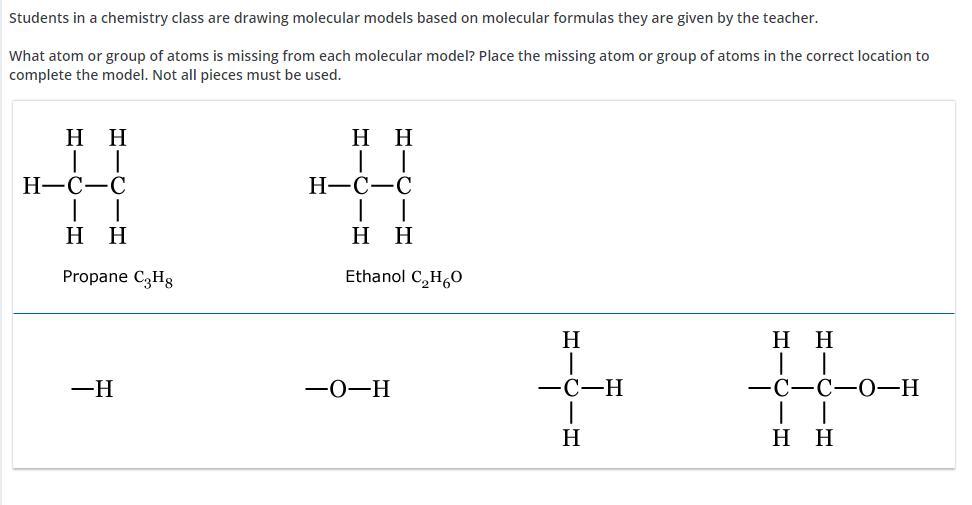
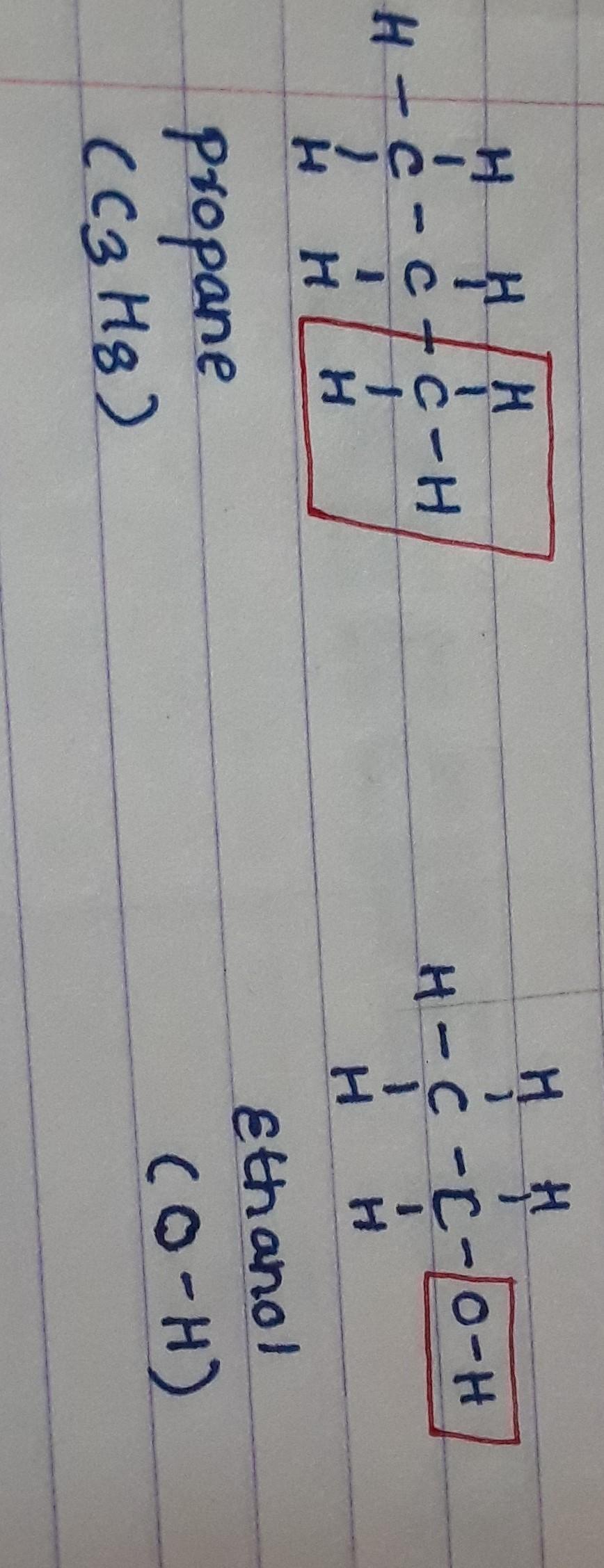
Answers
Explanation:
just try to arrange using the molecular formula.
