fe produces 14.37g of \(Fe_2O_3\) and 7g of \(O_2\) produce 46.84g of \(Fe_2O_3\)
What is Limiting Reagent ?The limiting reactant (or limiting reagent) is the reactant that gets consumed first in a chemical reaction and therefore limits how much product can be formed
Write the balanced equation for the reaction taking place:
4Fe + 3 \(O_2\) ==> 2 \(Fe_2O_3\) (rust)
Use the stoichiometry of the balanced equation and dimensional analysis to find limiting reagent & grams of \(Fe_2O_3\):
10 g Fe x 1 mol Fe/55.85 g x 2 mol \(Fe_2O_3\)/4 mol Fe x 160 g/mol
\(Fe_2O_3\) = 14.37 g \(Fe_2O_3\) (2 Significant figures)
7 g \(O_2\) x 1 mol \(O_2\)/32 g x 2 mol \(Fe_2O_3\)/3 mol \(O_2\) x 160 g/mol
\(Fe_2O_3\) = 46.84 g \(Fe_2O_3\) (2 Significant figures)
Hence, 10g of Fe produces 14.37g of \(Fe_2O_3\) and 7g of \(O_2\) produce 46.84g of \(Fe_2O_3\)
Learn more about Limiting Reagent here ;
https://brainly.com/question/1059823
#SPJ1
In a gas-liquid solution, which of these naturally takes a liquid state?
(A) the solute and the solution
(B) only the solute
(C) only the solvent
(D) the solute and the solvent
(E) the solvent and the solution
(F) only the solution
Answers
Answer:
the solvent and solution
Explanation:
Solutions having solute in gaseous state and solvent in liquid state, are called Gas - Liquid Solutions. For example - Solution (mixture) of oxygen in water, mixture of carbon dioxide in water. Coca cola, a beverage, is an example of gas - liquid solution, as it has carbon dioxide dissolved in water.
In a gas-liquid solution, the substances that naturally takes a liquid state is: (E) the solvent and the solution.
Dissolution can be defined as the process of dissolving or dissociating a solute in solid, liquid or gaseous phase into fragmented particles by a solvent in order to form a solution.
Gas-liquid solution that have solute in their gaseous state and solvent in their liquid state.
For liquid and gases, the substance to be dissolved must form a non-covalent bond with the solvent, in order to produce a solution.
In Science, a solute refers to a substance that is typically dissolved in a liquid solvent such as water, coffee, tea, etc., to produce a solution.
Additionally, a solute maybe completely ionized, partially ionized, or it may remain intact during the process of dissolution.
Read more: https://brainly.com/question/24057916
who knows india??????
Answers
Answer:
Me India is located in Asia and its capital name is New Delhi.
Explanation:
\(india \\ 1)india \: is \: the \: 7 \: th \: largest \: country \\ 2)home \: to \: old \: civilizations \\ 3)father \: of \: the \: nation \: mahathma \: gandi \\ 4)country \: is \: divided \: in \: to \: 28state \\ 5)1000of \: lnguages \: 100 \: of \: cultures \: \\ so \: india \: is \: very \: much \: attractive \\ very \: much \: special \\ 1947 \: august15 \: indias \: independce \: day \\ thank \: you \: \\ jai \: hind\)
plsss help ASAP!!!
BRAINLIEST

Answers
Answer:
-175.15
Explanation:
1.0 °C = 274.15 K
2.0 °C = 275.15 K
3.0 °C = 276.15 K
4.0 °C = 277.15 K
5.0 °C = 278.15 K
6.0 °C = 279.15 K
7.0 °C = 280.15 K
8.0 °C = 281.15 K
9.0 °C = 282.15 K
Complete and balance the following redox equation in acidic solution.
MnO₄ + Br → Mn² + Br₂
What is the sum of the smallest whole number coefficients?
Answers
The balanced redox equation in acidic solution is: 8H⁺ + MnO₄⁻ + 2Br⁻ → Mn²⁺ + 2Br₂ + 4H₂O. The sum of the smallest whole number coefficients in the balanced equation is 16.
How to balance a redox equation?
To balance the redox equation, we need to ensure that the number of atoms and charges are balanced on both sides of the equation. Here's the step-by-step process:
1. Assign oxidation numbers to each element:
MnO₄⁻: Mn = +7, O = -2
Br: Br = 0
Mn²⁺: Mn = +2
Br₂: Br = 0
2. Identify the elements undergoing oxidation and reduction:
Mn is being reduced from +7 to +2, so it undergoes reduction.
