Answers
Answer:
b, c and d (last three options)
Explanation:
It can't be the first one because:
On the left hand side there is one of everything and then on the right, ther is one zinc, two chloride and 2 hydrogen atoms. It is unbalanced
Second one:
there are 8 carbons on both sides, 20 hydrogens on both sides and 26 oxygen on both sides. It is balanced
Third
1 copper on both sides, 2 hydrogens on both sides and 1 oxygen on both sides. It is balanced
Fourth
4 silver on both sides and 2 oxygens on both sides
Related Questions
What is the molar concentration a a 12 % sodium chloride solution (MW 58.5)
Answers
The molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To determine the molar concentration of a 12% sodium chloride solution, we need to convert the given percentage concentration into molarity.
First, we need to understand that the percentage concentration refers to the mass of the solute (sodium chloride) relative to the total mass of the solution.
In this case, a 12% sodium chloride solution means that there are 12 grams of sodium chloride in 100 grams of the solution.
To convert this into molar concentration, we need to consider the molar mass of sodium chloride, which is 58.5 g/mol.
We can start by calculating the number of moles of sodium chloride in 12 grams:
Moles of sodium chloride = mass of sodium chloride / molar mass of sodium chloride
Moles of sodium chloride = 12 g / 58.5 g/mol = 0.205 moles
Next, we calculate the volume of the solution in liters using the density of the solution. Since the density is not provided, we assume a density of 1 g/mL for simplicity:
Volume of solution = mass of solution / density
Volume of solution = 100 g / 1 g/mL = 100 mL = 0.1 L
Finally, we calculate the molar concentration (Molarity) by dividing the number of moles by the volume in liters:
Molar concentration = moles of solute / volume of solution
Molar concentration = 0.205 moles / 0.1 L = 2.05 M
Therefore, the molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To learn more about molarity click here: brainly.com/question/31545539
#SPJ11
Please help me by giving me an drawing , it’s urgent !! Or writing explanation but drawing is easier.

Answers
Answer:
13 Protons, 14 Neutrons, and 13 electrons.
Explanation:
See Picture Below
I have labeled all the numbers and the diagram.
I hope it helped

Can the ratio of hydrogen to oxygen change if a solute is dissolved in the water
Answers
Answer:
Water molecules feature the combinations of hydrogen and oxygen atoms in a 2:1 ratio. Since they are present in a fixed ratio of mass, water molecules obey the law of constant proportions. Water is formed when two molecules of the diatomic hydrogen gas, combine with one molecule of the diatomic oxygen gas to produce two molecules of water
1. A) Calculate DO of water at 25 0 C, P 0 = 1.01x10 5 Pa; given: water vapor pressure (25 0 C) is 3.2x10 3 Pa and Henry constant of oxygen in water is 1.3x10 -8 mol/l.Pa b) How's DO content changed as increase or decrease water temperature? Explain.
Answers
Answer:
cfvgbhnjmk,
Explanation:
How many valence electrons do the halogens possess? group of answer choices
A. 2
B. 1
C. 7
D. 5
E. 6
Answers
The answer is : C.7
The Group 7A elements have seven valence electrons in their highest-energy orbitals (ns2np5).
Group 7A — The Halogens.
Group 7A (or VIIA) of the periodic table are the halogens: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). The name "halogen" means "salt former", derived from the Greek words halo- ("salt") and -gen ("formation").
The Group 7A elements have seven valence electrons in their highest-energy orbitals (ns2np5). This is one electron away from having a full octet of eight electrons, so these elements tend to form anions having -1 charges, known as halides: fluoride, F-; chloride, Cl-, bromide, Br-, and iodide, I-. In combination with other nonmetals, the halogens form compounds through covalent bonding.
