Answers
The balanced reaction equation is;
3X + 2YZ ----> XY2 + 2XZ
What is the meaning of a reaction equation?A reaction equation is a written representation of a chemical reaction using chemical formulas and symbols. It shows the reactants, the products, and the stoichiometry, which is the quantitative relationship between the reactants and products.
The general format for a reaction equation is:
Reactants → Products
The arrow represents the direction of the reaction, and the reactants are listed on the left side of the arrow, while the products are listed on the right side of the arrow.
Learn more about reaction equation:https://brainly.com/question/3588292
#SPJ1
Related Questions
Mention three scientist that defined acid
Answers
Answer:
1. Svante Arrhenius
2. Johannes Nicolaus Brønsted and Thomas Martin Lowry
3. Gilbert N. Lewis
Explanation:
The scientist mentioned above researched and made their tested hypothesis available for further research to be built on them and they do these researches to help human understanding of the field and therefore improve knowledge.
Through their different definitions of acid which includes the ability of hydrogen ion (H+) to be produced when a substance is separated from water, the ability of acid to donate a proton (H +), It can be concluded that acids contain hydrogen and they have the ability to donate that (H +) to other substance.
An example of a physical property of an element is the element's ability to what?
Answers
Explanation:
Appearance, melting point, density, solubility, polarity
issued this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your etext. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon.
Answers
The atomic ratio of carbon to oxygen in carbon monoxide (CO) is 1:1, and the atomic ratio of carbon to oxygen in carbon dioxide (CO₂) is 2:1.
Firstly, we can analyze the decomposition of carbon monoxide (CO) and carbon dioxide (CO₂) to determine the atomic ratios involved.
Let's denote the atomic ratio of carbon to oxygen in carbon monoxide as x, and the atomic ratio of carbon to oxygen in carbon dioxide as y.
According to the given data;
Decomposition of carbon monoxide (CO);
Oxygen produced = 3.36 g
Carbon produced = 2.52 g
We know that the atomic mass of carbon is 12 g/mol, and the atomic mass of oxygen is 16 g/mol. Using these values, we can calculate the number of moles for each element;
Number of moles of oxygen = mass / atomic mass = 3.36 g / 16 g/mol = 0.21 mol
Number of moles of carbon = mass / atomic mass = 2.52 g / 12 g/mol = 0.21 mol
Since the atomic ratio of carbon to oxygen in carbon monoxide is x, we can write the following equation;
0.21 mol C / (0.21 mol O) = x
Simplifying the equation, we have;
x = 1
Therefore, the atomic ratio of carbon to oxygen in carbon monoxide is 1:1.
Decomposition of carbon dioxide (CO₂);
Oxygen produced = 9.92 g
Carbon produced = 3.72 g
Following the same calculations as before;
Number of moles of oxygen = mass / atomic mass = 9.92 g / 16 g/mol = 0.62 mol
Number of moles of carbon = mass / atomic mass = 3.72 g / 12 g/mol = 0.31 mol
Since the atomic ratio of carbon to oxygen in carbon dioxide is y, we can write the following equation;
0.31 mol C / (0.62 mol O) = y
Simplifying the equation, we have;
y = 0.5
Therefore, the atomic ratio of carbon to oxygen in carbon dioxide is 1:0.5, which can be simplified to 2:1.
To know more about decomposition here
https://brainly.com/question/20418092
#SPJ4
--The given question is incomplete, the complete question is
"Missed this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your text. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon. Calculate the atomic ratio of carbon to oxygen in carbon monoxide, and carbon dioxide."--
What is the pOH of water?

Answers
Answer:
A. 7
(assuming the water is neutral)
please help me i need yall will mark brainliest and 30 points!

Answers
Reaction
2Fe(OH)₃ + 3H₂SO₄⇒Fe₂(SO₄)₃ + 6H₂O
because the limiting reactant is Fe(OH)₃, then the moles of all compounds will be based on moles of Fe(OH)₃
mole H₂SO₄ = 3/2 x mole Fe(OH)₃ = 3/2 x 3 mole = 4.5 mole
remaining mole of H₂SO₄ (as an excess reactant) = 6.4 - 4.5 = 1.9 mole
What does it mean to be an exact number? Give an example of an exact number.