Related Questions
What is mAngleMHJ?
A boron atom contains 6 neutrons. This isotope of boron will have
protons and a mass number of
Answers
Answer: 5 and 11
Explanation:
protons= 5
neutrons=6
mass number= 6+5=11
PLS ANSWER ASAP WILL GIVE BRAINIEST
Chlorine gas is produced by the reaction: 2 HCl --> H2 + Cl2
How many moles of HCl must react to produce 10.0 L of chlorine gas at STP?
Answers
Answer:
i believe its 1.56
Explanation:
30.2 g
32.6 g
36.5 g
32.6 g
The equation that shows the relationship between speed, wavelength, and frequency of electromagnetic waves is
Answers
Answer:
c = λ f
c is the speed of light, λ the wavelength in meters, and f equals the frequency in cycles per second.
Explanation:
Select all of the following which are a hazard of magnesium. a. Wash thoroughly after handling. b. Avoid breathing vapors. c. Keep away from heat and ignition sources. d. Use with adequate ventilation. e. Avoid contact with eyes, skin, and clothes. f. Harmful if swallowed. g. Keep container closed.
Answers
All of the above options are hazards of magnesium. Magnesium can make skin irritation, eye damage, and respiratory tract irritation if it comes into contact with the skin, eyes, or if it's devoured.
Magnesium can also burn spontaneously in air, and it reacts violently with water, acids, and oxidizing agents. Ingestion of magnesium can bring abdominal pain, puking, diarrhea, and in severe cases, cardiovascular and central nervous system goods. thus, it's critical to follow proper safety procedures when handling magnesium.
Similar as using particular defensive equipment, avoiding ignition sources, and using the substance in a well sounded area.
To learn more about Hazards of magnesium:
https://brainly.com/question/6202566
#SPJ4
BRAINLIEST
What is kinetic energy? Give an example of kinetic energy.
Answers
energy which a body possess by virtue of being in motion
ex: a river flowing at a certain speed comprises kinetic energy as water has a certain velocity and mass
I need help with this pls

Answers
Answer:
H - Cl2 +NaBr -> Br2+2NaCl
What is the bond order of N2+? Express the bond order numerically. Is N2+ paramagnetic or diamagnetic? paramagnetic diamagnetic neither
Answers
Bond order of N2+ is 2.5. It is a diamagnetic substance.
Bond order is termed as the number of chemical bonds between a pair of the atoms. For example: In case of acetylene the bond order between the two carbon atoms is 3, in diatomic nitrogen the bond order is 3, and the C-H bond order is 1.
The bond order of N2+ is 2.5.
Bond order = 1 / 2[Nb - Na] Where, Nb = no. of electrons in bonding molecular orbital and Na = number of electrons in antibonding molecular orbital.
Bond order = 9-4 / 2
= 2.5
N2+ is diamagnetic in nature because they do not have any unpaired electrons they are having 14 electrons.