To learn more about Halogens. visit: https://brainly.com/question/28204396
#SPJ4
The valence electron the halogens posses are 7
Valence electronThe electron in the outermost shell or energy level of an atom
HalogensThe Group 7A elements have seven valence electrons in their highestGroup 7A — The Halogens.Group 7A of the periodic table are the halogens: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). The name "halogen" means "salt former", derived from the Greek words halo- ("salt") and -gen ("formation").The Group 7A elements have seven valence electrons in their highest-energy orbitals (ns2np5). This is one electron away from having a full octet of eight electrons, so these elements tend to form anions having -1 charges, known as halides: fluoride, F-; chloride, Cl-, bromide, Br-, and iodide, I-. In combination with other nonmetals, the halogens form compounds through covalent bonding.Hence the valence electron of the halogen posses are 7
Learn more about the valence electron on
https://brainly.com/question/13552988
#SPJ4
• 2. Using the following formula Force (N) = mass (kg) * acceleration (m/s").
What is the force of a ball with a mass of 5kg fallinly to the ground at
10m/s.
Answers
Answer
50 N
Explanation
8. Classify stomach acid (HCL) on the basis of the number of ionizable hydrogens it has.
9. State the general properties of water-soluble acids.
Answers
Answer:
8. one ionizable hydrogen
9. Water-soluble organic acids, including dicarboxylic acids, such as malonic and glutaric acid,
Explanation:
8. A monoprotic acid is an acid that contains only one ionizable hydrogen. Hydrochloric acid and acetic acid are monoprotic acids. A polyprotic acid is an acid that contains multiple ionizable hydrogens
9. are a group of organic compounds often detected in atmospheric particles [Chebbi and Carlier, 1996; Kawamura and Kaplan, 1987; Saxena and Hildemann, 1996; Yang and Yu, 2008].
I hope this helps! It was a bit hard to find
Answer:
Answer:
8. one ionizable hydrogen
9. Water-soluble organic acids, including dicarboxylic acids, such as malonic and glutaric acid,
Explanation:
8. A monoprotic acid is an acid that contains only one ionizable hydrogen. Hydrochloric acid and acetic acid are monoprotic acids. A polyprotic acid is an acid that contains multiple ionizable hydrogens
9. are a group of organic compounds often detected in atmospheric particles
Explanation:
none
The volume changes from 1. 25L to 1. 15Lat a temperature of 313K, what is the final temperature?
Answers
Answer: 288 K
Explanation:
Based on the information provided, this question is related to Charles's Law which describes how a volume of a gas is directly proportional to its absolute temperature at constant pressure. This relationship can be described through the following ratios:
V1/T1 = V2/T2
V1, represents the initial volume of the gasT1, represents the initial temperature of the gasV2, represents the final volume of the gasT2, represents the final temperature of the gasSince the question is asking for the final temperature of the gas after its initial volume (V1 = 1.25 L) changes to (V2 = 1.15 L) from an initial temperature of (T1 = 313 K), we need to rearrange the above ratios and solve for T2:
T2 = (V2 * T1)/V1 = (1.15 L * 313 K)/(1.25 L) = 287.96 (3 sig. figs.) = 288 K
This answer makes sense because if we consider the ideal gas law (PV=nRT), we see that if pressure (P) and the amount of the gas (n, in moles) remain constant, then the volume (V) is directly proportional to temperature (T). By decreasing the volume of the gas in this problem, we will also decrease the temperature.
How many moles are 454 grams of iron
Answers
Answer:
8.1293534120006496
Explanation:
hope this helps
when ethane burns in air to form carbon dioxide and water ,heat energy is released. Explain why energy is released
Answers
Energy is released because it is a combustion reaction.
Combustion reaction -
Hydrocarbon bonds are broken during combustion events, and more energy is always released during the formation of water and carbon dioxide bonds than was consumed to break the initial hydrocarbon bonds. Burning materials that are primarily made of hydrocarbons produces energy because of this. This is an exothermic reaction.
2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O + Heat
(ethane)
In the process of burning, or combustion, the reactant takes up oxygen and oxidizes it, releasing energy in the form of heat and light. It happens quickly. Along with the energy created during burning, carbon dioxide and water vapor are also produced.
