Place the following atoms in order of increasing atomic radii: se, sb, br, and te
Answers
The order of increasing atomic radii for the given elements is: Br < Sb < Se < Te.
When we talk about atomic radii, we are referring to the size of an atom. The atomic radius increases as we move down a group in the periodic table, and it decreases as we move across a period. This is because as we move down a group, the number of electron shells increases, leading to a larger atomic radius.
Conversely, as we move across a period, the number of protons in the nucleus increases, leading to a stronger attractive force on the electrons, resulting in a smaller atomic radius.
In the case of the four elements given - selenium (Se), antimony (Sb), bromine (Br), and tellurium (Te) - we need to determine their position in the periodic table to determine the order of increasing atomic radii.
Starting from the top, we have selenium (Se) and tellurium (Te) in the same group, but Te has a larger atomic number, so it has more electron shells, resulting in a larger atomic radius. Next, we have antimony (Sb), which is in the same period as Te, but with a smaller atomic number, meaning it has a smaller atomic radius.
Finally, we have bromine (Br), which has the smallest atomic number and is also in the same period as Sb, so it has the smallest atomic radius.
Therefore, the order of increasing atomic radii for the given elements is: Br < Sb < Se < Te.
To know more about atomic radii, visit:
https://brainly.com/question/14086621#
#SPJ11
Related Questions
2. two kinds of carbonyl acceptor structures in addition to benzoate ester can be used in reaction with phenylmagnesium bromide to afford triphenylmethanol. what are they? hint: each of the three reacts with a different number of equivalents of the grignard reagent.
Answers
Two kinds of carbonyl acceptor structures in addition to benzoate ester are dialkyl carbonate and benzophenone.
Grignard reaction is an organometallic organic chemical reaction in which alkyl, allyl, vinyl or aryl-magnesium halides who are called as Grignard reagent are added to the carbonyl group in aldehyde or ketone. This reaction is important for the formation of carbon-carbon bonds. or basically they are mainly used for the formation of carbonyl compounds.
Triphenylmethanol can be synthesized by Grignard reaction using 3 different starting materials and also the different number of equivalents of Grignard reagent.
To know more about carbonyl compounds
https://brainly.com/question/14777564
#SPJ4
The reaction of benzaldehyde with acetone and sodium hydroxide produces ____________ This is an example of ____________ reaction.
Answers
The reaction of benzaldehyde with acetone and sodium hydroxide produces DibenzalacetoneThis is an example of an aldol condensation reaction.
A physical reaction is a reaction in which a change in the physical properties of matter or substances occurs. Physical properties include density, mass, and volume. The definition of a physical reaction is a reaction in which molecules undergo molecular rearrangements but do not change chemically.
A response, although technically a reaction, takes into account the desired outcome of the interaction. Reactions can produce positive or negative results, but reactions are designed to produce positive or negative results. To react is emotional, to react is emotional intelligence.
Learn more about the reaction here
https://brainly.com/question/11231920
#SPJ4
1. Standard free energy change for the reaction A + B is -15kJ/mole (AG° = - 15 kJ/mole). What is the equilibrium constant (
1. Standard free energy change for the reaction A B is -15kJ/mole (ΔGo’ = - 15 kJ/mole). What is the equilibrium constant (Keq =?)
2. Based on the above data, what is the actual free energy change for the reaction A B, when [A] = 10mM and [B] = 0.1mM?
3. When the reaction A+B C is at equilibrium, the concentration of reactants are as follows: [A] = 2mM, [B] = 3mM, and [C] = 9mM. What is the standard free energy for the reaction?
ΔGo’ = - RT lnKeq
ΔG = ΔGo’ + RT lnKeq
Where, ΔGo’ = biological standard free energy, J/mol
(Reactants = 1 M; Products = 1 M; T = 37 C or 310; 1 ATM; pH =7.0)
ΔG = overall free energy (or actual free energy in living system)
R = gas constant, 8.314 J/mol.K
T = temperature in K
Keq = equilibrium constant (ratio of products/reactants)
Answers
1. The equilibrium constant (Keq) is approximately 0.002 for the reaction A → B with a standard free energy change of -15 kJ/mol.
