please help asap!
3. A double replacement reaction occurs between two solutions of lead (II) nitrate and potassium bromide. Write a
balanced equation for this reaction-identifying the product that will precipitate, and the product that will remain in
solution.
a) Write the balanced equation for this double replacement reaction.
b) If this reaction starts with 32.5 g lead (II) nitrate and 38.75 g potassium bromide, how many grams of the
precipitate will be produced? Remember to use the limiting reactant to calculate the amount of precipitate
formed.
c) How many grams of the excess reactant will remain?

Answers
Answer:
Explanation:
a) The balanced equation for the double replacement reaction between lead (II) nitrate and potassium bromide is:
Pb(NO₃)₂(aq) + 2KBr(aq) → PbBr₂(s) + 2KNO₃(aq)
In this reaction, lead (II) bromide (PbBr₂) will precipitate, while potassium nitrate (KNO₃) will remain in solution.
b) To determine the amount of precipitate produced, we need to first determine the limiting reactant. We can do this by calculating the number of moles of each reactant and comparing it to the stoichiometry of the balanced equation.
The molar mass of lead (II) nitrate is 331.21 g/mol and the molar mass of potassium bromide is 119.00 g/mol.
The number of moles of lead (II) nitrate is 32.5 g / 331.21 g/mol = 0.0981 mol The number of moles of potassium bromide is 38.75 g / 119.00 g/mol = 0.3256 mol
According to the balanced equation, one mole of lead (II) nitrate reacts with two moles of potassium bromide to produce one mole of lead (II) bromide. This means that if all the lead (II) nitrate were to react, it would require 0.0981 mol * 2 = 0.1962 mol of potassium bromide.
Since we have more than enough potassium bromide (0.3256 mol > 0.1962 mol), lead (II) nitrate is the limiting reactant.
The number of moles of lead (II) bromide produced will be equal to the number of moles of lead (II) nitrate consumed, which is 0.0981 mol.
The molar mass of lead (II) bromide is 367.01 g/mol, so the mass of lead (II) bromide produced will be 0.0981 mol * 367.01 g/mol = 36.0 g.
c) To determine the amount of excess reactant remaining, we need to subtract the amount consumed from the initial amount.
The number of moles of potassium bromide consumed is half the number of moles of lead (II) nitrate consumed, which is 0.0981 mol / 2 = 0.04905 mol.
The mass of potassium bromide consumed is 0.04905 mol * 119.00 g/mol = 5.84 g.
The mass of potassium bromide remaining is 38.75 g - 5.84 g = 32.91 g.
Related Questions
Water is intentionally present in a state where its molecules are far apart during a change of state is molecule slow down which change of state has most likely taking place
Answers
Answer:
melting
Explanation:
alculate the specific heat of the metal, if the heat the water absorbed was 424.7 J.
Answers
Answer:
424.7J-312J=the specific amount of heat passing through the metal
0.72 moles SO2 how many molecules
Answers
0.72 moles SO2 contains 4.33 × \(10^{23}\) molecules.
What is molecule?A molecule refers to a group of two/more atoms bounded together by attractive forces known as chemical bonds. Depending on the context, the term may include ions that meet this criterion. A molecule is made up of one or more atoms. If they contain multiple atoms, those atoms can be the same (the oxygen molecule has two oxygen atoms) or different (the water molecule has two hydrogen atoms and one oxygen atom). Biomolecules such as proteins and DNA are made up of thousands of atoms. A group of two/more atoms of the same/different elements chemically bonded together is called a molecule.For example, two hydrogen atoms and one oxygen atom react to form a water molecule.To learn more about molecule from the given link :
https://brainly.com/question/19922822
#SPJ1
Hydrogen chloride gas and oxygen react to form water vapor and chlorine gas. What volume of chlorine would be produced by this reaction if of oxygen were consumed
Answers
Reaction of O₂ with HCl is as follow,
4 HCl + O₂ → 2 H₂O + 2 Cl₂
Let suppose we are given with 1 mole of HCl gas, Then,
According to eq,
89.6 L (4 moles) of HCl when reacted produced = 44.8 L (2 moles) Cl₂ gas
So,
22.4 L (1 mole) of HCl will produce = X L of Cl₂ gas
Solving for X,
X = (22.4 L × 44.8 L) ÷ 89.6 L
X = 11.2 L of Cl₂ Gas
Explain how these results show that chlorine is more reactive than bromine and
lodine.