Answers
Answer:
An exact number is a value that is known with complete certainty. Like for Examples of exact numbers are counted numbers of objects or certain unit conversions. For example, there are exactly 3 feet in 1 yard. There are exactly 12 eggs in a dozen. A class may contain exactly 25 students.
Explanation:
Hope this helps.
Have a good night ma´am/sir.
Be safe!
If 246,000 J of energy are required to raise 10 kg of sodium from 15°C to 35°C, what is the specific heat capacity of sodium?
Answers
The specific heat capacity of sodium is 6.15 J/(g°C).The specific heat capacity of a substance is the amount of heat energy required to raise the temperature of one unit of mass by one degree Celsius. In this case, we are given that 246,000 J of energy are required to raise 10 kg of sodium from 15°C to 35°C.
To find the specific heat capacity of sodium, we can use the formula:
Q = mcΔT
Where Q is the heat energy absorbed, m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature.
Plugging in the values given, we get:
246,000 J = 10 kg x c x (35°C - 15°C)
Simplifying:
246,000 J = 200 kg x c x 20°C
Solving for c:
c = 246,000 J / (200 kg x 20°C)
c = 6.15 J/(g°C)
The specific heat capacity of a substance is the amount of heat energy required to raise the temperature of one unit of mass by one degree Celsius. In this case, we are given that 246,000 J of energy are required to raise 10 kg of sodium from 15°C to 35°C.
Therefore, the specific heat capacity of sodium is 6.15 J/(g°C).
learn more about energy
https://brainly.com/question/15318867
#SPJ11
Find the volume of a cereal box that has the dimensions of 35.2 cm x 220 cm x 6.0 cm
Answers
Answer:
Volume = 35.2×220×6.0 = 46464 centimeters³
Explanation:
What does replacement mean?
Answers
Answer: Replacement means a person or thing that takes the place of another; a substitute.
If a container holds 5.00 moles of gas and the total pressure is 5.00 atm., what is the partial pressure for each mole of gas?
Answers
Answer:
1 atm
Explanation:
From the question given above, the following data were obtained:
Total mole = 5 moles
Total pressure = 5 atm
Partial pressure for each mole =.?
Next, we shall determine the mole fraction for each mole of the gas. This is illustrated below:
Since the container holds 5 moles of gas, then the mole fraction for each mole of the gas will be 1/5.
Finally, we shall determine the partial pressure for each mole of the gas as follow:
Mole fraction for each mole = 1/5
Total pressure = 5 atm
Partial pressure for each mole =.?
Partial pressure = mole fraction × total pressure
Partial pressure = 1/5 × 5
Partial pressure = 1 atm
Thus, the partial pressure for each mole of the gas is 1 atm.
Study the information in the table.
what conclusion can be reached from this data?

Answers
Answer:
I can't get you what you are telling
Convert each measurement into scientific notation. For example, 8.73 Ms is the same as 8.73 x 106 because the “M” stands for 106
107pg
(I'm assuming I have to convert the 107pg but I am unsure of how to answer this)
Answers
Answer:
\(107pg = 107 \times {10}^{ - 12}g =1.07 \times {10}^{ - 10} g\\ because \: pico, \: p = {10}^{ - 12} .\)
The diagram on the left shows a ball on the end of a string being whirled in a circle. The diagram on the right shows the whirling ball as
viewed from above.
(View from above)
After several whirls, the string is released when the ball is at Q. Which of these diagrams shows the direction in which the ball will fly
the instant the string is released?
Answers
Answer:B, because this picture move to B
how many moles of fluorine atoms are contained in 3.0 moles of sulfur hexafluoride?
Answers
There are 18 moles of fluorine atoms in 3.0 moles of sulfur hexafluoride. Sulfur hexafluoride (SF6) is a covalent compound made up of one sulfur atom and six fluorine atoms.
