Outline the best method for preparing the following aldehyde from an appropriate alcohol in one step. Draw the starting alcohol and select the best reagent.
The structure is a 6 carbon ring where carbon 1 is bonded to an aldehyde
Answers
To prepare the desired aldehyde with a 6-carbon ring and an aldehyde group on carbon 1, starting with cyclohexanol is a suitable approach.
Cyclohexanol is a 6-carbon ring compound with an alcohol group (OH) attached to carbon 1. To convert the alcohol group into an aldehyde group, the oxidation of the primary alcohol is required.
In this case, the best reagent to use for the oxidation of cyclohexanol to the corresponding aldehyde is PCC (pyridinium chlorochromate).
PCC is a mild oxidizing agent that selectively oxidizes primary alcohols to aldehydes without further oxidation to carboxylic acids. It allows for a controlled oxidation, preventing overoxidation of the aldehyde to a carboxylic acid.
The reaction using PCC as the oxidizing agent can be carried out in one step. The PCC reagent is typically dissolved in a suitable solvent, and the cyclohexanol is added to the reaction mixture.
The reaction proceeds, converting the alcohol group to an aldehyde group while maintaining the 6-carbon ring structure.
By using cyclohexanol as the starting alcohol and PCC as the reagent, you can achieve the desired aldehyde product with a 6-carbon ring and an aldehyde group on carbon 1 in a single step.
This method provides a reliable and efficient way to selectively oxidize the primary alcohol to the corresponding aldehyde without the risk of overoxidation.
To learn more about oxidation, refer below:
https://brainly.com/question/16976470
#SPJ11
Related Questions
question shown in picture tysm!!!

Answers
Answer:
the answer is the second one.
A. potassium carbonate
formula
Answers
Answer:
The formula for Potassium Carbonate is K2CO3
Examine the models shown above.where is potential energy the greatest
Answers
Answer:
A
Explanation:
Because it is the greatest
True or false??? (Hydrogen bonds are the attraction between the partially positive regions of oxygen with the partially positive regions of oxygen on different molecules.)
Answers
Answer:
true
Explanation:
A type of ion that is needed in our bodies and explain briefly what it does.
Answers
Answer:
Body fluid has electrolytes, they are chemicals that dissolve in water and then produce charged ions.
Electrolytes play an important role in our body and there are many of them.
The main electrolytes include chloride, potassium, calcium, sodium, and magnesium.
For example Sodium ions (Na+)
Function in our body: regulate osmotic pressure and the content of water of our body, transmit nerve signals, contract muscle, etc.
That is one type of ion.
Scientist are studying photosynthesisIn in a forest ecosystem that has plants animals and decomposers
Answers
Answer:
Ecosystem is food web of primary and secondary producers and consumers.
Explanation:
In an ecosystem, there exist primary producers, primary consumers, secondary consumers, and tertiary consumers such as detritivorous such as viruses and bacteria that act on the living bodies and recycle the nutrients back to plants through the soil. The primary producers such as all green plants and others make their own food being autotrophs they use sunlight and carry out photosynthesis process. On the depend on the herbivorous they eat grass etc. The secondary consumers are carnivores as heterotrophs that feed on herbivores.both sulfur and galena are softer than glass. using just the minerals, how can you determine which of the two minerals is harder?
Answers
The hardness of a mineral is typically measured using Mohs hardness scale, which assigns a numerical value to the ability of a mineral to scratch another mineral. According to the Mohs hardness scale, glass has a hardness of 5.5. Therefore, if both sulfur and galena are softer than glass, it means that their hardness falls below 5.5 on the Mohs hardness scale.
The determine which of the two minerals is harder, we can conduct a simple scratch test. If we take a piece of galena and a piece of sulfur and try to scratch each with a nail, we will notice that galena is harder and cannot be scratched easily. This is because galena has a higher Mohs hardness value than sulfur. Galena has a Mohs hardness of 2.5-2.75, while sulfur has a Mohs hardness of 1.5-2.5. This means that galena is harder than sulfur and can scratch it easily. Therefore, if we use the scratch test method, we can easily determine that galena is harder than sulfur. In conclusion, the hardness of a mineral is an important characteristic that can be determined using the Mohs hardness scale or simple scratch tests. In this case, we can determine that galena is harder than sulfur by conducting a scratch test using a nail. Both sulfur and galena have their unique properties and importance, but their hardness can be determined easily.
learn more about mineral here.
https://brainly.com/question/30472865
#SPJ11
Gallium is a metallic element in Group III. It has similar properties to aluminium.