2. The actual free energy change (ΔG) for the reaction A → B is approximately -27,240 J/mol when [A] = 10 mM and [B] = 0.1 mM.
3. The standard free energy change (ΔGo') for the reaction A + B → C is approximately -10,117.23 J/mol.
1. The equilibrium constant (Keq) can be determined using the equation: ΔGo' = -RT ln(Keq), where ΔGo' is the standard free energy change, R is the gas constant (8.314 J/mol.K), and T is the temperature in Kelvin.
Given that ΔGo' = -15 kJ/mol, we need to convert it to Joules by multiplying by 1000:
ΔGo' = -15 kJ/mol = -15,000 J/mol.
Assuming the temperature is 310 K, we can calculate Keq as follows:
ΔGo' = -RT ln(Keq)
-15,000 J/mol = -(8.314 J/mol.K)(310 K) ln(Keq)
Simplifying the equation:
ln(Keq) = -15,000 J/mol / (8.314 J/mol.K * 310 K)
ln(Keq) ≈ -5.97
Taking the exponential of both sides:
Keq ≈ e^(-5.97)
Calculating Keq:
Keq ≈ 0.002
Therefore, the equilibrium constant (Keq) for the reaction A → B is approximately 0.002.
2. To determine the actual free energy change (ΔG) for the reaction A → B, we can use the equation: ΔG = ΔGo' + RT ln(Keq), where ΔG is the overall free energy change, R is the gas constant (8.314 J/mol.K), T is the temperature in Kelvin, and Keq is the equilibrium constant.
Given that [A] = 10 mM and [B] = 0.1 mM, we can calculate the actual free energy change as follows:
ΔG = -15,000 J/mol + (8.314 J/mol.K)(310 K) ln(0.1/10)
Simplifying the equation:
ΔG ≈ -15,000 J/mol + (8.314 J/mol.K)(310 K) ln(0.01)
Calculating ΔG:
ΔG ≈ -15,000 J/mol + (8.314 J/mol.K)(310 K)(-4.605)
ΔG ≈ -15,000 J/mol - 12,240 J/mol
ΔG ≈ -27,240 J/mol
Therefore, the actual free energy change (ΔG) for the reaction A → B, when [A] = 10 mM and [B] = 0.1 mM, is approximately -27,240 J/mol.
3. To calculate the standard free energy change (ΔGo') for the reaction A + B → C, we can use the equation: ΔGo' = -RT ln(Keq), where ΔGo' is the standard free energy change, R is the gas constant (8.314 J/mol.K), T is the temperature in Kelvin, and Keq is the equilibrium constant.
Given the concentrations at equilibrium: [A] = 2 mM, [B] = 3 mM, and [C] = 9 mM, we can calculate the standard free energy change as follows:
First, let's calculate the ratio of products to reactants based on their concentrations:
[A] = 2 mM, [B] = 3 mM, and [C] = 9 mM
Keq = ([C]^coefficient[C] * [A]^coefficient[A] * [B]^coefficient[B]) / ([A]^coefficient[A] * [B]^coefficient[B])
Keq = (9^1 * 2^0 * 3^0) / (2^1 * 3^1)
Keq = 9 / 6
Keq = 1.5
Now, we can calculate ΔGo' using the equation:
ΔGo' = -RT ln(Keq)
Assuming the temperature is 310 K, and using the gas constant R = 8.314 J/mol.K:
ΔGo' = -(8.314 J/mol.K)(310 K) ln(1.5)
Calculating ΔGo':
ΔGo' ≈ -(8.314 J/mol.K)(310 K)(0.405)
ΔGo' ≈ -10,117.23 J/mol
Therefore, the standard free energy change (ΔGo') for the reaction A + B → C, when the concentrations are [A] = 2 mM, [B] = 3 mM, and [C] = 9 mM, is approximately -10,117.23 J/mol.
1. The equilibrium constant (Keq) is approximately 0.002 for the reaction A → B with a standard free energy change of -15 kJ/mol.
2. The actual free energy change (ΔG) for the reaction A → B is approximately -27,240 J/mol when [A] = 10 mM and [B] = 0.1 mM.