Hence heat and light which are forms of energy are released during combustion reaction.
To learn more about Combustion reaction refer- https://brainly.com/question/13251946
#SPJ9
The average atomic mass of zinc is 65.37 amu, and it's atomic number is
30. How many ELECTRONS does zinc have?
Answers
Answer:
30
Explanation:
The number of protons determines an element's atomic number and is used to distinguish one element from another. ... Together, the number of protons and the number of neutrons determine an element's mass number.
The number of electrons in a neutral atom is equal to the number of protons. The mass number of the atom (M) is equal to the sum of the number of protons and neutrons in the nucleus. The number of neutrons is equal to the difference between the mass number of the atom (M) and the atomic number (Z).
How long is a sunflower seed? Select the best estimate.
15 meters
15 kilometers
15 millilmeters
15 centimeters
Answers
Answer:
15 millimeters
Explanation:
the other answer choices are too large.
Answer:
15 millimeters
Explanation:
The other options are too long.
A clownfish uses a sea anemone as a safe place to live. While living there, the clownfish lures in food for the anemone. This is an example of what type of relationship?
Answers
Ground-level ozone in most major u. S. Cities results primarily from:.
Answers
Ground-level ozone in most major U.S. cities results primarily from the reaction of nitrogen oxides (NOx) and volatile organic compounds (VOCs) in the presence of sunlight.
These compounds are released by a variety of sources, including cars, trucks, industrial facilities, and power plants.
In the presence of sunlight, NOx and VOCs undergo a complex series of reactions to form ozone, which is a harmful air pollutant that can cause respiratory problems and other health issues.
Ground-level ozone is a major component of smog and can also contribute to climate change.
Reducing emissions of NOx and VOCs is an important step in reducing ground-level ozone in urban areas.
To know more about ozone, refer here:
https://brainly.com/question/27911475#
#SPJ11
PLEASE HELP
This tree species was preserved as a fossil in the Arizona desert. The species is now extinct. Describe what the environment was like when the tree lived and how it is different now. Then, infer why this type of tree and other living things that lived with it are extinct
Answers
When the tree species was preserved as a fossil in the Arizona desert, the environment was likely different compared to the present. Based on the fact that the tree species is now extinct, infer that the conditions that supported its existence have significantly changed.
What leads to extinction?In the past, the environment where the tree lived may have had different climatic conditions, such as temperature, precipitation patterns, and humidity levels. It may have been part of a specific ecosystem or habitat that provided suitable resources for the tree's growth and survival. The tree species may have had adaptations that allowed it to thrive in that particular environment.
The extinction of the tree species may have had cascading effects on other living things that were associated with it. For example, other plant species that relied on the tree for shade or as a host for epiphytic growth might have also suffered or disappeared. Similarly, animals that depended on the tree for food, shelter, or nesting sites could have been affected by its extinction.
Find out more on extinction here: https://brainly.com/question/1027555
#SPJ1
Dec
Match each concept to the scientist who supported it.
Isaac Newton
Heinrich Hertz
Light is a wave because this property can
explain interference
Light is a wave because it bends when it
hits barriers and openings.
Light is made of particles because it travels
only in straight paths
Light is a wave made of electric and
magnetic fields
Light is a wave that has similar properties
James Clerk Maxwell
Christiaan Huygens
Thomas Young
as radio waves

Answers
Answer:the pic
Explanation:

In ionic bonding, atoms _____.
share electrons
are connected by strong electrical forces
lose protons and form ions
stop moving completely
Answers
Answer:
lose protons and form ions
Explanation:
I thjink hope this helps
PLease mark brainliest
When using the nernst equation to find cell potential under nonstandard conditions,what is one of the first steps?

Answers
the nernst equation to determine cell potential in unusual circumstances allows for the precise measurement of equilibrium constants and connects the measured cell potential to the reaction quotient.