Answers
Chlorine is more reactive than bromine because it replaces both bromine and iodine.
How chlorine is more reactive than bromine?Fluorine is the most sensitive while on the other hand, the astatine is the least reactive as compared to other elements. The chlorine displaces both bromine and iodine, and bromine displaces iodine because of its high reactivity. The element that replaces other atom is considered as more reactive.
The order of reactivity is that the chlorine is more reactive than bromine, which indicates that chlorine is more reactive than iodine.
So we can conclude that chlorine is more reactive than bromine due to high reactivity.
Learn more about Chlorine here: https://brainly.com/question/24218286
#SPJ1
HELP PLZ AND THANKS WILL MARK YOU AS BRAINLIEST

Answers
Answer:
D
Explanation:
How many shells are there in the Bohr model of a hydrogen atom?
Answers
Answer:
Explanation:
When looking at the periodic table, the period (row) corresponds to the number of shells or PEL (principled energy level).
Hydrogen is in the first period and thus has one shell.
Step 7: Put the Metal in the Water and Measure Temperature Changes (Copper)
Answers
When copper is placed in water, it reacts with the water molecules to form copper(II) ions and hydrogen gas. The reaction is exothermic, which means it releases heat energy into the surroundings. By measuring the temperature changes that occur, we can determine the amount of heat that is released by the reaction.
The temperature changes can be measured using a thermometer. We can place the copper metal in a container of water and take the initial temperature reading. Then, we can add the copper to the water and record the temperature change over time. By monitoring the temperature changes, we can observe the exothermic reaction taking place.
The heat released by the reaction between copper and water has many practical applications, including in the design of power plants and in the production of steam for heating and electricity generation. Therefore, understanding the heat released during this reaction is important for a variety of scientific and engineering fields.
In conclusion, step 7 of putting copper metal in water and measuring the temperature changes allows us to observe and measure the heat released by the exothermic reaction between copper and water, which has important applications in various scientific and engineering fields.
Know more about exothermic here:
https://brainly.com/question/2924714
#SPJ11
Answer:
Aluminum
100 C22.4 C27.1 C4.7 C72.9 Ccopper
100 C22.7 C24.6 C1.9 C75.4 CIron
100 C22.5 C24.9 C2.4 C75.1 CLead
100 C22.6 C23.3 C0.7 C76.7 CThe Final Slide:
Aluminum- 0.90
Copper- 0.35
Iron- 0.44
Lead- 0.12
Explanation:
I hope this helps! :))))
Balance the following equations and then write the net ionic equations. (Use the lowest possible whole number coefficients. Include states-of-matter in your answers. Use instead of to balance equations. Write weak electrolytes in their undissociated form. Omit any coefficients of 1).
A. (NH4)3PO4(aq) + Bi(NO3)3(aq) + BiPO4(s) + NH4NO3(aq).
B. AGOH(s) + H2SO4 (aq) + Ag, SO4(s) + H2O.
C. HCIO2 (aq) + Mn(OH)2(s) + Mn(C102)2 (aq) + H2O.
Answers
Answer:
Explanation:
A ) (NH₄)₃PO₄(aq) + Bi(NO₃)₃(aq) = BiPO₄(s) + 3 NH₄NO₃(aq).
B₁³⁺ + PO₄⁻³ = Bi ( PO₄ )
B ) AgOH(s) + H₂SO₄ (aq) = AgSO₄(s) + H₂O.
Ag⁺ + OH⁻ + H⁺ + 2 SO₄⁻² (aq) = Ag(SO₄)₂(s) + H₂O.
C ) HCIO₂ (aq) + Mn(OH)₂(s) = Mn(Cl0₂)₂ (aq) + H₂O.
H⁺ + OH⁻ = H₂O .
985.2 moles of nitrogen, how many moles of ammonia can produce?
Answers
Answer:
985.2 moles of nitrogen can produce 1970.4 moles of ammonia.
Explanation:
The balanced chemical equation for the production of ammonia from nitrogen is:
N2 + 3H2 → 2NH3
From the balanced equation, we can see that 1 mole of nitrogen reacts with 3 moles of hydrogen to produce 2 moles of ammonia.
So, to determine how many moles of ammonia can be produced from 985.2 moles of nitrogen, we need to use the mole ratio from the balanced chemical equation as follows:
985.2 moles N2 x (2 moles NH3 / 1 mole N2) = 1970.4 moles NH3
Therefore, 985.2 moles of nitrogen can produce 1970.4 moles of ammonia.