Br is being oxidized from 0 to +2, so it undergoes oxidation.
3. Balance the number of atoms by adding water molecules and H⁺ ions:
Add 4 H₂O to the left side to balance the oxygen atoms.
4. Balance the charges by adding electrons:
Add 5 e⁻ to the left side to balance the charge on the MnO₄⁻ ion.
5. Make the number of electrons lost in oxidation equal to the number gained in reduction:
Multiply the reduction half-reaction by 5 to balance the electrons.
6. Combine the half-reactions and cancel out common terms:
8H⁺ + MnO₄⁻ + 2Br⁻ → Mn²⁺ + 2Br₂ + 4H₂O
The sum of the smallest whole number coefficients in the balanced equation is 8 + 1 + 2 + 1 + 2 + 4 = 16.
To know more about redox equation, refer here:
https://brainly.com/question/31048013
#SPJ4
In a chemical equation, the arrow (indicating a reaction) points in which
direction?
•
A. To the bottom
• B. To the right
O C. To the left
O D. To the top
Answers
The arrow in a chemical equation is an important component as it indicates the direction of the chemical reaction. This direction is from left to right, which means that the reactants are transformed into the products. The reactants are placed on the left side of the equation, and the products are placed on the right side of the equation.
A chemical equation is a symbolic representation of a chemical reaction. It includes the reactants on the left side of the equation and the products on the right side, separated by an arrow indicating the direction of the reaction. The arrow represents the conversion of the reactants into the products. It is important to note that the arrow only represents the direction of the reaction, not the speed or rate of the reaction. The speed of the reaction can be influenced by various factors, such as temperature, pressure, and catalysts.
In a chemical equation, the arrow (indicating a reaction) points to the right. This arrow represents the direction of the reaction, with the reactants on the left side of the arrow and the products on the right side. A chemical equation shows a chemical reaction occurring between reactants and forming products. The arrow in the equation points to the right, separating the reactants from the products, and symbolizing the conversion of reactants into products. The reactants are written on the left side of the arrow, and the products are written on the right side. This convention helps to easily understand the reaction taking place and the substances involved.
To know more about reaction visit:
https://brainly.com/question/28984750
#SPJ11
whats the molar mass of CO
Answers
hope that helped
Answer:
28.01
Explanation:
this is the molar mass
True or False.. Light from an unshaded bulb radiates in all directions??
Answers
Its is true that light from an unshaded bulb radiates in all directions
which of the following statements about the mass of an object is correct
a. mass changes with location
b. mass remains constant
c. mass changes with altitude
d. mass changes with gravity
Answers
Answer: Mass remains constant
Explanation:
why in simple diffusion do molecules naturally move from areas where there is a higher concentration to areas where there is a lower concentration?
Answers
In simple diffusion, molecules move across the cell membrane from high to low concentration, meaning that the molecules move from areas where they are more concentrated to areas where they are less concentrated. This is known as the concentration gradient.
The molecules tend to move in this direction because of the natural tendency to reach a state of equilibrium. This means that molecules will distribute themselves evenly in an area over time.
The direction of the movement of the molecules in simple diffusion is a result of Brownian motion, which is the movement of particles in a fluid or gas as a result of their random collision with each other. Brownian motion causes the particles to move from an area of high concentration to an area of low concentration until equilibrium is reached.
The movement of molecules by simple diffusion does not require energy input because it is a passive process. Therefore, it is an efficient way for molecules to move across the cell membrane when they need to reach areas with a lower concentration.
In conclusion, molecules naturally move from areas of higher concentration to areas of lower concentration in simple diffusion because they follow the concentration gradient, which is the natural tendency to reach a state of equilibrium. The movement is caused by Brownian motion, which is the random collision of particles with each other. The process is passive and does not require energy input.
Learn more about Brownian motion from the given link:
https://brainly.com/question/28441932
#SPJ11
At constant pressure and volume, the density of a gas _____ as the molar mass increases. A gas made of heavier particles will have a _____ density than a gas composed of lighter particles under the same conditions of temperature and pressure.
Answers
Answer:
Increases, higher
Explanation:
Remember that the formula for Density is D=mass/volume.
If volume remains the same, as mass increases the density will also increase as the numerator of this math problem increases while the denominator remains the same.
(a) At constant pressure and volume, the density of a gas increases as the molar mass increases.
(b) A gas made of heavier particles will have a greater density than a gas composed of lighter particles under the same conditions of temperature and pressure.