To know more about bond order here
https://brainly.com/question/29853110
#SPJ4
A hockey player is wearing ice skates and pushes against the wall propelling her backward on the ice. According to Newton's third law of motion, which of the following best compares the forces acting between the wall and the hockey player?
A.
The forces are equal and applied in the same direction.
B.
The forces are different sizes and applied in the opposite direction.
C.
The forces are equal and applied against gravity.
D.
The forces are equal and applied in the opposite direction.
Answers
its D
random characters AAAJIJRIAJKLGIJHEOGEINDKIKAN
Objects with more (less or more) molecules have
heat energy than objects with (greater or fewer)
molecules? fill in the blanks
Answers
Because the more molecules the more heat is produced.
B. What is the equation that illustrates the relationship between wave velocity, frequency, and wavelength? (1 point)
Answers
The equation that illustrates the relationship between wave velocity, frequency, and wavelength is: v = λ * f
v represents the wave velocity, which is the speed at which the wave travels through a medium. It is usually measured in meters per second (m/s).
λ (lambda) represents the wavelength of the wave, which is the distance between two consecutive points of similar phase on the wave. It is usually measured in meters (m).
f represents the frequency of the wave, which is the number of complete cycles or oscillations of the wave that occur in one second. It is usually measured in hertz (Hz).
The equation v = λ * f is known as the wave equation or the wave speed equation. It states that the wave velocity is equal to the product of the wavelength and the frequency of the wave.
This equation implies that as the frequency of a wave increases, the wavelength decreases, and vice versa, while keeping the wave velocity constant. In other words, if the frequency increases, the wave becomes more compressed and the distance between consecutive points of similar phase decreases.
On the other hand, if the frequency decreases, the wave becomes more spread out, and the distance between consecutive points of similar phase increases.
For more such questions on equation visit:
https://brainly.com/question/28818351
#SPJ8
Is pH = 11 an acid, a base, or neutral?
Answers
Answer:
Hi! A substance with a pH of 11 is considered to be a base. Substances with a pH of 1-6 are considered to be acidic, substances with a pH of 7 are considered to be neutral, and substances with a ph of 8-14 are considered to be basic.
Have a great day! - Mani :)
Answer:
Base.
Explanation:
The pH scale includes the numbers 0 - 14, in which:
0 - 6 = acid, the smaller the number, the more acidic.
7 = neutral
8 - 14 = base, the greater the number, the more basic.
In this case, 11 falls within base, so your answer will be a base.
Camk1 and camk2 are in the same gene family. camk1 is a kinase that phosphorylates target proteins in the cytosol. what can you infer about camk2?
Answers
Based on the information provided, we can infer that camk2 is also a kinase, since it belongs to the same gene family as camk1. Camk2 is likely to have a similar function as camk1, which is phosphorylating target proteins in the cytosol.
Gene families are groups of genes that share a common ancestry and have similar functions. Since camk1 and camk2 are in the same gene family, they are likely to have similar characteristics and functions. Camk1 is specifically mentioned as a kinase that phosphorylates target proteins in the cytosol.
Therefore, it is reasonable to infer that camk2, being in the same gene family, would also be a kinase and have a similar function of phosphorylating target proteins in the cytosol. In summary, camk2 is inferred to be a kinase that phosphorylates target proteins in the cytosol, similar to camk1.
To know more about phosphorylating visit:
https://brainly.com/question/30278433
#SPJ11
A 15.0 ml sample of gas at 10.0 degree Celsius and 760 torr changes to a pressure of 1252 torr at 35.0 degree Celsius. What is the volume of the gas?
Answers
Answer:
9.91 mL
Explanation:
Using the combined gas law equation as follows;
P1V1/T1 = P2V2/T2
Where;
P1 = initial pressure (torr)
P2 = final pressure (torr)
V1 = initial volume (mL)
V2 = final volume (mL)
T1 = initial temperature (K)
T2 = final temperature (K)
According to the information provided in this question;
V1 = 15.0mL
V2 = ?