3. The standard free energy change (ΔGo') for the reaction A + B → C is approximately -10,117.23 J/mol.
To learn more about energy, visit
https://brainly.com/question/31055237
#SPJ11
what 1-bromobutane undergoes dehydrohalogenation by an elimination reaction when heated in the presence of base?
Answers
When 1-bromobutane undergoes dehydrohalogenation by an elimination reaction when heated in the presence of base, the organic product formed is 1- butene.
Dehydrohalogenation is defined as a reaction in which a hydrogen atom and a halogen atom are removed from adjacent atoms in a molecule forming an alkene or an alkyne. When this is reacted with strong base such as hydroxide ion, cyclohexyl chloride suffers dehydrohalogenation by a concerted E2 reaction mechanism. An elimination reaction is defined as a type of organic reaction in which two substituents are removed from a molecule in either a one or two step mechanism. The one step mechanism involved is known as the E2 reaction and the two-step mechanism is known as the E1 reaction. When 1-bromobutane undergoes dehydrohalogenation in the presence of a base it undergo elimination reaction.
To learn more about Dehydrohalogenation
https://brainly.com/question/13947468
#SPJ4
The correct question is,
1-bromobutane undergoes dehydrohalogenation by an elimination reaction when heated in the presence of base. what is the identity of the organic product?
Which object absorbs the most visible light?a. a clear bowlb. a white rockc. a black t-shirtd. a red apple
Answers
A black t-shirt absorbs the most visible light. This is because black is a color that absorbs all visible light, which is why it appears to be a dark color.
Correct option is A.
When light hits a black t-shirt, the material absorbs the photons, which is why it looks dark and doesn't reflect light like a white t-shirt or a white rock would. When visible light enters a black t-shirt, it is absorbed by the molecules in the material, and is converted into heat. This is why a black t-shirt can feel warmer than other colored t-shirts.
A clear bowl, a white rock and a red apple all reflect visible light, so they don't absorb as much as a black t-shirt. All these objects are able to absorb some visible light, but a black t-shirt absorbs the most. A black t-shirt absorbs more visible light than any other object because it is a darker color, and darker colors absorb more light.
Correct option is A.
know more about photons here
https://brainly.com/question/20912241#
#SPJ11
A compound with chemical formula na2cx3 has formula mass 106 amu .. what is the atomic mass of element x
Answers
The element "X" is "O" (oxygen).
Calculation:Given,
Chemical formula = Na₂CX₃
Formula mass = 106 amu
Molar mass of Na = 23 amu
Molar mass of C = 12 amu
To find,
Element X =?
We will equate the equation as follows,
2(23) + 12 + 3(y) = 106
46 + 12 + 3y =106
58 + 3y = 106
3y = 106 - 58
3y = 48
y = 48/3
y = 16
We know that Oxygen has molecular mass of 16. Therefore the element "X" is "O".
Learn more about molar mass here:
https://brainly.com/question/22997914
#SPJ4
What is the smallest unit of cellular organization?
Answers
Answer:
A cell
Explanation:
Patience, a popular card game in Mendeleev's time, is also known as Go Fish.
A. True
B. False
Answers
Answer:
B. False
Explanation:
step by step explanation
Answer:
False
Explanation:
Seleccione una actividad humana y el impacto generado debe realizar un resumen sobre la actividad seleccionada y dos medidas de prevención para evitar la alteración de los ciclos.
Answers
Answer:
Industrial activity increases the emission of greenhouse gases in the atmosphere, especially carbon dioxide (CO2), and it negatively affects climate by increasing global warming.
Two actions to face the global warming:
1- To substitute the use of fossil fuels by alternative clean energy sources such as, for example, hydroelectric, geothermal, solar and wind energy resources.
2- The government's policies decided to develop electric cars and to stimulate healthy habits such as walking instead of the use of conventional fossil-fueled transport modes.
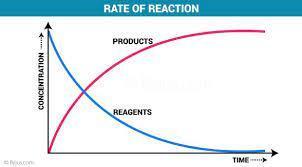
Click on the graph to show how rate of reaction is usually related to activation energy
Which one




Answers
Answer: Graph 3
Explanation:
Activation energy is the level of energy required by a chemical reaction for the reaction to proceed.