Ideal gas equationThe relationship between density and molar mass is given in the following equations;
PV = nRT
PV = (m/M) RT
PV = (ρV/M)RT
P = (ρ/M)RT
P = (ρRT)/M
PM = ρRT
where;
P is pressureρ is densityM is molar massThus, we can conclude that, at constant pressure and volume, the density of a gas increases as the molar mass increases.
A gas made of heavier particles will have a greater density than a gas composed of lighter particles under the same conditions of temperature and pressure.
Learn more about density of gases here: https://brainly.com/question/18992505
6. If the broken wire of the top branch
is connected, what will be true of the
lightbulbs? sc.6.P.13.1
A All of the bulbs would be lit.
B
None of the bulbs would be lit.
C Only the top bulb would be lit.
D Only the top two bulbs would be lit.
I NEED THIS !!
Answers
Answer: When two light bulbs are connected in parallel, which is true? A. The total resistance is less than the resistance of either bulb alone. B. The Voltage provided
Explanation:
Answer:
D. Only the top two bulbs would be lit.
Explanation:
When you connect the top light bulb, both the top and middle light bulbs will be lit. But, the bottom light bulb will not be lit because it is not connected. In order for a light bulb to be lit, it has to be connected on both sides by wire.
< Hope this helps! :)
a. Suppose your best fit line is: y = 7978x – 0.006. If your unknown sample has a %T of 68.0, what is the solution concentration? Show your work.
b. The sample above was obtained after 2.00 mL of the original 100-mL solution was diluted to 50.00 mL (Part II, Step 4) and 2.25 mL of this solution was diluted again with 5.25 mL of water to give 7.50 mL of total volume (Part III, Step 2). Working backwards, what was the original concentration of your unknown solution in the 100-mL flask? Show your work.
Answers
The solution concentration is approximately 0.008525, or 0.8525% (assuming the concentration is expressed as a percentage).
To determine the solution concentration using the given best fit line equation and %T value, we can rearrange the equation to solve for x, which represents the solution concentration.
The equation given is: y = 7978x - 0.006
Let's substitute %T = 68.0 for y in the equation:
68.0 = 7978x - 0.006
Now, solve for x by isolating it
7978x = 68.0 + 0.006
7978x = 68.006
x = 68.006 / 7978
x ≈ 0.008525
Know more about solution concentration here;
https://brainly.com/question/28480075
#SPJ11
What are two ways in whitch electrons in atoms are in constant motion
Must be two
WIll GIVE BRAINLIST
Answers
Answer:
1. an electron moving around the nucleus
2. an electron moving on its axis
Explanation:
The best way to remember how atoms move is by comparing them to a solar system. Just like a solar system atoms have a core, in this case the nucleus, and each atom rotates around the nucleus just like planets revolve around a star. They also rotate just like a plant does.
1. Which of the following is not homogeneous? (A) Brass
(B) Calamine lotion
(C) Dilute hydrochloric acid
(D) Air
Answers
1. Which of the following is not homogeneous? (A) Brass
(B) Calamine lotion
(C) Dilute hydrochloric acid
(D) Air
All of them are homogeneous, I looked them up individually, And it says they are all homogeneous.
Hope this helps and let me know if I'm wrong!
Only certified electricians are allowed to work on facility electrical system?
Answers
How many mL of 2.5 M K2SO4 are required to obtain 1.25 grams of the compound?
Answers
2.87mL of 2.5 M K2SO4 are required to obtain 1.25 grams of the compound.
HOW TO CALCULATE VOLUME?The volume of a substance can be calculated by dividing the number of moles of the substance by its molarity. That is;
volume = no. of moles ÷ molarity
According to this question, 2.5M K2SO4 are required to obtain 1.25 grams of the compound. The volume can be calculated as follows:
Molar mass of K2SO4 = 174.26 g/mol
moles of K2SO4 = 1.25g ÷ 174.26g/mol
moles of K2SO4 = 0.00717moles
Volume = 0.00717moles ÷ 2.5
Volume = 0.00287 L = 2.87mL.
Therefore, 2.87mL of 2.5 M K2SO4 are required to obtain 1.25 grams of the compound.