P1 = 760 torr
P2 = 1252 torr
T1 = 10°C = 10 + 273 = 283K
T2 = 35°C = 35 + 273 = 308K
Using P1V1/T1 = P2V2/T2
760 × 15/283 = 1252 × V2/308
11400/283 = 1252V2/308
Cross multiply
11400 × 308 = 283 × 1252V2
3511200 = 354316V2
V2 = 3511200 ÷ 354316
V2 = 9.91 mL
a solution of hydrochloric acid contains 1.5 moles of the solute in 2.0 liters of solution. calculate the molarity of this solution
Answers
Answer:
0.75 M
Explanation:
Molarity = moles of solute / liters of solution
so;
Molarity = \(\frac{1.5 Moles}{2.0 Liters}\)
According to molar concentration, the molarity of this solution is 0.75 molar.
What is molar concentration?Molar concentration is defined as a measure by which concentration of chemical substances present in a solution are determined. It is defined in particular reference to solute concentration in a solution . Most commonly used unit for molar concentration is moles/liter.
The molar concentration depends on change in volume of the solution which is mainly due to thermal expansion. Molar concentration is calculated by the formula, molar concentration=mass/ molar mass ×1/volume of solution in liters.
In terms of moles, it's formula is given as molar concentration= number of moles /volume of solution in liters.Substitution in formula gives, molarity= 1.5/2=0.75 M
Thus, the molarity of this solution is 0.75 molar.
Learn more about molar concentration,here:
https://brainly.com/question/15532279
#SPJ2
What is the new atom called when one proton is added to Phosphorus
Answers
Answer:
The answer is Sulfur
Explanation:
Atomic number is a proton.
The atomic number of Phosporus is 15. ( 15 protons)
If you add one proton, it will become 16.
The element with the atomic number of 16 is sulfur.
What is the mass of sodium hydroxide in a solution that has a total mass of 5,500g and is 22% NaOH by mass
Answers
Solution = 5,500g
NaOH = 22% of 5,500g
Therefore mass of NaOH is :
0.22 × 5,500g
1,210g
Meaning 1,210 grams inside the solution is Sodium hydroxide
The mass of sodium hydroxide in a solution that has a total mass of 5,500g and is 22% NaOH by mass is 121,000 g.
How do we calculate mass percent?Mass percent of any substance present in any solution will be calculated as:
Mass % = (Mass of substance) / (Mass of solution)
In the question given that,
Mass percent of NaOH = 22%
Mass of solution = 5,500 g
On putting these values on the above equation we get,
Mass of NaOH = (22)×(5,500) = 121,000 g
Hence required mass of NaOH is 121,000 g.
To know more about mass percent, visit the below link:
https://brainly.com/question/10031774
How does Lori make the solution?

Answers
Answer:
I would say the top one but im not confident in the answer
What is the limiting reagent when 16 grams of ch4 is reacted with 64 grams of o2? what remains after the reaction?.
Answers
From the given reaction, Oxygen serves as the limiting reagent. Methane remains after the reaction.
What is a limiting reagent?A reactant that is completely consumed at the end of a chemical reaction is known as the limiting reagent. Since the reaction cannot proceed without this reagent, the amount of product that can be produced is constrained.
Because its quantity restricts the number of products that can be generated, the reagent that is totally consumed or reacted is known as the limiting reagent.
CO₂ + 2H₂O ⇒ CH₄+ 2O₂
One mole of methane will totally react with two moles of oxygen to produce one mole of CO₂ and two moles of water, according to the stoichiometric equation given above.
64g of oxygen and 16g of methane will totally react in such case.
When methane is burned, the weight ratio of the reactions between CH₄ and O₂ is (16:64)=1:4.
Only 32 g of oxygen are provided in the question, though. Since we only have 32 when 64 is divided, oxygen will be the limiting reagent in this scenario.
Oxygen serves as the limiting reagent.
Methane is the extra reagent.