To determine how many moles of fluorine atoms are contained in 3.0 moles of SF6, we need to first calculate the number of moles of SF6, and then multiply it by the number of fluorine atoms in one molecule of SF6. The molar mass of SF6 can be calculated by adding the atomic masses of one sulfur atom and six fluorine atoms. The atomic mass of sulfur is 32.06 g/mol, and the atomic mass of fluorine is 18.998 g/mol. Therefore, the molar mass of SF6 = (1 x 32.06 g/mol) + (6 x 18.998 g/mol) = 146.06 g/mol
Finally, we can calculate the number of moles of fluorine atoms in 3.0 moles of SF6 by multiplying the number of moles of SF6 by the number of fluorine atoms in one molecule of SF6. One molecule of SF6 contains six fluorine atoms, so Number of moles of fluorine atoms = 3.0 mol SF6 x 6 mol F / 1 mol SF6 = 18 mol F Therefore, there are 18 moles of fluorine atoms in 3.0 moles of sulfur hexafluoride.
To know more about moles visit ;
https://brainly.com/question/30885025
#SPJ11
when collecting a gas over water why is it important to make sure that the water line on the inside of the eudiometer matches that of the outside?
Answers
It important to make sure that the water line on the inside of the eudiometer matches that of the outside in order to obtain the actual pressure of the created gas.
Now, the issue is that water, regardless of temperature, has a specific vapour pressure. In other words, water will begin to evaporate whenever it is in contact with a gaseous phase up to a specific point where the partial pressure of the water vapour in the gas phase reaches a certain value. The pressure in the eudiometer will be lower than the atmospheric pressure when the water level in the eudiometer is lower than that in the cylinder. And so, in order to account for the difference between the two and also be able to obtain appropriate results, you must first determine the difference between the water levels or subtract them before converting them to millimeters of mercury (mmHg).
To know more about eudiometer visit
https://brainly.com/question/18994460
#SPJ4
5.27x10^45 molecules of h20 is how many moles
Answers
Answer:
6.02 × 10^23 molecules = 1 mole
5.27 × 10^45 molecules = x
x = 5.27 × 10^45/ 6.02 × 10^23 × 1
= 8.754 × 10^21 mol
I don't know if it's correct but based on the question that was the only way I saw how to work it out
Convert the following word equations into formula equations by changing element and compound names into chemical formulas.
1. sodium + oxygen → sodium oxide
2. copper + silver nitrate → copper(II) nitrate + silver
3. aluminum chlorate + barium sulfate → aluminum sulfate + barium chlorate
4. bromine + calcium iodide → calcium bromide + iodine
5. zinc sulfide + magnesium arsenate → zinc arsenate + magnesium sulfide
6. cobalt + lead(II) nitrite → cobalt(III) nitrite + lead
7. potassium + water → potassium hydroxide + hydrogen
Answers
Answer:
Na + O.2 = NaO.2 whereas Cu + AgNo3 = Cu.2No.3 +AG
The given reactions can be converted to equations as follows:
1. 2 Na + O2 → 2 Na2O
2. Cu + 2 AgNO3 → Cu(NO3)2 + 2 Ag
What is a balanced chemical equation ?A balanced chemical equation is a representation of a chemical reaction using chemical formulas of reactants and products. It shows the number of atoms of each element on both the reactant and product sides of the equation.
The equation is balanced when the number of atoms of each element is the same on both sides of the equation.
The given reactions can be written as balanced equations as follows:
2 Na + O₂ → 2 Na₂O2. Cu + 2 AgNO₃ → Cu(NO₃)₂ + 2 Ag2 Al(ClO₃)₃ + 3 BaSO4 → Al2(SO₄)₃ + 3 Ba(ClO₃)₂Br₂ + CaI₂ → CaBr₂ + I₂ZnS + Mg₃(AsO₄)2 → Zn₃(AsO₄)₂ + 3 MgSCo + 2 Pb(NO₂)₂ → Co(NO₂)₂ + 2 Pb2 K + 2 H₂O → 2 KOH + H₂Find more on balanced chemical equations :
https://brainly.com/question/30702275
#SPJ2
Will 10g of k2s04 completely dusdolve in 100 g of water at 50 degrees celsius
Answers
Yes, 10g of K₂SO₄ will completely dissolve in 100g of water at 50° Celsius.