A higher Activation energy will translate to a lower reaction rate because less molecules will be able to achieve this required energy needed for them to react. A lower activation energy however will enable a faster reaction time because more molecules will achieve the required energy.
Higher Activation energy = Lower Reaction rate
Lower Activation energy = Higher reaction rate
This is what Graph 3 shows so it is correct.

How many moles of carbon atoms are there in 0.500 mole of C2H6?
a. 6.02x 10^23 moles
b. 3.00 moles
c.1.00 mole
d. 0.500 mole
Answers
The moles of carbon atoms are there in 0.500 mole of C₂H₆ is:
c. 1.00 mole
To determine the number of moles of carbon atoms, we need to use the mole ratio between C₂H₆ and carbon atoms. The mole ratio is based on the balanced chemical equation, which shows the relationship between the number of moles of different elements or compounds.
In this case, the mole ratio between C₂H₆ and carbon atoms is 1:2. This means that for every 1 mole of C₂H₆, there are 2 moles of carbon atoms.
Using this mole ratio, we can set up a conversion factor to calculate the moles of carbon atoms:
0.500 mole C₂H₆ * (2 moles C / 1 mole C₂H₆)
By multiplying the given quantity (0.500 mole C₂H₆) by the conversion factor, we cancel out the mole unit for C₂H₆ and end up with the moles of carbon atoms.
Calculating this expression gives us:
0.500 mole C₂H₆ * (2 moles C / 1 mole C₂H₆) = 1.00 mole C
Therefore, there are 1.00 mole of carbon atoms in 0.500 mole of C₂H₆.
To know more about carbon atoms here
https://brainly.com/question/13990654
#SPJ4
State the method used to separate;
1. Water from aqueous magnesium sulfate
2. Potassium chloride from aqueous potassium chloride
3. Silver chloride from a mixture of silver chloride and Water
4. Glucose from a mixture of Glucose and maltose
Options: filtration, diffusion, fractional distillation, crystallisation, chromatography. (each may be used once, multiple times or not at all)
Answers
The methods used to separate:
What is Separation in Chemistry?
In chemistry, separation refers to the process of separating the constituents of a mixture into distinct entities.
This can be accomplished by the use of several techniques such as filtering, crystallization, distillation, and chromatography, which are selected based on the qualities of the substances being separated and the required level of purity.
Separation is an essential feature of chemical analysis that is utilized in a variety of applications such as purifying substances, identifying and measuring the components of a mixture, and preparing samples for future investigation.
Learn more about Separation:
https://brainly.com/question/13619907
#SPJ1
Vincent graduated from college with a degree in horticulture and now owns a local plant nursery. He maintains a blog titled, "How to Live a Happy Life,” and writes that people can lead happier lives by varying the routes they usually take home from work. Which statement best evaluates this claim? This claim is scientific because it is based on Vincent’s personal experience. This claim is scientific because other people have taken his advice and are now happier. This claim may not be accurate because the blog is produced by someone who is not an expert in the field of human psychology. This claim may not be accurate because most people follow the same route home.
Answers
Answer:
this claim may not be accurate because the blog is produced by someone who is not an expert in the field of human psychology.
Explanation:
Answer:
this claim may not be accurate because the blog is produced by someone who is not an expert in the field of human psychology.
Explanation: give the branliest to the otherperson :)
the henry's law constant for h2 is 8.1×10−4 matm at 25∘c. what pressure of hydrogen is needed to maintain a h2 concentration of 0.42 m?\
Answers
518.5 atm pressure of hydrogen is needed to maintain a \(H_2\) concentration of 0.42 m
The given Henry's law constant for \(H_2\) is 8.1 × 10^-4 M atm^-1 at 25°C. To find the pressure of hydrogen needed to maintain a \(H_2\) concentration of 0.42 M, we can use Henry's law.
The equation for Henry's law is:
C = kH*P
where C is the concentration of gas in moles per liter, P is the partial pressure of the gas in atmospheres, and kH is Henry's law constant in M/atm.
Plugging the values in Henry's law equation, we get:
0.42 = (8.1 × 10^-4)P
Dividing both sides by (8.1 × 10^-4), we get:
P = (0.42)/(8.1 × 10^-4)
P = 518.5 atm
Hence, the pressure of hydrogen needed to maintain a \(H_2\) concentration of 0.42 M is 518.5 atm.