We mix 88 grams of oxygen gas with 33 grams
of argon gas in a volume of 2400 mL at 62◦C.
What will be the final pressure of the gas
mixture?
Answers
The final pressure of the gas mixtures is 72.48 atm.
The given parameters;
mass of the oxygen = 88 gramsmass of the argon, = 33 gramsvolume of the gaseous mixture, V = 2400 mL = 2.4 LThe number of moles of the gases is calculated as follows;
\(number \ of \ moles \ of \ oxygen = \frac{88}{16} = 5.5 \ moles\\\)
\(number \ of \ moles \ of \ argon = \frac{33}{40} = 0.825 \ moles\\\)
The total number of moles of the gas mixture;
= 5.5 + 0.825
= 6.325 moles
The final pressure of the mixture is calculated as follows;
PV = nRT
\(P = \frac{nRT}{V} \\\\P = \frac{6.325\ mol \times (0.0821 \ L.atm/mo.K) \times (62+273)}{2.4 \ L} \\\\P = 72.48 \ atm\)
Thus, the final pressure of the gas mixtures is 72.48 atm.
Learn more here:https://brainly.com/question/3238925
Fe(OH)2(s) + O2(g) +OH-Fe(OH)3(s) + H2O balance in basic solution
Answers
Fe(OH)₂(s) + O₂(g) +OH → Fe(OH)₃(s) + H₂O balance in basic solution is 4Fe(OH)₂ (s) + O₂(g) + 2H₂O → 4Fe(OH)₃(s)
A basic solution is an aqueous solution containing more OH⁻ ions than H⁺ ion
The unbalanced redox equation is as follows:
Fe(OH)₂(s) + O₂(g) +OH → Fe(OH)₃(s) + H₂O
In the chemical reaction all atoms other than hydrogen and oxygen are balanced and the oxidation number of fe changes from 2 to 3 and the change in the oxidation number is 1 and the oxidation number of fe changes from 0 to -2 and for 2O atoms the change in the oxidation number is 4 and then balance the equation with increase in the oxidation number and with decrease in the oxidation number
Fe(OH)₂(s) and Fe(OH)₃(s) with 4 then,
4Fe(OH)₂(s)+O₂(g) → 4Fe(OH)₃(s)
To balance O atoms, add 2 water molecules on LHS,
4Fe(OH)₂ (s) + O₂(g) + 2H₂O → 4Fe(OH)₃(s)
Know more about basic solution
https://brainly.com/question/16867271
#SPJ1
Stamples of heterogeneous equilibria. FeO(s) + CO(g) = Fe(s) + CO₂(g) II. H₂(g) L₂(g) = 2HI(g) III. CO₂(g) + C(s) = 2CO(g) IV. N₂(g) 3H₂(g) + 2NH3(g) Identify I.
Answers
An example of heterogeneous equilibrium is:
I. FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g)What is heterogeneous equilibrium?Heterogeneous equilibrium refers to an equilibrium state in a chemical reaction where the reactants and products exist in different physical states or phases. It occurs when substances in different phases, such as solids, liquids, and gases, are involved in a chemical reaction.
Considering the given equations:
The equation I: FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g) represents a heterogeneous equilibrium.
This is because the reactants and products involve different phases (solid and gas). FeO is a solid (s), CO is a gas (g), Fe is a solid (s), and CO₂ is a gas (g). The reaction involves the conversion of a solid and a gas to another solid and a gas, and the equilibrium is established between these different phases.
Learn more about heterogenous equilibrium at: https://brainly.com/question/25257772
#SPJ1
Part 2:Construct a graph of temperature versus time. Put temperature on the y-axis and time on the x-axis. Graph all three sets of data on the same graph. Use a different colored pencil for each test material. Provide a key on the graph relating the pencil color to the test material. Calculate the change in temperature from start to finish for the air and each of the samples and record on your data sheet. Which water sample lost the least amount of heat energy over the 6-minute time interval? Was the rate of heat loss constant during each experiment? How can you tell? Which material was the best insulator? Which material was the least effective insulator? Write a summary paragraph discussing this experiment and the results. Summarize the conclusions that you can draw from this experiment. Explain why the design of the bird's nest provides good insulation for the eggs
Answers
The change in temperature from start to finish for the air and each of the samples would be calculated by subtracting the initial temperature from the final temperature. For example, if the initial temperature of the air was 20°C and the final temperature was 15°C, the change in temperature would be -5°C.