Learn more about volume at: https://brainly.com/question/1578538
a. Provide an equation for the acid-catalyzed condensation of ethanoic (acetic) acid and 3- methylbutanol (isopentyl alcohol). Please use proper condensed structural formulas. Compare this product with the ester that you would isolate from the esterification of 4-methylpentanoic acid with methanol. Provide an equation for this reaction as well. Are these products isomers and if so what type of isomer are they?
b. Plan how you will do this organic synthesis, i.e. what is the limiting reactant (acetic acid or isopentyl alcohol) and what would be in excess. To begin, consult the Reagents Table in the lab experimental section and determine which compound is the limiting reactant. Show work for any necessary calculations to receive full credit.
Answers
A. These two products are isomers, specifically structural isomers, because they have the same molecular formula but different arrangements of atoms.
B. We should use a slight excess of ethanoic acid to ensure that all of the 3-methylbutanol is consumed.
A.
The equation for the acid-catalyzed condensation of ethanoic acid and 3-methylbutanol is:
\(CH_3COOH + (CH_3)_2CHCH_2CH_2OH - > CH_3COO(CH_2)_2CH(CH_3)_2 + H_2O\)
The product formed is isopentyl acetate, which is an ester.
The equation for the esterification of 4-methylpentanoic acid with methanol is:
\(CH_3COOH + CH_3OH - > CH_3COOCH_3 + H_2O\)
The product formed is methyl 4-methylpentanoate, which is also an ester.
B.
To determine the limiting reactant, we need to compare the amount of each reactant present and calculate how much product can be formed from each.
First, we need to convert the given volume of 3-methylbutanol to mass:
density of 3-methylbutanol = 0.81 g/mL
mass of 3-methylbutanol = density x volume = 0.81 g/mL x 5.00 L = 405 g
Next, we calculate the number of moles of each reactant:
moles of ethanoic acid = 25.0 mL x 1 L/1000 mL x 1.049 g/mL / 60.05 g/mol = 0.00436 mol
moles of 3-methylbutanol = 405 g / 88.15 g/mol = 4.60 mol
Based on these calculations, 3-methylbutanol is the limiting reactant because it has fewer moles available for the reaction.
For more question on isomers click on
https://brainly.com/question/26298707
#SPJ11
a. The equation for the acid-catalyzed condensation of ethanoic acid and 3-methylbutanol is:
CH3COOH + (CH3)2CHCH2OH → (CH3)2CHCOOCH2CH(CH3)2 + H2O
The product is isopentyl acetate, which is an ester. The condensed structural formula for isopentyl acetate is:
CH3COOCH2CH(CH3)2
The equation for the esterification of 4-methylpentanoic acid with methanol is:
CH3COOH + CH3OH → CH3COOCH3 + H2O
The product is methyl 4-methylpentanoate, which is also an ester. The condensed structural formula for methyl 4-methylpentanoate is:
CH3COOCH2CH(CH3)CH2CH3
These products are isomers because they have the same molecular formula but different structures. Specifically, they are structural isomers.
b. To determine the limiting reactant, we need to calculate the moles of each reactant. The molar mass of ethanoic acid is 60.05 g/mol and the molar mass of 3-methylbutanol is 88.15 g/mol.
Assuming we have 1 mole of each reactant:
- Moles of ethanoic acid = 1 mole / 60.05 g/mol = 0.01665 mol
- Moles of 3-methylbutanol = 1 mole / 88.15 g/mol = 0.01134 mol
Since we need 1 mole of ethanoic acid for every 1 mole of 3-methylbutanol to react completely, we can see that ethanoic acid is the limiting reactant. This means that isopentyl alcohol would be in excess.
To perform this organic synthesis, we would mix ethanoic acid and 3-methylbutanol together in the presence of an acid catalyst (such as sulfuric acid) and heat the mixture to promote the reaction. The product (isopentyl acetate) could then be isolated and purified using techniques such as distillation or extraction.
Learn more about organic synthesis click here:
https://brainly.com/question/14274397
#SPJ11
describe the experimental procedure for obtaining the data needed and the calculations required to determine the proportion of water in a hydrated compound. explain the reason for each step.
Answers
To determine the proportion of water in a hydrated compound, the following experimental procedure and calculations are needed:
1. Weigh the sample of the hydrated compound and record its mass.
2. Heat the sample in an oven for a certain period of time, usually at 100°C, until all the water is evaporated.
3. Weigh the sample again and record the mass.
4. Calculate the difference in mass between the two weights to determine the mass of water that has been removed.
5. Divide the mass of water removed by the mass of the sample before heating to determine the proportion of water in the hydrated compound.
the reason for each step:
1. Weigh the sample of the hydrated compound and record its mass: This is done to obtain the initial mass of the hydrated compound before the water is removed.