The solubility of K₂SO₄ at 50 degrees Celsius is 17g per 100g of water. Since the amount of K₂SO₄ being dissolved (10g) is less than the solubility at this temperature (17g), it will completely dissolve.
To calculate solubility, you need to know the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature. This is known as the solubility limit. Once you know this, you can use the following equation to calculate solubility:
Solubility = (mass of solute) / (mass of solvent)
Learn more about solubility at https://brainly.com/question/23946616
#SPJ11
What are the products of the following neutralization reaction? (No need to balance)
H3PO4 + Ba(OH)2 --> ??? + ???
A.)H2O + Ba3(PO4)2
B.)H30+ + BaPO4
C.)H30+ + Ba3(PO4)2
D.)H2O + BaPO4

Answers
H3PO4 + Ba(OH)2 --> Ba3(PO4)2 + H2O
so, (A) H2O + Ba3(PO4)2 is your answer.
Describe a situation where two objects have the same mass and the same amount of thermal energy but different temperatures.
Answers
Answer:
The material that makes up the substance is a third factor that affects how much thermal energy a sense has.
PLS HELPPPPPPP and explain how to do it

Answers
Answer: 3.613x10^24
Explanation: Avogadro’s number represents the number of atoms in one mole of substance: 6.022x10^23 atoms/mole. Multiplying the number of moles by this number reveals that, in this sample, 3.613x10^24 atoms of methane are present.
Answer: A
Explanation: When using dimensional analysis, always put similar unit-ed numbers diagnolly from each other so they 'cross out'
For example, If you had 1 mol of gold and you wanted to convert it to grams using gold's molar mass, 196.97 g/mol, it would look like this:
Start with the given number over nothing:
\(\frac{1 mol Au}{} *\frac{ }{ }\)
Then add your conversion, in this case the molar mass, with the MOLES on the BOTTOM, so they are diagnol with the 1 mol of Au
\(\frac{1 mol Au}{ } *\frac{196.7 g Au}{1 mol}\)
Always have the top right in your dimensional analysis be in the units that you are solving for. In this case, we went from moles to grams, so grams is in the top right.
Hope this helps!
Also, 6.02 x 10²³ is otherwise known as avogadros number- it is used when you are converting something from moles to molecules (or particles). For example, if instead we were going from moles of gold to molecules of gold, our dimensional analysis would look like this:
\(\frac{1 mol Au}{ } *\frac{6.02 x 10^2^3 molecules Au}{1 mol}\)
subatomic particles that are associated with negative charges and surround the protons and neutrons in an atom, are called
Answers
Electrons.
Neutrons and Protons are contained with the positive nucleus, whilst negatively charged electrons surround it.
The density of water is 1 g/cm3(1 gram per cubic centimeter); using this fact as a reference & Table c , how would you determine if an object sinks or float
Answers
Answer:
Explanation:
Any densities less than 1g/cm3 will float, while objects with densities over 1g/cm3 will sink.
what are the steps of the election process in order )?
Answers
There are 9 steps of the election process Call for Nominations, Preliminary Reviews, Ballot Petition Rules, Nominee Interviews, Committee Meeting, Slate of Candidates Announcement, Voting Opens etc..
Request for Nominations
The nomination process is made public by the Academy.
introductory reviews
Candidates are required to submit biographical sketches that contain digital images, films they have recorded themselves, and curriculum vitae or resumes.
Election Petition Rules
Check out the requirements for adding a petitioner to the general election ballot.
interviewing nominees
Virtual meetings with the Nominating Committee are held.