Learn more about Henery's law here:
https://brainly.com/question/14908970
#SPJ11
UNA ONDA SE MUEVE CON UNA FRECUENCIA DE 2 (HZ), Y SU RAPIDEZ DE PROPAGACIÓN ES DE 20 (m/s). ¿Cuál ES LA LONGITUD DE ONDA.
Answers
I need some help answering this question for my chemistry homework.

Answers
The mini marshmallows would extend to a height of 1.843 x 10 km above the surface of Colorado if they covered the state without any space between them.
What will be the height of an Avogadro number of Marshmallows?To find the height of an Avogadro number of Marshmallows, first, we need to find the total volume of all the mini marshmallows.
Each mini marshmallow has a diameter of 0.635 cm and a height of 2.54 cm, so its volume can be calculated as:
V = πr²h
Volume = π * (0.3175 cm) * (2.54 cm)
Volume = 0.8217 cm³
Avogadro's number is 6.02 x 10²³, so the total volume of all the mini marshmallows can be calculated as:
Total volume = 6.02 x 10²³ * 0.8217 cm³
Total volume = 4.947 x 10³ cm³
Now we can use the land area of Colorado to find the height to which the mini marshmallows would extend if packed together without any space between them.
1.037 x 10⁵ mi² is equal to (1.037 x 10⁵ mi² * 2.59 x 10⁶ = 2.685 x 10¹¹ m²
If the mini marshmallows cover this area without any space between them, their height can be calculated as:
Height = total volume/area
Height = (4.947 * 10²³ cm³) / (2.685 x 10¹¹ cm²)
Height = 1.843 x 10² cm
Converting to kilometers, we get:
Height = (1.843 x 10¹ ²cm) / (100,000 cm/km)
Height = 1.843 x 10⁷ km
Learn more about Avogadro's number at: https://brainly.com/question/14138110
#SPJ1
which statement correctly describes the relationship between intermolecular forces and the vapor pressure of a substance? multiple choice question. a substance with weak intermolecular forces vaporizes more easily and therefore has a low vapor pressure. a substance with strong intermolecular forces will have a high vapor pressure because of the strong interaction between its molecules. a substance with weaker intermolecular forces vaporizes more easily and has a high vapor pressure. molecules with strong intermolecular forces interact more strongly with the atmosphere, resulting in a high vapor pressure.
Answers
A substance with weak intermolecular forces vaporizes more easily and therefore has a low vapor pressure. (option 1)
The strength of intermolecular forces between molecules determines how tightly they are held together in the liquid state. In general, substances with weaker intermolecular forces require less energy to break the bonds holding them together and vaporize more easily. As a result, they have a higher tendency to escape into the gas phase and have a higher vapor pressure.
On the other hand, substances with stronger intermolecular forces require more energy to overcome the forces holding them together and have a lower tendency to vaporize. Therefore, they have a lower vapor pressure. So, the correct statement is "A substance with weak intermolecular forces vaporizes more easily and therefore has a low vapor pressure."
Learn more about Intermolecular Forces:
https://brainly.com/question/2193457
#SPJ4
Complete Question:
which statement correctly describes the relationship between intermolecular forces and the vapor pressure of a substance? multiple choice question.
a substance with weak intermolecular forces vaporizes more easily and therefore has a low vapor pressure. a substance with strong intermolecular forces will have a high vapor pressure because of the strong interaction between its molecules. a substance with weaker intermolecular forces vaporizes more easily and has a high vapor pressure. molecules with strong intermolecular forces interact more strongly with the atmosphere, resulting in a high vapor pressure.how many moles of sodium hydroxide are present in 50.00 ml of 0.09899 m naoh?
Answers
There are approximately 0.00495 moles of sodium hydroxide present in the 50.00 mL solution.
To find the moles of sodium hydroxide (NaOH) in a 50.00 mL solution with a concentration of 0.09899 M, you can use the formula:
moles = volume (L) × concentration (M)
First, convert the volume from mL to L:
50.00 mL = 0.05000 L
Now, multiply the volume in liters by the concentration:
moles = 0.05000 L × 0.09899 M
moles ≈ 0.00495 mol
Therefore, there are approximately 0.00495 moles of sodium hydroxide present in the 50.00 mL solution.