Under unusual circumstances, the cell potential can be calculated using the Nernst Equation. It allows for the precise measurement of equilibrium constants, including solubility constants, and connects the observed cell potential to the reaction quotient.
Only when there is no net current flowing through the electrode does the Nernst equation also hold true. When current flows, the ion activity at the electrode surface changes, and the measured potential also includes extra overpotential and resistive loss components.'
Read more about cell potential at
https://brainly.com/question/31747319
#SPJ4
Please help with sodium and fluorine

Answers
Answer:
Sorry I wish I could help u.. I think this picture is correct
Explanation:

Which force is equal but opposite to the one shown?
A. The normal force that the air applies to the kitten
B. The force that the kitten applies to Earth
C. The force of gravity that acts on the kitten
D. The force that the branch applies to the kitten
Answers
The force equal but opposite to the one shown is : ( D ) The force that the branch applies to the kitten
Opposite and equal forcesThe force of gravity/weight of an object is equal and opposite to the reactive force because it cancels the effect of the weight of the object on the surface. In option D the force that the branch applies to the kitten resting on it is equal and opposite to the weight of the kitten.
Hence we can conclude that The force equal but opposite to the one shown is The force that the branch applies to the kitten.
Learn more about reactive forces : https://brainly.com/question/1013858
#SPJ1
Your questio lacks the diagram but I have provided a general answer within the contest of your question
Fossil fuels were formed when living things (plants and animals) died and their remains were broken down. This is an example of _______.
A. Combustion
B. Decomposition
C. Photosynthesis
D. Cellular Respiration
Answers
How is the process of natural selection different from the process of selective breeding?
Answers
Answer:
Explanation:
The difference between the two is that natural selection happens naturally, but selective breeding only occurs when humans intervene. For this reason selective breeding is sometimes called artificial selection. Different varieties of plants and animals with desired characteristics can be developed by selective breeding.
What is the role of Cl2 in the following
reaction?
CL + NaOH Nacl+ NaCO3+H2O
Answers
Answer:
Cl2 acts as an oxidizing agent
Explanation:
The balanced equation is
Cl2+NaOH⟶NaCl+NaClO3+H2O
Cl2 acts as a reactant in this chemical reaction
Cl2 receives one electron and thus reduces itself from Cl2 to 2 Cl−. This indicates that 2Cl- will oxidize some other element and hence Cl2 act as an oxidizing agent.
Simon has collected three samples from the coral reef where he observes marine life. He must determine whether
each one is a pure substance or a mixture.
Appearance
When heated
Sample A
Sample B
Sample C
Sample A is a
Clear liquid
Clear, blue
liquid
Opaque, whitish
liquid
Evaporates completely at
70°C
Boils at 90°C, leaving blue
crystals behind
Boils at 100°C, leaving
white crystals behind
Sample B is a
When left over time
Appearance does not change
Appearance does not change
Dust appears to settle to the
bottom
and Sample C is a
Dona
Answers
From the collected three samples from the coral reef we can conclude that:
SAMPLE A - pure substance.
SAMPLE B - homogeneous mixture.
SAMPLE C - heterogeneous mixture.
Pure substances and mixtures are the two broad categories into which matter can be divided.
A sort of matter known as a pure substance has qualities that are constant throughout the sample and a stable composition that makes it the same everywhere (meaning that there is only one set of properties such as melting point, color, boiling point, etc. throughout the matter).
A mixture is said to be homogenous if its composition is constant throughout. Because the dissolved salt is evenly distributed throughout the whole salt water sample, the salt water described above is homogenous.
A combination is said to be heterogeneous if its composition is not constant throughout. Vegetable soup is a complex concoction. Each mouthful of soup will have differing percentages of the various veggies and other ingredients.