To learn more about limiting reagent from given link
https://brainly.com/question/23661051
#SPJ4
Electromagnetic vs. Mechanical Waves 1?
Answers
Answer:
example of electromagnetic waves water waves and sound wave.
Types of mechanical waves are transverse and longitudinal wave...
13. An organic compound is found to contain 77.42% of C, 7.53% of H and
nitrogen. The mass of 1.12L of its vapour at NTP is 4.65g. Determine
the
empirical and molecular formula of the compound.
Answers
Answer
7.53% 97% if you divide it you can get the answer
Explanation:
Mass and energy are conserved:
Question 3 options:
A)
only in physical changes.
B)
in chemical changes and physical changes.
C)
always in physical changes and sometimes in chemical changes.
D)
only in chemical changes.
Answers
what is an emulsion? how does using brine help with an emulsion?
Answers
An emulsion is a mixture of two immiscible liquids, meaning they do not mix easily, such as oil and water. Brine, can help with the formation and stabilization of an emulsion.
In an emulsion, one liquid, called the dispersed phase, is distributed as small droplets throughout the other liquid, called the continuous phase. This distribution is usually achieved by the use of an emulsifying agent, which helps stabilize the emulsion by reducing the surface tension between the two immiscible liquids. Brine, is a highly concentrated salt solution. When added to the mixture, brine can influence the properties of the continuous phase by increasing its density and viscosity.
This results in a more stable emulsion as the droplets of the dispersed phase are less likely to coalesce or separate. Moreover, the presence of salt in the brine can also act as an electrolyte, modifying the interfacial tension between the two immiscible liquids. This change in interfacial tension can help to stabilize the emulsion, preventing the dispersed phase droplets from merging and the emulsion from breaking.