2. Heat the sample in an oven for a certain period of time, usually at 100°C, until all the water is evaporated: This step is required to evaporate the water from the hydrated compound and leave the anhydrous compound.
3. Weigh the sample again and record the mass: This is done to obtain the mass of the anhydrous compound, which will be used in calculations to determine the proportion of water.
4. Calculate the difference in mass between the two weights to determine the mass of water that has been removed: This step is necessary to determine the exact amount of water that has been removed from the hydrated compound.
5. Divide the mass of water removed by the mass of the sample before heating to determine the proportion of water in the hydrated compound: This calculation is used to determine the proportion of water in the hydrated compound before it was heated.
To know more about proportion of water refer here:
https://brainly.com/question/28391374#
#SPJ11
What does it mean for the energy levels in an atom to be quantized? Describe how this affects where electrons can be found.
Answers
Answer:
Energy of an electron is quantinized means the electrons can possess only specific individually separated values.
These energy levels are stable and are stationary along with different energy value. The electron remains in the state until energy is absorbed or released.
The electrons absorb energy when it moves from lower energy state to higher energy state. The electrons lose energy when it moves from higher energy state to lower energy state and thus emit radiations corresponding to the energy gap and is called as quanta.
draw all of the stereoisomers of 2-chloro-5-methylheptane. use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, where applicable.
Answers
The stereoisomers of 2-chloro-5-methyl heptane Iis in the 2 chloro 5 methyl hectare.
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism wherein molecules have an equal molecular method and series of bonded atoms, however, fluctuate inside the 3-dimensional orientations in their atoms in area.
Stereoisomers are isomers that fluctuate inside the spatial arrangement of atoms, in preference to the order of atomic connectivity. one in every of their most exciting sort of isomers is the replicate-image stereoisomer, a non-superimposable set of molecules that are a replicate image of one another. Stereoisomers are isomeric molecules with identical chemical formulas but exceptional atomic arrangements. for this reason, they own similar chemical and physical homes.
Learn more about stereoisomers here:-https://brainly.com/question/24128816
#SPJ4

Using the table below, identify the unknown material that has a mass of 15,06 g and a
volume of 0.78 ml
Substance Density (g/cm) Substance Density (g/cm")
Ice (00 0.917 Human fat 0.94
Water (4.0°C) 1.0000 Cork 0.22-0.26
Gold
19.31
Table sugar 159
Helium (250) 0.000 164 Balsa wood 0.12
Air (250) 0.001 185 Earth 5.54
Ole
Water
Gold
Hellum
O Air
lo

Answers
The unknown material : gold
Further explanationDensity is a quantity derived from the mass and volume
Density is the ratio of mass per unit volume
Density formula:
\(\large {\boxed {\bold {\rho ~ = ~ \frac {m} {V}}}}\)
ρ = density
m = mass
v = volume
mass of unknown the material : 15.06 g
volume = 0.78 ml
The density :
\(\tt \rho=\dfrac{m}{V}\\\\\rho=\dfrac{15.06}{0.78}=19.31~g/ml\)
materials that match the density: gold
Why might having mud in an ecosystem be important?
Mud can replace producers in an ecosystem.
Pigs love to wallow in mud.
Mud can replace consumers in an ecosystem.
An insect needs mud wallows to breed; that insect pollinates several flowers in that ecosystem.
plsssssssssssssssssssssssssssssssssssssssssssssssssssssssssssssssssssssssssssssssssssssss answer with one of the options
Answers
An insect needs mud wallows to breed; that insect pollinates several flowers in that ecosystem.
Having mud in an ecosystem is important because Insects are very important as primary or secondary decomposers in the ecosystem. These insects need mud wallows to breed. That insect pollinates several flowers in that ecosystem.
Why do insects like mud?Muddy sediments recycle organic matter back into nutrients for plant photosynthesis. Butterflies and other insects search for moisture, salt, and other nutrients. It's not just damp patches of soil that draw butterflies. Some are attracted by wet sand and dung.An insect needs mud wallows to breed.How do insects play an important role in the ecosystem?Insects like Bees are some of the most important pollinators in the ecosystem.Without bees, most of the plants we rely on would not be able to produce most of the food we eat.Therefore, the mud in an ecosystem is most important as it provides a place for insects to breed and these insects play a major role in the ecosystem like pollination.
Learn more about insect pollination here:
https://brainly.com/question/11204416
#SPJ2