Commission Meeting
The Nominating Committee meets in late fall to review the materials submitted by candidates, taking into account which positions are open on the ballot, and evaluating key personal, interpersonal, and demonstrated leadership skills as they relate to the qualifications and skill set needs for each position.
Release of the Candidate Slate
Announcing candidates for posts includes posting supporting information online, in the Academy's electronic weekly newsletter, and on social media, including images, self-recorded films, biographical sketches, and answers to questions posed by the Nominating Committee.
Voting begins
Members take part in computerised voting during the month of February for the national Academy elections.
Announcing the results of the election
Following the tallying of all votes and the notification of candidates, the Academy informs members of the election results.
Review of the election and nomination processes
The process for nomination and election is assessed at each stage. The Nominating Committee, the Academy, and the applicants are all asked for their opinions.
Learn more about election process here:
https://brainly.com/question/2172915
#SPJ4
22. How many atoms are there in 344.75 g of gold nugget? a. 1.05 x 10 to the power of 24 atoms b. 1.05 x 10 to the power of 23 atoms c. 6.02 x 10 to the power of 23 atoms d. 197 atoms Is B the answer
Answers
Answer:
1.053×10²⁴ atoms of gold
Explanation:
Hello,
Gold nugget are usually the natural occurring gold and they contain 85% - 90% weight of pure gold.
In this question, we're required to find the number of atoms in 344.75g of a gold nugget.
We can use mole concept relationship between Avogadro's number and molar mass.
1 mole = molar mass
Molar mass of gold = 197 g/mol
1 mole = Avogadro's number = 6.022 × 10²³ atoms
Number of mole = mass / molar mass
Mass = number of mole × molar mass
Mass = 1 × 197
Mass = 197g
197g is present in 6.022×10²³ atoms
344.75g will contain x atoms
x = (344.75 × 6.022×10²³) / 197
X = 1.053×10²⁴ atoms
Therefore 344.75g of gold nugget will contain 1.053×10²⁴ atoms of gold