In summary, an emulsion is a mixture of two immiscible liquids, such as oil and water, where one is dispersed as small droplets throughout the other. Brine can assist in emulsion formation and stabilization by altering the density, viscosity, and interfacial tension of the mixture, resulting in a more stable emulsion.
Know more about emulsion here:
https://brainly.com/question/6711819
#SPJ11
which of the following best describes a neutron? A. It is located in the electron cloud and has a negative charge. B. It is located in the electron cloud and has no charge. C. It is located in the nucleus and has no charge. D. It is located in the nucleus and has no positive charge.
Answers
C. It is located in the nucleus and has no charge
Explanation:
here ya go :)
Wave motion
Vibration
What does the wave shown in the photograph transport from left to right?
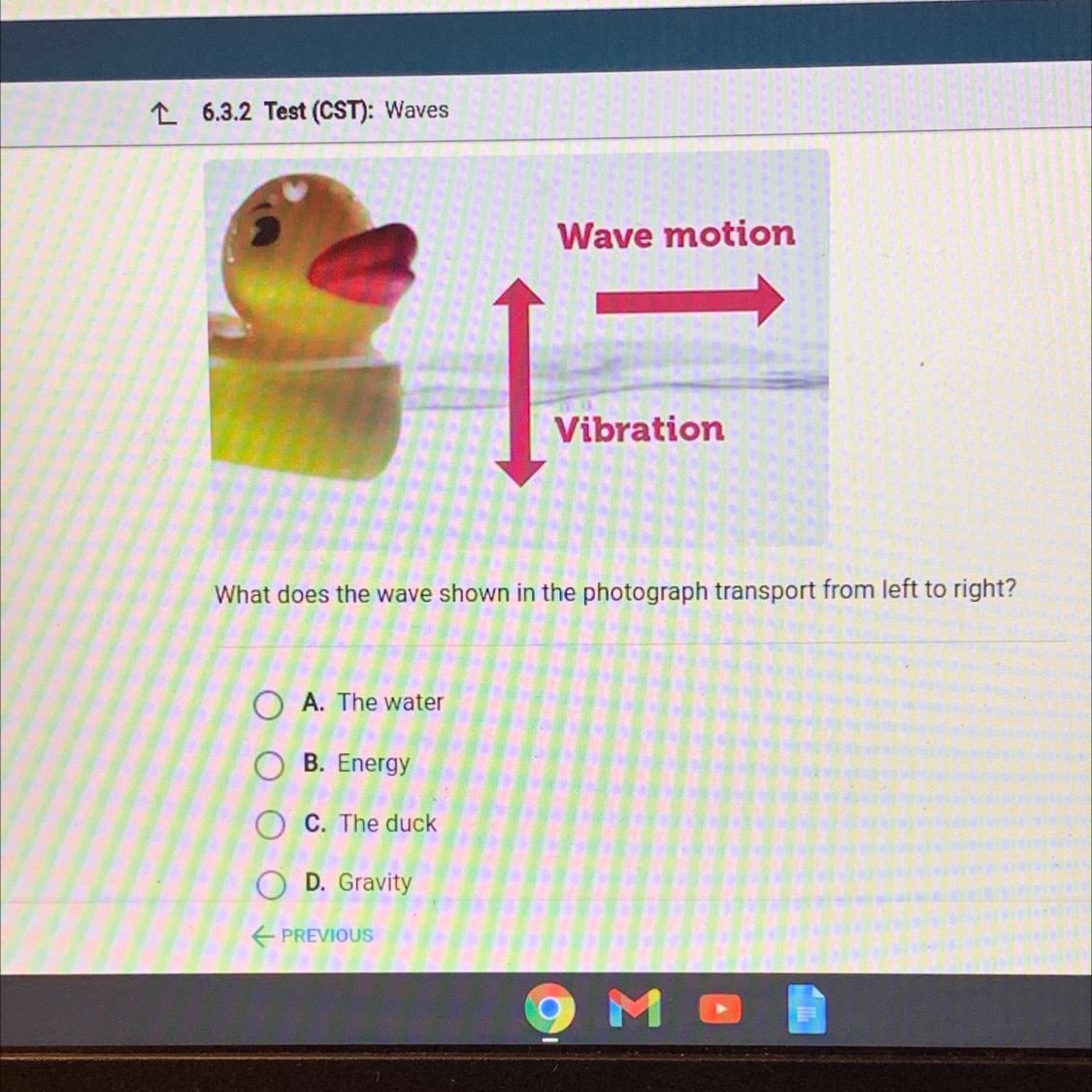
Answers
Answer:
B. Energy
Explanation:
I took the quiz apix
What is the buffer component ratio, ([CH3CH2COO-]/[CH3CH2COOH]) of a propanoate buffer that has a pH of 4.32. Ka of CH3CH2COOH is 1.3 x 10^-5.?
Answers
The buffer component ratio \([CH_3CH_2COO^-]/[CH_3CH_2COOH]\)of the propanoate buffer with a pH of 4.32 is approximately 0.278.
To calculate the buffer component ratio\([CH_3CH_2COO^-]/[CH_3CH_2COOH]\), we need to use the Henderson-Hasselbalch equation:
\(pH = pKa + log_{10}([A-]/[HA])\)
Given:
pH = 4.32
pKa = \(-log_{10}(Ka)\)=\(-log_{10}(1.3 * 10^-5)\) = 4.89
Now, let's rearrange the Henderson-Hasselbalch equation to solve for the buffer component ratio:
\(log_{10}([A-]/[HA]) = pH - pKa\)
Substitute the values into the equation:
\(log_{10}([A-]/[HA]) = 4.32 - 4.89\)
Now, we can solve for the buffer component ratio by taking the antilog of both sides:
\([A-]/[HA] = 10^{(4.32 - 4.89)}\\\[A-]/[HA] = 10^{(-0.57)}\\\[A-]/[HA] = 0.278\)
Therefore, the buffer component ratio \([CH_3CH_2COO^-]/[CH_3CH_2COOH]\)of the propanoate buffer with a pH of 4.32 is approximately 0.278.