(a) (i) Describe the structure and bonding in a metallic element.
Answers
Metallic elements exist in a solid-state and they are opaque, have a shiny surface, good conductors of electricity and heat, malleable and ductile, and are dense. The structure of metals is formed by atoms that are held together by metallic bonds. These atoms have loosely bound valence electrons that can be shared between the neighboring atoms.
Therefore, the outermost shells of these atoms are incomplete due to the sharing of valence electrons, forming a lattice structure known as a metallic bond.Metallic elements have a unique crystal structure that occurs in two forms. The most common type of metal crystal structure is the body-centered cubic structure where the atoms are arranged in a cube with one atom located at the center of the cube. The other type of metal crystal structure is the face-centered cubic structure, where each corner of the cube is an atom and there is an additional atom at the center of each face of the cube .Metallic bonding occurs due to the delocalized electrons that exist in the metal structure. The valence electrons from each atom are free to move throughout the entire metal lattice. Therefore, these electrons form a "sea of electrons" that is shared by all the atoms in the lattice. This results in the metal structure having high thermal and electrical conductivity.Metals are known for their ductility and malleability properties. These properties are due to the metallic bonding that exists in the metal structure. Since the valence electrons are shared, they can easily move past one another, allowing the metal to be hammered into different shapes without breaking.The properties of metals vary depending on their structure and bonding. Gallium, being a metallic element in Group III, has similar properties to aluminum. Therefore, it has a similar metallic bond structure with delocalized electrons that provide the metal with its unique properties.For such more question on valence electrons
https://brainly.com/question/371590
#SPJ8
A hot air balloon is an example of what type of system? *
Answers
Answer:
The balloon transport system or Hot Air Balloon is one of several transportation methods in RuneScape. During the Enlightened Journey quest it can be used for travel between two locations. After the quest is complete, four more locations are available to be unlocked by completing respective journeys to those locations.
Explanation:
transportation
Which elements are;
a)solids,
b)liquids
c)gases
at 25°C
Answers
The mineral lead leaves a streak on a piece of pottery, but the mineral topaz does not. Which mineral has a greater hardness? answers; They have the same hardness, lead, topaz
Answers
Answer:
The answer would be topaz
What is the Lewis structure N3 negative?
Answers
Each nitrogen atom has one lone pair of electrons, and each nitrogen atom is connected to the other two nitrogen atoms by a single bond.
The Lewis structure for N3 negative - is:
N
|
N - N - N
|
N
To draw the Lewis structure for N3-, we need to follow these steps:
Calculate the total number of valence electrons present in N3-. Nitrogen (N) is in Group 15 of the periodic table, so it has 5 valence electrons. The negative charge means that there is an extra electron, giving us a total of 3 × 6 + 1 = 19 valence electrons.
Determine the central atom. In this case, there are three nitrogen atoms in N3-. Since they are all the same, it doesn't matter which one we choose as the central atom. We'll choose the nitrogen in the middle.
Draw a skeletal structure. Connect the three nitrogen atoms with single bonds to form a linear molecule.
Add electrons to the structure. Place lone pairs around each nitrogen atom to fill their octets. Since we have 19 valence electrons, we'll have to use a lone pair on one of the nitrogen atoms.
Check the formal charges. Each nitrogen atom has 4 valence electrons in the structure, so they all have a formal charge of -1. This gives us a total charge of -3, which matches the charge on the N3- ion.
Learn more about Lewis structure here:
https://brainly.com/question/20300458
#SPJ4
20
Which of the following is an unbalanced equation?*
(1 Point)
a. CaCO3 --> CaO + CO2
O b. 2PbO 2 + 2H 2 -->2Pb +2H2O
O c. 2Na+ 2H 2 O-->2NASH + H 2
d. CS 2 + 20 2 --> CO 2 + 250 2
Answers
Answer:
c2Na+2H 2 0 -2NASH +H 2
Explanation:
i think its wrong
What is the new concentration of a solution of CaSO3 if 10.0 mL of a 2.0 M CaSO3 solution is diluted to 100 ml?
Answers
Answer: The new concentration of a solution of \(CaSO_{3}\) is 0.2 M 10.0 mL of a 2.0 M \(CaSO_{3}\) solution is diluted to 100 mL.
Explanation:
Given: \(V_{1}\) = 10.0 mL, \(M_{1}\) = 2.0 M
\(V_{2}\) = 100 mL, \(M_{2}\) = ?