To learn more about volume, refer below:
https://brainly.com/question/1578538
#SPJ11
Which Hazardous Material class includes compressed gases, dissolved gases, and gases liquefied by compression or refrigeration?
Answers
The Hazardous Material class that includes compressed gases, dissolved gases, and gases liquefied by compression or refrigeration is Class 2: Gases. This class is further divided into three divisions:
1. Division 2.1 - Flammable Gases: These are gases that can burn in the presence of an ignition source. Examples include propane, butane, and hydrogen.
2. Division 2.2 - Non-Flammable, Non-Toxic Gases: These are gases that do not burn and are not toxic, but may still pose risks due to their physical properties, such as high pressure or low temperature. Examples include nitrogen, helium, and carbon dioxide.
3. Division 2.3 - Toxic Gases: These are gases that are harmful or even fatal when inhaled. Examples include chlorine, ammonia, and phosgene.
The proper handling, storage, and transportation of these gases are essential to minimize the risks associated with their hazardous properties. Regulations and guidelines are in place to ensure the safety of those working with and around these materials.
for more information on Hazardous Materials: https://brainly.com/question/29738119
#SPJ11
HELP ASAP PLEASE CHEMISTRY
The rate of reaction was measured during a chemical reaction. After the first 3 seconds, the rate of reaction was 1.8 x10−6 M/s. Which of the following would you expect after another 3 seconds?
The rate would be lower, and the concentration of products would be higher.
The rate would be lower, and the concentration of products would be lower.
The rate would be higher, and the concentration of products would be higher.
The rate would be higher, and the concentration of products would be lower.
Answers
Answer:
The rate would be higher, and the concentration of products would be lower.
Explanation:
The correct answer is The rate would be lower, and the concentration of products would be higher.
The rate of reaction refers to the speed with which reactants are converted into products.
The rate of reaction decreases as the reaction progresses because more reactants are being converted into products.
As such, the reactant concentration decreases and the concentration of products increases.
Hence, after three seconds, the rate would be lower, and the concentration of products would be higher.
https://brainly.com/question/23761331
How many grams of iron (III) oxide can be produced from 2.50 g of oxygen reacting with iron, according to the
following equation?
4 Fe (s) + 3 02 (g) -->2 Fe₂O3(s)
Answers
3 moles of oxygen gives 2 moles of the product. Then, 2.50 g or 0.07 moles of oxygen gas will give, 0.04 moles or 14.8 g of Fe₂O₃.
What is Fe₂O₃ ?Metals are easily reactive towards atmospheric oxygen and they form their oxides. Fe reacts with oxygen to form one of its oxide in the + 3 oxidation state that is Fe₂O₃.
Here, 3 moles of oxygen gives 2 moles of the oxide.
molar mass of oxygen gas = 32 g/mol
no.of moles in 2.5 g = 2.5 /32 = 0.07 moles.
0.07 moles produce, 0.07 × 2 /3 = 0.04 moles.
molar mass of Fe₂O₃ = 319.2 g.
then, mass of 0.04 moles = 0.04 × 319.2 = 14.8 g.
Therefore, 2.5 g of oxygen gas will give 14.8 g of the product.
Find more on Fe₂O₃:
https://brainly.com/question/24236942
#SPJ1
Suppose you analyze a 35.1 g sample of bleach and determine that there are 2.21 g of sodium hypochlorite present. What is the percent of sodium hypochlorite in the bleach sample
Answers
The percent of sodium hypochlorite in the bleach sample is 6.29%. Bleach typically contains a solution of sodium hypochlorite, which is a compound that has strong oxidizing properties.
What is Bleach?
Bleach is a chemical solution that is used as a whitening or cleaning agent. It is commonly used to remove stains, whiten fabrics, and disinfect surfaces in households, commercial establishments, and industrial settings.