To learn more about mixtures visit the link:
https://brainly.com/question/24898889?referrer=searchResults
#SPJ9
:))))))))))))))))))))))))))
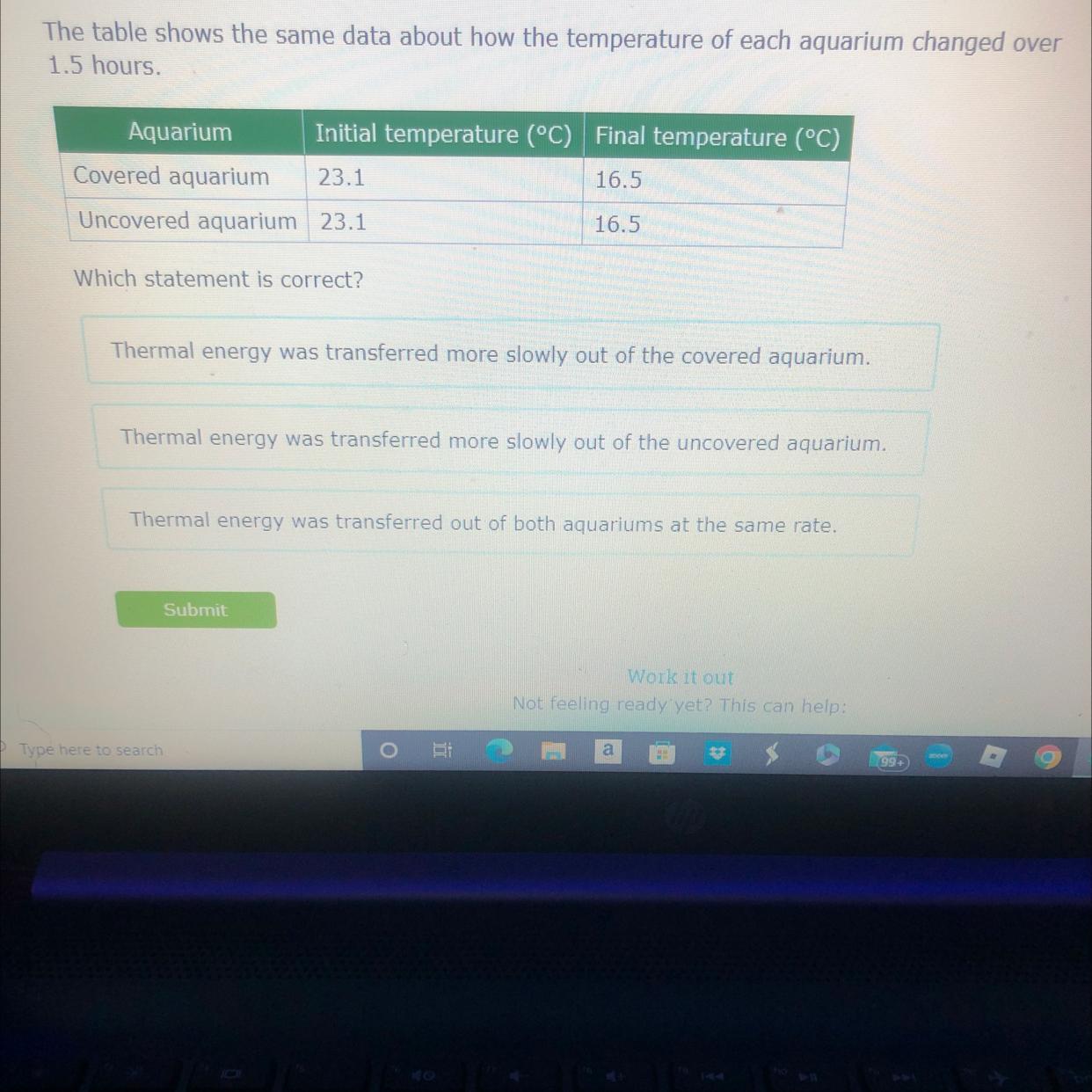
Answers
Answer:
Thermal Energy was transferred out of both Aquariums at the same rate
Answer:
Thermal energy was transformed at the same rate
Explanation:
:))))))))
A. Briefly explain how a loss of water by evaporation would affect the initial calculation of the solubility of your salt.