Learn more about the Henderson-Hasselbalch equation at:
https://brainly.com/question/26746644
#SPJ4
after successfully isolating solid copper in part b of this experiment, bernice is wondering if there are other acids that could be used in place of the acids available in part b of this experiment. which of the following acids could be used instead of the provided acids (h2so4 and h3po4) to isolate solid copper in part b of this experiment? select all that apply
o. HBr
o. HNO3
o. H2S
o. H2CO3
Answers
HNO3 and HBr can also be used instead of the provided acids (h2so4 and h3po4) to isolate solid copper in this experiment. Solid copper can be isolated by reacting it with acid. This is achieved in two stages: stage one, where copper reacts with sulfuric acid to produce copper sulfate and hydrogen gas, and stage two, where copper sulfate is reduced to copper using hydrogen gas.
Therefore, in part b of the experiment, H2SO4 and H3PO4 are used. HNO3 and HBr can also be used instead of H2SO4 and H3PO4 to isolate solid copper. H2S and H2CO3 cannot be used as the acids to isolate solid copper. 'Hence, the correct options are : HNO3 and HBr Therefore, both HBr and HNO3 could be used in place of the acids (H2SO4 and H3PO4) to isolate solid copper in part b of this experiment.
Know more about sulfuric acid here:
https://brainly.com/question/1107054
#SPJ11
The human body is about 9.5% carbon atoms, with a small fraction of that carbon being radioactive carbon-14. Carbon dating is a method of determining the age of things made of once living materials. What information would be needed to find the age of a human fossil or the Dead Sea Scrolls?
Answers
Answer:The age can be determined by utilizing carbon-14, a radioactive isotope of carbon.
Explanation:
F app F fric F norm F grav Which statement best describes the forces shown in this picture? A. The force of friction and the gravitational force are balanced. B. The force of friction is less than the applied force. C. The normal force and the gravitational force are balanced. D. All four forces are acting on the student.
Answers
He is not falling nor about to fall which means he’s perfectly balanced. So this means the force in the photo and friction in this photo is in a balanced condition, balancing the boy.
How forces are balanced?In the given picture, the applied force and the force of friction are balanced. if two forces act in the direction to make the object in a balanced state.
If the forces are in a balanced state it does not change the motion of the object, and if it is in an unbalanced state, it causes to change in the state of the motion.
Therefore, the force of friction and the gravitational force are balanced is the correct option.
Learn more about forces, here:
https://brainly.com/question/28458730
#SPJ1
The given question is incomplete, so the complete question image is attached in the image below.

What will be the aproximate final volume of a solution prepared by diluting 25 mL of 8.25 M sodium hydroxide to a concentration of 2.40 M? A. 96 mL B. 25 mL C. 86 mL D.1.38 x 10^2 mL
Answers
The correct option is (C). The approximate final volume of a solution prepared by diluting 25 mL of 8.25 M sodium hydroxide to a concentration of 2.40 M is 86 mL (option C).
To find the final volume of the solution, we can use the formula:
C1V1 = C2V2
Where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
Plugging in the values we know, we get:
(8.25 M) (25 mL) = (2.40 M) (V2)
Solving for V2, we get:
V2 = (8.25 M x 25 mL) / 2.40 M
V2 = 86.25 mL
Therefore, the approximate final volume of the solution prepared by diluting 25 mL of 8.25 M sodium hydroxide to a concentration of 2.40 M is 86 mL (option C).
Learn more about final volume at: https://brainly.com/question/22012954
#SPJ11
when carbon is heated in a limited supply of oxygen, a gas is obtained.
1 .what is the name of this gas
Answers
Answer:
carbon monoxide
I think its correct but I am not sure
Answer: when carbon is heated in air carbon dioxide is formed, so is incomplete combustion which results in carbon monoxide.
Explanation: but when carbon dioxide reacts with more oxygen carbon monoxide is formed i guess.
I AM A BIT SURE BUT HOPE THIS HELPSSSS!!!!