To calculate the percent of sodium hypochlorite in the bleach sample, we need to use the formula:
percent of sodium hypochlorite = (mass of sodium hypochlorite / mass of bleach sample) x 100%
Using the given values, we can substitute:
percent of sodium hypochlorite = (2.21 g / 35.1 g) x 100%
Simplifying this expression gives:
percent of sodium hypochlorite = 6.29%
Therefore, the percent of sodium hypochlorite in the bleach sample is 6.29%.
To know more about Bleach, visit;
https://brainly.com/question/30587051
#SPJ4
Which of the following elements has the lowest electronegativity?
A. Barium
B. Magnesium
C. Strontium
D. Calcium
Answers
Answer:
A. Barium
Explanation:
hope this helps! :)
The first scale of electronegativity was developed by Linus Pauling and on his scale barium has a value of 0.89 on a scale running from from about 0.7 (an estimate for francium) to 2.20 (for hydrogen) to 3.98 (fluorine).
Use the equation below to solve the problem that follows.
2H2 (g) + O2 (g) → 2H2O (g)
When David reacts 13.8 grams of hydrogen gas with excess oxygen, 87.0 grams of water are formed. Calculate his percent yield of water.
Answers
Answer: 142.8%
Explanation:
13.8g H2 * (1 mol H2 / 2 g H2) * (2 mol H2O/ 2 mol H2) * (18 g H2O/1 mol H2O) = 124.2 g H2O
we just calculated our theoretical amount that should be formed and we are given the actual
percent yield: actual/theo * 100% = 124.2/87 * 100% = 142.8%
How many grams of hydrogen gas are produced if 3.0 moles of magnesium are reacted with excess hydrochloric acid?
Answers
Answer k
Explanation: better luck
Calculate the number of moles of 2.00g of K2SO4
Answers
Which three things does a plant need to create a glucose molecule in photosynthesis?
Choose all correct answers.
water
oxygen
carbon dioxide
sunlight
Answers
Lauren has a big social studies test coming up. She knows that she needs to spread out her study sessions over a period of time. She develops a schedule in which each session is divided up evenly among her available days. What strategy is Lauren is using? rewarding pacing cramming getting help.
Answers
The study strategy Lauren is using in spreading her study sessions over a period of time is pacing, which helps the student develop a schedule focused on their own study pace.
Pacing Study SessionsThis study strategy of distributing the study into short sessions rather than studying the entire content through one long session is more effective in retaining content and learning.
What happens is that Lauren is using mass repetition processing, which can be compared to a longitudinal wave in physics, with spaces in between, concentrating the initial review close to the proof to ensure retention and avoid forgetting.
Through pacing, Lauren achieves greater motivation to carry out her studies in a concentrated and focused way, helping her to retain and preserve knowledge.
The correct answer is:
PacingFind out more information about pacing here:
https://brainly.com/question/988371
Answer: what there trying to say is c pacing
Explanation:
White-tailed deer are mammals. Mammals have a reproductive strategy
that is not found in all animals.
Which two statements provide a cause and effect of the reproductive
strategy of white-tailed deer that helps them survive?
A. It requires two parents.
B. It requires only one parent.
C. It causes very fast growth and development.
D. It produces genetic variation within a population.
E. It results in individuals being identical to one parent.

Answers
This lack of genetic variation can lead to a decrease in the population’s ability to survive and adapt to changing environments, resulting in a population that is more at risk of extinction.
Which two statements provide a cause and effect of the reproductive strategy of white-tailed deer that helps them survive?A. It requires two parents. - CauseD. It produces genetic variation within a population. - EffectThe reproductive strategy of white-tailed deer helps them survive because it requires two parents.This helps ensure that the young deer receive the care and protection of both parents, which increases their chance of survival.Additionally, the two-parent strategy of the deer produces genetic variation within a population.This variation allows for natural selection to take place and for the population to adapt to changing environments. As a result, members of the population are more likely to survive and reproduce, creating a more resilient population.In contrast, a one-parent strategy, such as parthenogenesis, produces individuals that are identical to one parent.This lack of genetic variation can lead to a decrease in the population’s ability to survive and adapt to changing environments, resulting in a population that is more at risk of extinction.To learn more about The reproductive strategy refer to:
https://brainly.com/question/10413000
#SPJ1
Earth materials that are soluble in water or react to a weak acid commonly have what type of appearance?.