The water sample that lost the least amount of heat energy over the 6-minute time interval would be the one with the smallest change in temperature. In the example above, if the change in temperature for water sample 1 was -3°C and the change in temperature for water sample 2 was -4°C, water sample 1 would have lost the least amount of heat energy.
The rate of heat loss was not constant during each experiment because the slope of the lines on the graph is not constant. The slope of the line represents the rate of heat loss, and if the slope is not constant, the rate of heat loss is not constant.
The material that was the best insulator would be the one with the smallest change in temperature. In the example above, water sample 1 would be the best insulator because it had the smallest change in temperature.
Learn more about insulating materials:
https://brainly.com/question/29346083
#SPJ11
please help GIVING brainliest

Answers
Answer:
heat wave
Explanation:
A high pressure system is a whirling mass of cool, dry air that generally brings fair weather and light winds. When viewed from above, winds spiral out of a high-pressure center in a clockwise rotation in the Northern Hemisphere.
Which metal will spontaneously react with Zn²⁺(aq), but will not
spontaneously react with Mg²⁺ (aq)?
Answers
Mn(s) metal will spontaneously react with Zn²⁺(aq), but will not
spontaneously react with Mg²⁺ (aq)
The Eo value of an electrochemical cell determines its spontaneity. Positive Eo electrochemical cells are spontaneous, and vice versa.
The relevant Eo of the half-cell in this instance are as follows for Mn(s) metal
Zn2+/Zn = -0.763v for Eo
2.37v for Eo Mg2+/Mg.
Mn2+/Mn = -1.18v for Eo.
Consequently, the equation for an Eo cell (with Zn as one of the half-cells) is: Eo Zn2+/Zn - Eo Mn2+/Mn = -0.763 - (-1.18) = 0.417v.
On the other hand, the equation for an Eo cell (with Mg as one of the half-cells) is: Eo Mg2+/Zn - Eo Mn2+/Mn = -2.37 - (-1.18) = -1.19v.
As a result, Mn(s) metal will spontaneously react with Zn2+(aq), but not with Mg2+ (aq)
To learn more about Mn(s) metal please visit -
https://brainly.com/question/13153267
#SPJ1
Determine the pH at the equivalence point in the titration of 50.0 ml of 0.300 m ch3cooh with .3 M NaOH. the Value of Ka for CH3COOH is 1.8 x 10^-5
Answers
The pH at the equivalence point in the titration of 50.0 ml of 0.300 M CH₃COOH with 0.3 M NaOH is 8.61.
At the equivalence point in the titration of CH₃COOH with NaOH, the pH is determined by the hydrolysis of the salt formed, which is CH3COO⁻ and Na+.
The equation for the hydrolysis reaction is:
CH3COO⁻ + H2O ⇌ CH3COOH + OH⁻
We can use the Ka value for CH₃COOH to find the Kb value for CH3COO⁻ using the equation:
Kw = Ka x Kb
Where Kw is the ion product constant for water (1.0 x 10⁻¹⁴).
Kb = Kw/Ka
= (1.0 x 10⁻¹⁴)/(1.8 x 10⁻⁵)
= 5.56 x 10⁻¹⁰
Now we can use the Kb value to find the concentration of OH⁻ at the equivalence point using the equation:
Kb = ([OH⁻][CH3COOH])/[CH3COO⁻].
Since the concentrations of CH3COOH and CH3COO⁻ are equal at the equivalence point, we can simplify the equation to Kb = ([OH⁻]²)/[CH₃COO⁻].
Hence,
[OH-] = \(\sqrt{Kb(CH3COO-)}\)
=\(\sqrt{5.56(10^{-10})(0.3) }\)
= 4.06 x 10⁻⁶ M
Use the concentration of OH⁻ to find the pH at the equivalence point using the equation pOH = -log[OH-] and the relationship pH + pOH = 14.
pOH = -log(4.06 x 10⁻⁶)
= 5.39
pH = 14 - 5.39
= 8.61
Therefore, the pH at the equivalence point in the titration of 50.0 ml of 0.300 M CH3COOH with 0.3 M NaOH is 8.61.