As per the atoms the 344.5 g of gold nuggets have a 1.05x10 and have a power of 24 atoms.
Gold nugget are usually the natural occurring gold and they contain 85% - 90% weight of pure gold. In this question, we're required to find the number of atoms in 344.75g of a gold nugget. 1.053×10²⁴ atoms of gold.Learn more about the atoms are there.
brainly.com/question/18510197.
i need to know the answer ASAP PLEASE
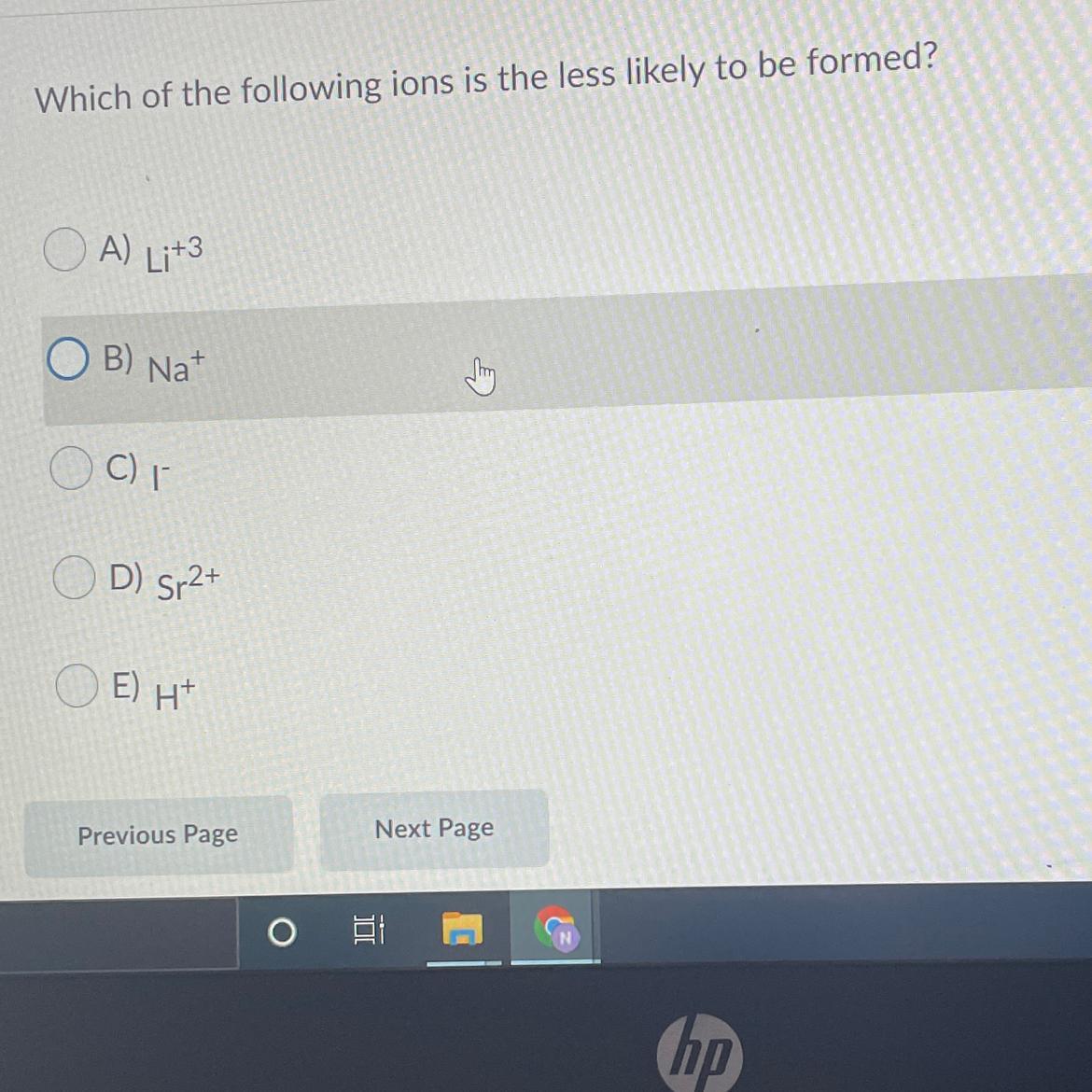
Answers
Answer:
E....H+
Explanation:
coz when hydrogen ions are formed they automatically join to form hydrogen
I WILL GIVE 15 POINTS PLS I NEED THIS BEFORE THE CLASS FINISHES IN 30 MINN
The diagram above shows the repeating groups of atoms that make up two samples. Will the properties of the two samples likely be the same or different? (Examples of properties are smell, color, and the temperature at which a substance melts.)

Answers
Answer:
likely be the same
Explanation:
this is because we have one color that both atoms share (green). both sample 1 and sample 2 have green and another color. yet, since they share one color, they are likely similar
Calculate the ph of this solution. round to the nearest hundredth. poh = 3.45 ph =
Answers
The potential of hydrogen pH of the solution with the given value of pOH to the nearest hundredth is 10.55.
What is pH of solution?
The pH of a solution is defined as the logarithm of the reciprocal of the hydrogen ion concentration [H+] of the given solution.
It is expressed as;
pH = -log[ H⁺ ]
Also,
pH + pOH = 14
Given that;
pOH = 3.45pH = ?We simply substitute our values into the expression above.
pH + pOH = 14
pH + 3.45 = 14
pH = 14 - 3.45
pH = 10.55
Therefore, the potential of hydrogen pH of the solution with the given value of pOH to the nearest hundredth is 10.55.
Learn more about pH & pOH here: brainly.com/question/17144456
#SPJ4
Answer:
10.55
Explanation:
pH = 10.55
in need of help very soon please.

Answers
Answer:
hello, i hope this helps.
Explanation:
1 - group
2 - period
3 - periodic table
4 - family
5 - octet rule
6 - valence electrons
What do you call an animal with multiple organs ?
Answers
Answer: Hermaphrodite
hope this is helpful