B. Would this initial evaporation affect the calculated solubility of your salt at each subsequent experimental saturation temperature, or just the initial temperature? Explain.
Answers
Answer:
Following are the solution to this question:
Explanation:
Please find the complete question in the attached file.
In point a:
When some water evaporates from its mixture, then when a thick layer is initiated, it reduces salt solubility, but sodium remains unclear throughout the solution (ppt solte).
In point b:
Ksp is measured by calculating the mass of its solvent reduction process, decreasing salt solubility and hence decreasing Ksp.
In point c:
From of the graph \(\Delta \ H \ \ or \ \ \Delta \ S\) are calculated by \(\frac{1}{T}\) v/s lnKsp
(decrease) LnKsp may shift,
\(\Delta \ H = -slope \times R \\\\\Delta \ S = intercept \times R\)
So, both are decreasing

If the kinetic rate constant for the decay of a radioactive nuclide is 0. 00892 years, what is the half-life of this isotope?
Answers
If the kinetic rate constant for the decay of a radioactive nuclide is 0. 00892 years,the half-life of the radioactive isotope is 77.7 years.
The decay rate constant, k is related to the half-life (t1/2) of a radioactive substance by the formula:k = 0.693/t1/2Where:k is the rate constant,t1/2 is the half-life of the radioactive substance.From the above formula, the half-life of a radioactive substance can be calculated as:t1/2 = 0.693/kGiven:k = 0.00892 yearsSubstituting this value into the formula above gives:t1/2 = 0.693/0.00892= 77.7 years. Therefore, the half-life of the radioactive isotope is 77.7 years.
learn more about radioactive
https://brainly.com/question/28106187
#SPJ11
if a chemical reaction produces 20.0 grams of product, but by stoichiometry it is supposed to have 25.0 grams of product; what is the percent yield of the reaction?
Answers
The percent yield of the reaction is 80.0%.
To determine the percent yield of a reaction, you need to compare the actual yield to the theoretical yield and express it as a percentage.
The percent yield is calculated using the formula,
Percent Yield = (Actual Yield / Theoretical Yield) * 100
In this case, the actual yield is given as 20.0 grams, and the theoretical yield, based on stoichiometry, is supposed to be 25.0 grams.
Plugging these values into the formula, we can calculate the percent yield,
Percent Yield = (20.0 / 25.0) * 100
= 0.8 * 100
= 80.0%
The percent yield of the reaction is 80.0%.
A percent yield of 100% would indicate that the actual yield is equal to the theoretical yield, meaning the reaction proceeds with maximum efficiency.
In this case, the percent yield of 80.0% suggests that the reaction yielded 80.0% of the expected amount of product based on stoichiometry. Factors such as incomplete reactions, side reactions, and losses during product isolation can contribute to a percent yield lower than 100%.
Learn more about reaction from the given link:
https://brainly.com/question/11231920
#SPJ11
I need help with this

Answers
Cu-63 and Cu-65 have 34 and 36 neutrons, respectively, in each atom. 27- Because copper has an atomic number of 29, which essentially implies that an atom of copper has 29 protons and 29 electrons, an atom of copper has a total of 29 electrons.
The stable isotope of copper is copper-63, which has a nuclear spin of 3/2, a relative atomic mass of 62.929601, and a natural abundance of 69.2 atoms. a trace element of a heavy metal having the atomic symbols Cu, 29, and 63.55. This atom has the mass number 63 because it is a copper-63 atom. Every atom of a particular element has the same atomic number, which may be determined as the element's number on the predictable.
Learn more about copper here-
https://brainly.com/question/19761029
#SPJ9
1500 torr is how many kPa
Answers
Answer:
200 kPa
(it is actually 199.999, but rounding up it is 200)
hope this helps!
Answer:
1500 Torr is about 200 kPa