Formula used to calculate the new concentration is as follows.
\(M_{1}V_{1} = M_{2}V_{2}\)
Substitute the values into above formula as follows.
\(M_{1}V_{1} = M_{2}V_{2}\\10.0 mL \times 2.0 M = M_{2} \times 100 mL\\M_{2} = 0.2 M\)
Thus, we can conclude that the new concentration of a solution of \(CaSO_{3}\) is 0.2 M 10.0 mL of a 2.0 M \(CaSO_{3}\) solution is diluted to 100 mL.
if 6 moles of a a compound produce 84 J of energy, what is the h reaction in j/mol
Answers
The enthalpy of the reaction is 14 J/mol.
The enthalpy of a reaction (ΔH) is the amount of energy transferred between a system and its surroundings during a chemical reaction at constant pressure, measured in joules per mole (J/mol). This value is important because it can tell us whether a reaction is exothermic or endothermic, as well as give us information about the strength of chemical bonds within the reactants and products.To calculate the enthalpy of a reaction, we need to know the amount of energy released or absorbed (Q) and the number of moles of the compound involved in the reaction (n). We can use the equation:
ΔH = Q/n
Given that 6 moles of a compound produce 84 J of energy, we can calculate the enthalpy of the reaction as follows:
ΔH = Q/n
ΔH = 84 J / 6 mol
ΔH = 14 J/mol
This means that for every mole of the compound involved in the reaction, 14 J of energy is transferred between the system and the surroundings. Since the value is positive, we can conclude that the reaction is endothermic, meaning that it requires an input of energy to occur.It is worth noting that the enthalpy of a reaction can depend on a number of factors, such as temperature, pressure, and the specific conditions under which the reaction occurs. As such, it is important to take these factors into account when calculating or predicting enthalpy values.
for such more questions on enthalpy
https://brainly.com/question/14047927
#SPJ8
pls answer question will mark brainliset tyty

Answers
The option (1) water supply i.e. the biotic factor that affect the human most.
Competition is a biotic element that prevents population increase in an aquatic habitat since it involves living things.Competition is an interaction between individuals or species where the presence of another reduces the fitness of one. A factor may be the limited availability of at least one resource (such as food, water, and territory) used by both.Abiotic elements including as depth, sunlight, nutrients, and oxygen availability have an impact on population increase in aquatic ecosystems.An ecosystem's overall health is also influenced by biotic factors such as the diversity of consumers and the presence of autotrophs, or self-sustaining organisms like plants. Abiotic variables have an impact on an organism's capacity for survival and reproduction. Abiotic barriers prevent the formation of population .
To know more about biotic factor
https://brainly.com/question/27430655
#SPJ1
i need help asap. which are the products?

Answers
Answer:
A
Explanation:
The products of a chemical equation is what is being experimented with. However, the results in which you get are known as your solution.
i will give the brainliest for who answer this questio but no guessing
Write the common names of group IVA, VIIA and VIII A _____________________ ,
____________________ , _____________________
Answers
Answer:
IVA: nonmetal/other metals VIIA:halogens VIII: Nobel Gases
Explanation:makes sense
Classifying Characteristics of Rocks

Answers
Answer:
The texture of a rock is a result of its mineral composition
Most rocks contain more than one type of sediment
Explanation:
If 30.0 grams of calcium metal react with 18.0 grams of oxygen gas, your calculations show that ________ grams of CaO could be produced from the 30.0 grams of calcium and __________ grams of CaO could be produced from the 18.0 grams of oxygen. The theoretical yield of calcium oxide from this reaction is ________.
Answers
Answer:
42g if CaO can be produced from 30g and 31.5g of CaO will be produced from 18g of O
Explanation:
40g of CA =56g
30g =×
cross multiple
=56*30/40
=42g
32g of O =56g
18g =×
cross multiple
=56*18/32
=31.5g
theoretical yield
42-31.5= 10.5
just guessing
Use the equation below to answer the following question. How many grams of potassium chloride (KCl) are produced if 25 g of potassium chlorate (KClO3) decompose?
KClO3 → 2KCl + 3O2
10. g KCl
15 g KCl
16 g KCl
21 g KCl
Answers
The mass of KCl produced is 30.37 g. KClO3 → 2KCl + 3O2We have 25 grams of KClO3. We need to find the number of grams of KCl produced after the decomposition of 25 grams of KClO3.