Learn more about pH here: https://brainly.com/question/172153
#SPJ11
What is the predominant form of ethylenediamine at pH 6.184 ?
H2NCH2CH2NH+3
H2NCH2CH2NH2
H+3NCH2CH2NH+3
Answers
The predominant form of ethylenediamine at pH 6.184 is H2NCH2CH2NH+3.
At pH 6.184, which is slightly acidic, the amino groups in ethylenediamine (H2NCH2CH2NH2) can partially protonate, resulting in the formation of ammonium ions (H+3NCH2CH2NH+3). The equilibrium between the neutral form and the protonated form is pH-dependent.
Therefore, at pH 6.184, the predominant form of ethylenediamine is H2NCH2CH2NH+3.
Condensation polymers that contain the amide functional group are called ?
Answers
Answer:
Polyamides
(not entirely sure if this is right)
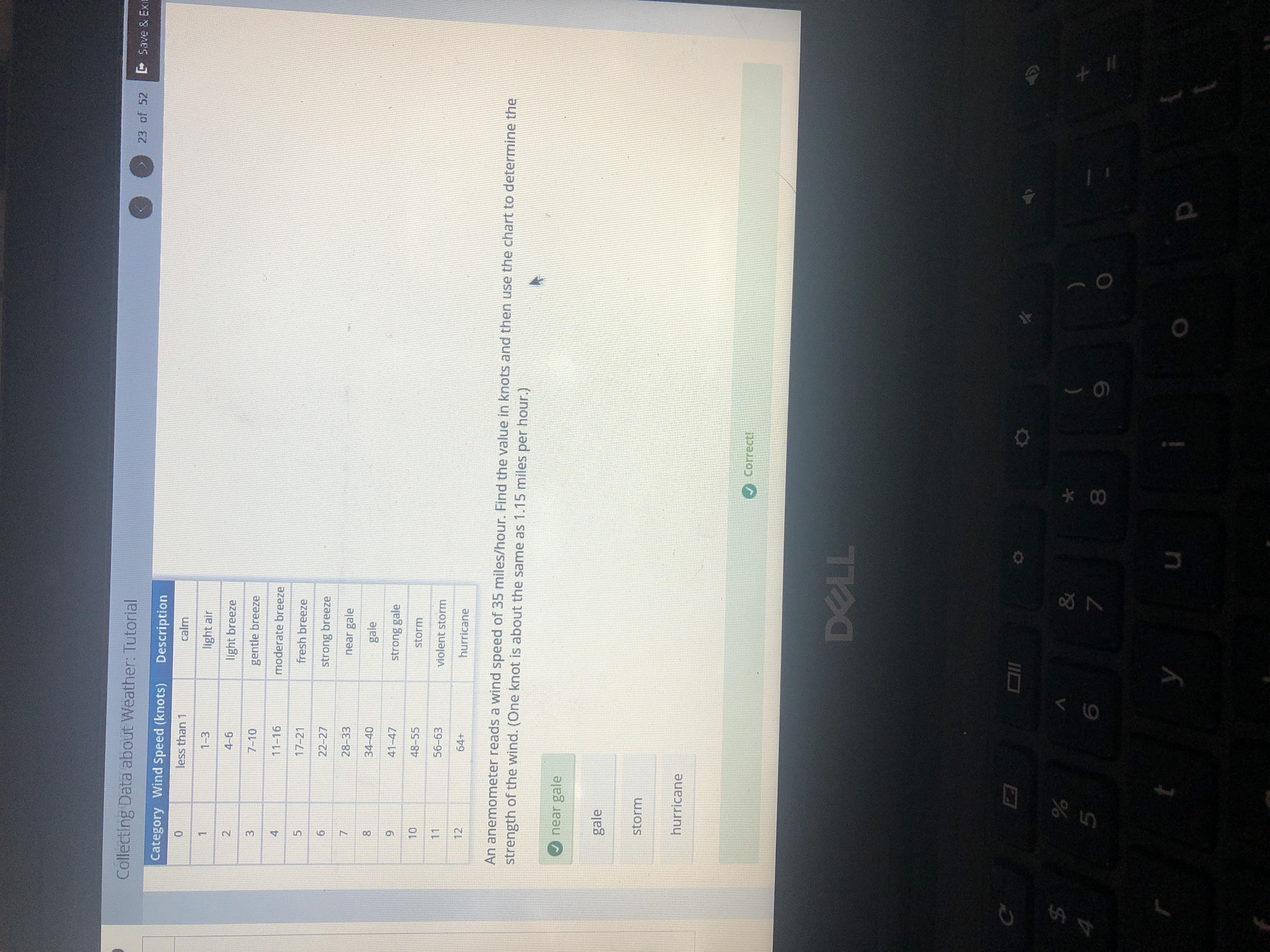
An anemometer reads a wind speed of 35 miles/hour. Find the value in knots and then use the chart to determine the strength of the wind. (One knot is about the same as 1.15 miles per hour.)
Answers
Answer: near gale
Explanation:
Which is the best example for genetic diversity?
Answers
Answer:
Genetic Diversity Examples
Different breeds of dogs. ...Different varieties of rose flower, wheat, etc.There are more than 50,000 varieties of rice and more than a thousand varieties of mangoes found in India.Genetic diversity is the total number of genetic characteristics in the genetic makeup of a species, it ranges widely from the number of species to differences within species and can be attributed to the span of survival for a species.
Explanation:
i hope this helps u.
The solubility of the ionic compound MX3, having a molar mass of 288 g/mol, is 3.60 x 10-2 g/L. Calculate the KSP of the compound.
Answers
\(K_{sp}\) of the compound is found to be 5.04 ×\(10^{-10}\).
Solubility :Solubility can be define as the amount of a substance that dissolves or mixes in a given amount of solvent at specific conditions.
Solubility equilibrium
Ksp = \([A^{+} ]^{a}\) \([B^{-} ]^{b}\)
Ksp = solubility product constant
A+ = cation in an aquious solution
B- = anion in an aqueous solution
a, b = relative concentrations of a and b
Given,
Solubility = s = 3.60 × \(10^{-2}\) g/L
molar mass = 288 g/ mol
∴ s= 3.60 × \(10^{-2}\) g/L ÷ 288 g/ mol = 1.25 ×\(10^{-4}\) mol/ L
Reaction:
MX3 ⇄ M + 3X
s 3s
\(K_{sp}\) =[ \(M^{+3}\)] [ \(X^{-1}\)\(]^{3}\) = solubility product
∴ \(K_{sp}\) =\([s]^{} [3s]^{3}\)
∴ \(K_{sp}\) = 3 \(s^{4}\)
∴ \(K_{sp}\) = 3 × (3.60 × \(10^{-2}\) \()^{4}\)
∴ \(K_{sp}\) = 503.8848 ×\(10^{-8}\) = 5.04 ×\(10^{-10}\)
Learn more about solubility here .....
https://brainly.com/question/23946616
#SPJ1
100 grams of iron (Fe) reacts with sulfuric acid (H2SO4) in the following equation.
Fe + H2SO4 Fe2(SO4)3 + H2
36. The number of grams of iron (III ) sulfate [Fe2(SO4)3] that will be produced is __ grams.
a
275
b
357
Answers
b
b357
Answer:
2Fe +3 H2SO4 Fe2(SO4)3 + 3H2
2*56g. 3*98g➔400g. +6g
we have
2*56 g of Fe gives 400g of iron (III ) sulfate
now
100g of Fe gives=400/112*100=357g of iron (III ) sulfate
\(\:\)
B is your answer.!
A solution is made from 0.5 moles of sodium dichromate. If the volume of the solution is 1 Liter, what is the molarity of the solution
2 M
0.5 M
4 M
1 M
Answers
Answer:
4 M
Explanation:
See the steps and explanation in the picture

Calculate the kinetic energy of a mole of oxygen gas molecules that have a speed
of 10.0 m/s.
Answers
Answer:
1600J
Explanation:
1 mole of oxygen gas (O2) has a mass of 32g i.e. the molar mass = 32g/mol
Kinetic energy (K.E) = ½ × m × v²
Where;
m = mass (g)
v = speed or velocity (m/s)
From the information given in this question;
m = 32g
V = 10m/s²
K.E = ½ × 32 × 10²
K.E = 16 × 100
K.E = 1600J
Which of the following are valid reasons you must repeat the standard curve (calibration plot) for crystal violet this week? I The spectrophotometer may not be the same instrument as last week. II The cuvette will not be the same as last week. III, A spectrophotometer may give different results on different days. a. I and III only b. I and II only c. II and III only d. I, II, and III
Answers
Option c) is correct . The cuvette will not be same as last week or spectrophotometer may give different results on different days.