We can find the molar mass of KClO3. K has a molar mass of 39.10 g/mol, Cl has a molar mass of 35.45 g/mol, and O has a molar mass of 16.00 g/mol.Molar mass of KClO3 = 39.10 + 35.45 + (3 × 16.00) = 122.55 g/molNow, we can find the number of moles of KClO3:25 g of KClO3 ÷ 122.55 g/mol = 0.2037 mol
We can see from the balanced chemical equation that the stoichiometric coefficient of KCl is 2. This means that 2 moles of KCl is produced for every 1 mole of KClO3.So, the number of moles of KCl produced = 2 × 0.2037 = 0.4074 mol Finally, we can find the mass of KCl produced:Mass of KCl = Number of moles of KCl × Molar mass of KCl= 0.4074 mol × 74.55 g/mol = 30.37 g.
To know more about decomposition visit:-
https://brainly.com/question/14843689
#SPJ11
When a car driver puts their foot on the brake pedal, the brake fluid is pushed down a narrow tube connected to the brake. Since brake fluid is hard to squash, the brakes are put on immediately. What is the scientific term for squash?
Answers
When a car driver puts their foot on the brake pedal, the brake fluid is pushed down a narrow tube connected to the brake. Since brake fluid is hard to squash, the brakes are put on immediately the scientific term for squash is compressibility.
What is compressibility?The scientific term for squash is "compressibility". In the case of brake fluid, it has very low compressibility, which means that it cannot be easily compressed or squashed, resulting in immediate and effective brake application when pressure is applied to the brake pedal.
The term "compressibility" describes how much a particular volume of matter shrinks under pressure. A solid or a liquid under pressure essentially maintains its volume. The solid or liquid's constituent atoms, ions, or molecules are in close proximity to one another.
Learn more about driver at:
https://brainly.com/question/30774200
#SPJ1
balancing chemical equations

Answers
2HgO—>2Hg+O2
Given the translation (0,5), translate ordered pairs (9, 0) and (2,-4).
Answers
Answer:
(9,5) and (2,1)
Explanation:
The atomic number and mass number for calcium 39 are 20 and 39. how many protons are in one atom?
Answers
Number of protons n one atom of Calcium 39 is 20.
Number of protons in any element is equal to its atomic number. Since the atomic number of calcium is 20, hence, the number of protons is also 20. To balance the positive charge, the atoms contain same number of electrons. Thus, number of electrons in given atom of calcium will also be 20.
The number of neutrons is calculated by performing subtraction between mass number and number of protons. The number of neutrons is indicative of the isotope of atom, as it changes with the mass of an atom.
Calcium is an alkaline earth metal. The smallest unit of matter is atom which can occur either in neutral state or in ionized state.
Learn more about calculation of number of protons, neutrons and electrons-