What is calibration plot?
This curve is used in analytical chemistry for the determination the concentration of an unknown sample solution.
Some errors might get during measurements
1. didn't wait for instrument to calibrate. (Before showing 0.00 have placed cuvette in it)
2. Not handled the cuvette in correct way.
3. May changed the cuvette. (Size of cuvette)
For more information about calibration curve please visit:
https://brainly.com/question/14662384
#SPJ4
a weather balloon is inflated to a volume 2.2 10square3 L with 374g of helium. what is the density of helium in grams per liter
Answers
Answer:
Density = 0.17 g/L
Explanation:
It is given that,
Volume of the inflated balloon filled with Helium, \(V=2.2\times 10^3\ L\)
Mass, m = 374 g
We need to find the density of helium. It is equal to its mass per unit volume. It can be given by :
d =m/V
\(d=\dfrac{374\ g}{2.2\times 10^3\ L}\\\\=0.17\ g/L\)
So, the density of helium in the balloon is 0.17 g/L.
The heat capacity of air is much smaller than that of water, and relatively modest amounts of heat are needed to change its temperature. This is one of the reasons why desert region, although very hot during the day, are bitterly cold at night. The heat capacity of air at room temperature and pressure is appoximately 21 J/K*mol. How much energy is required to raise the temperature of a room of dimensions 5.5m x 6.5m x 3.0m by 10 degrees Celsius? If losses are neglected, how long will it take a heater rated at 1.5 kW to achieve that increase given that 1 W = 1 J/s?
Answers
Answer:
\(Q=9.2x10^5J\)
\(t=614s=10.2min\)
Explanation:
Hello,
In this case, we can compute the energy by using the following formula for air:
\(Q=nCp\Delta T\)
Whereas the moles of air are computed via the ideal gas equation at room temperature inside the 5.5m x 6.5m x 3.0m-room:
\(n=\frac{PV}{RT}\\\\V=5.5m*6.5m*3.0m=107.25m^3*\frac{1000L}{1m^3}=107250L\\ \\n=\frac{1atm*107250L}{0.082\frac{atm*L}{mol*K}*298.15K}\\ \\n=4386.8mol\)
Now, we are able to compute heat, by considering that the temperature raise is given in degree Celsius or Kelvins as well:
\(Q=4386.8mol*21\frac{K}{mol*K}*10K \\\\Q=9.2x10^5J\)
Finally, we compute the time required for the heating by considering the heating rate and the required heat, shown below:
\(t=\frac{9.2x10^5J}{1.5\frac{kJ}{s}*\frac{1000J}{1kJ} } \\\\t=614s=10.2min\)
Regards.
An equilibrium mixture of N2, 02, and NO gases at 1500 K is determined to consist of
6.4 x101-3 mol/1 oF N2, 1.7 x 101-3 mol/ of 02 , and 1.1 × 10 ^-5 mol/1 of NO. What is the equilibrium constant for the system at this temperature?
Answers
The equilibrium constant for the system at this temperature is\(1.17 × 10^-31 mol^2/L^2\).
For the chemical equation:
N2(g) + O2(g) ⇌ 2NO(g)
The equilibrium mixture at a temperature of 1500 K is determined to contain 6.4 × 10^-3 mol/L of N2,\(1.7 × 10^-3\)mol/L of O2 and 1.1 × 10^-5 mol/L of NO. First, we need to calculate the concentration of N2 and O2 required to produce
1.1 × 10^-5 mol/L of NO:
2NO(g) = N2(g) + O2(g)
Given that there are 1.1 × 10^-5 mol/L of NO, the number of moles of N2 and O2 are equal since the stoichiometric ratio is 1:1. Therefore:
\(1.1 × 10^-5 mol/L\) = [N2][O2]Kc = \(([NO]^2)/([N2][O2])Kc\)= \((1.1 × 10^-5 mol/L)^2/(6.4 × 10^-3 mol/L)(1.7 × 10^-3 mol/L)Kc\) =
1.17 × 10^-31 mol^2/L^2.
for such more questions on equilibrium
https://brainly.com/question/5081082
#SPJ8
What is the first step when using a microscope?
A. Place the microscope slide on the stage
B. Plug the microscope in and turn it on
C. Switch to the low power objective lens
D. Secure the slide to the stage clips
Answers
Answer:
I think the answer is B :)
Answer:
the first step is B
Explanation: I'm on this lesson