https://brainly.com/question/9177915
#SPJ4
Mass = 35g
Density = 5 g/cm3
∙What is the Volume?
Answers
Answer:
7cm3
.........
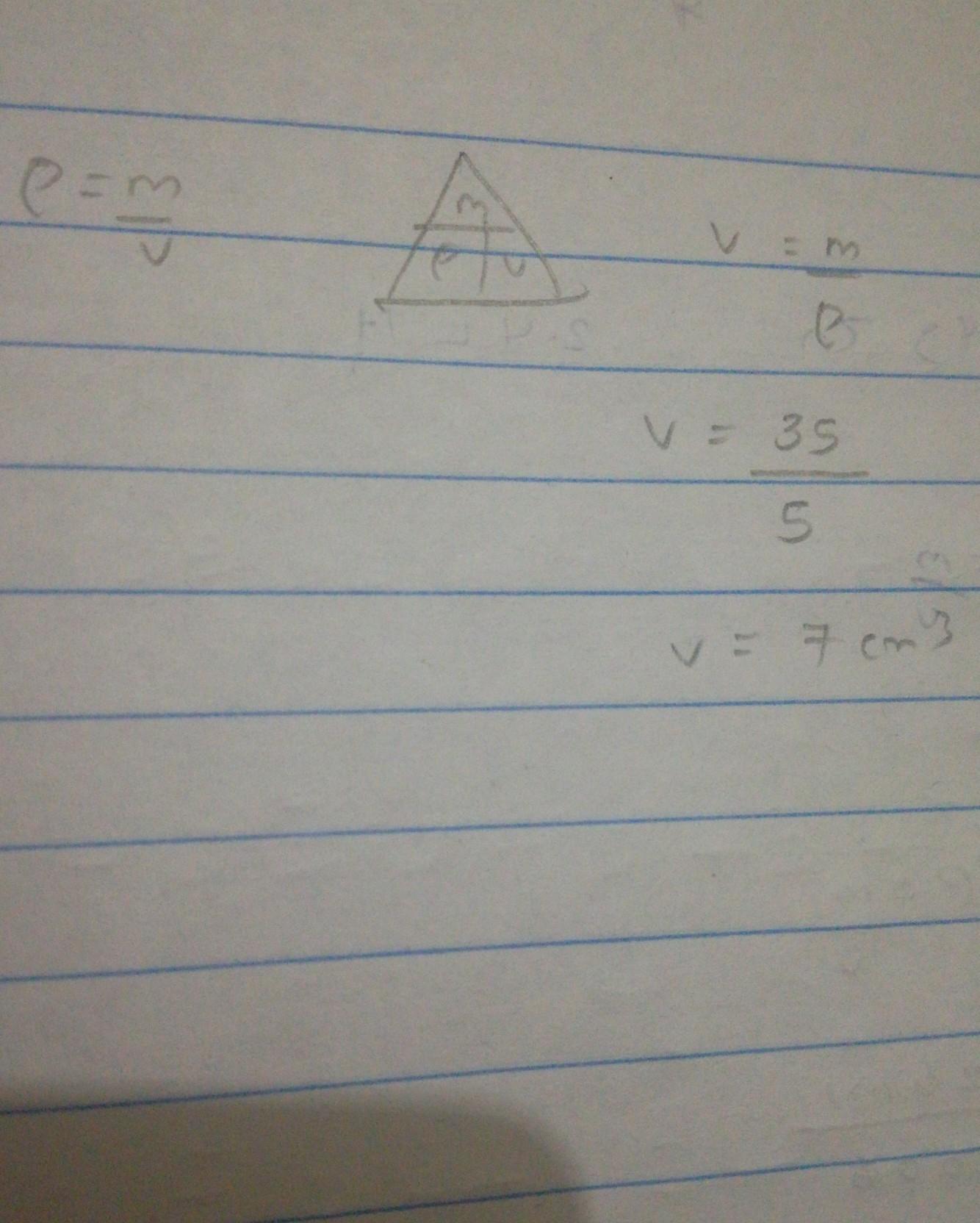
Which characteristics would be present in an air mass that develops over the Caribbean Sea near the equator?

Answers
answer: warm and wet
In an isolated system, the total heat given off by warmer substances equals the total heat energy gained by cooler substances (qhot water = -qcold liquid). which process in your activity was endothermic
Answers
In an isolated system, the total heat given off by warmer substances equals the total heat energy gained by cooler substances. The process that gained energy was endothermic.
Chemical reactions are said to be endothermic if the reactants take in heat energy from their environment in order to generate products. These reactions bring a reduction in the temperature of the space around them, which results in a cooling effect. Endothermic reactions can also occur in the context of physical processes; for example, ice cubes can melt into liquid water by absorbing heat energy from their surroundings. In simple the process that absorbs heat is endothermic.
You can also learn about Endothermic reactions from the following question:
https://brainly.com/question/23184814
#SPJ4
the most unique property of carbon is its ability to group of answer choices bond to carbon. bond to nitrogen. bond to oxygen. form four bonds.
Answers
the most unique property of carbon is its ability to form four bonds.
Carbon is unique in its ability to form four covalent bonds with other atoms, including other carbon atoms. This ability allows for the formation of long chains, rings, and complex structures, making carbon the basis for all life on Earth. Carbon can also form double or triple bonds, allowing for even more diversity in the types of molecules that can be created. While nitrogen and oxygen can also form multiple bonds, carbon's ability to form four bonds is what sets it apart.
Carbon has four valence electrons, which allows it to form four covalent bonds with other atoms. This property enables carbon to create a wide variety of complex molecules, including forming long chains and rings by bonding to other carbon atoms. This versatility is crucial for the formation of various organic compounds and is a key reason why carbon is the backbone of life on Earth.
To know more about unique property visit:
https://brainly.com/question/30296004
#SPJ11
Find the amount of CO2 obtained
a. from the burning of a mole of carbon
b. of one kilomol of carbon
Answers
Answer:
See explanation
Explanation:
The equation of the reaction is;
C (s) + O2(g) ------> CO2(g)
a) From the equation we can see that it is a 1:1 reaction hence, 1 mole of carbon yields 1 mole of CO2
b) Similarly, 1 * 10^3 moles of Carbon also yields 1 * 10^3 moles of CO2.