One of the uses of methanol (CH3OH) in dilute form is as a windshield washer antifreeze. In pure form, methanol has a molar concentration of 24.7M. How many moles are contained in 500 mL of solution?
Answers
Mole itself is the number of particles contained in a substance
1 mole = 6.02.10²³ particles
Mole : the ratio of the amount of substance mass and its molar mass
\(\large{\boxed{\boxed{\bold{mole=\frac{mass}{molar\:mass}}}}\)
Molarity is a way to express the concentration of the solution
Molarity shows the number of moles of solute in every 1 liter of solute or mmol in each ml of solution
\(\large{\boxed {\bold {M ~ = ~ \frac {n} {V}}}\)
Molar concentration of methanol=24.7 M
Volume of solution = 500 ml = 0.5 L
\(\tt mol=M\times V\\\\mol=24.7\times 0.5\\\\mol=12.35\)
Related Questions
When did carbon dioxide in the atmosphere first reach 400 ppm and thus well exceed its natural range of 180 to 280 ppm?
Answers
Carbon dioxide (CO2) in the atmosphere first reached 400 parts per million (ppm) in the spring of 2013.
This milestone was observed at the Mauna Loa Observatory in Hawaii, which has been continuously monitoring atmospheric CO2 levels since the late 1950s.
The natural range of CO2 concentrations in the atmosphere over the past 800,000 years has been between 180 and 280 ppm, but due to human activities such as burning fossil fuels and deforestation, atmospheric CO2 levels have been rising steadily since the industrial revolution.
The increase in atmospheric CO2 levels has significant implications for climate change, as CO2 is a potent greenhouse gas that contributes to global warming and climate instability.
Learn more about carbon dioxide in the atmosphere at
https://brainly.com/question/1686797
#SPJ4
What happens to the shape and function of a protein if one of the amino acids is replaced with a different type of amino acid?.
Answers
Depending on how the changed amino acid performs in the body. A protein's ability to function may be completely lost if another amino acid is substituted, where as substituting one may have no discernible impact.
The fundamental molecule used to create proteins is known as an amino acid. Twenty distinct amino acids are available. A gene encodes the sequence of one or more chains of amino acids (known as polypeptides) that make up a protein. While some amino acids (called essential amino acids) can be produced by the body, others (called non-essential amino acids) cannot and must be received through diet. In marketing for food products, the phrase "amino acid" has crept into everyday speech. Some foods, like eggs or quinoa, have been listed as having "large levels of amino acids." And from this point on, you will recognize those labels as referring to a crucial protein component that is functional rather than static.
Learn more about Amino acid here:
https://brainly.com/question/15687833
#SPJ4
what two parameters are plotted in an absorbance spectrum?
Answers
The two parameters that are commonly plotted in an absorbance spectrum are the wavelength of light (measured in nanometers, or nm) on the x-axis, and the absorbance (or optical density) on the y-axis.
What is measured by an absorbance spectrum?The amount of light absorbed by a sample is calculated as a function of wavelength in absorbance spectroscopy, also referred to as absorption spectroscopy. This can provide crucial details about an atom's or molecule's electronic structure.
What does the term "absorption spectrum" mean?Various radiation is absorbed by chemicals and elements when it passes through them, depending on the chemical or element in question. In the emission spectrum, coloured lines can be observed exactly where the dark line pattern appears.
To know more about spectrum visit:-
https://brainly.com/question/6836691
#SPJ1
Neural reorganization underlies improvement in stroke-induced motor dysfunction by music-supported therapy
Answers
The study titled "Neural reorganization underlies improvement in stroke-induced motor dysfunction by music-supported therapy" investigates the role of music-supported therapy in improving motor dysfunction caused by stroke.
The researchers found that this therapy induces neural reorganization in the brain, leading to significant improvements in motor function among stroke patients.
The study focused on individuals who had experienced a stroke and subsequently suffered from motor dysfunction. Music-supported therapy, which involves engaging patients in music-based exercises and activities, was employed as an intervention. The researchers used neuroimaging techniques such as functional magnetic resonance imaging (fMRI) to assess changes in brain activity and connectivity before and after the therapy.
The results revealed that music-supported therapy led to neural reorganization within the brain. This reorganization involved the activation of alternative neural pathways, compensation for damaged areas, and improved connectivity between brain regions associated with motor control. As a result, the participants demonstrated significant improvements in their motor function.
The findings of this study suggest that music-supported therapy can facilitate neural plasticity and functional recovery in individuals with stroke-induced motor dysfunction. By engaging the brain's adaptive capacities, this therapy helps rewire neural circuits and promote the restoration of motor abilities. This research highlights the potential of music as a therapeutic tool for stroke rehabilitation and provides insights into the underlying mechanisms of its effectiveness.
Learn more about research here ;
brainly.com/question/24174276
#SPJ11
which basic postulates of Dalton's atomic theory are modified by the modern atomic theory
Answers
Answer:
Dalton's atomic theory has been modified as
Explanation:
Atoms of same element may not have same atomic mass. For example, some atoms of chlorine have atomic mass 35 amu while others have atomic mass 37 amu. Such atoms of same element having different atomic masses are called isotopes
Answer:
On the basis of new experimental facts, Dalton's atomic theory has been modified as: ... Atoms of same element may not have same atomic mass. For example, some atoms of chlorine have atomic mass 35 amu while others have atomic mass 37 amu. Such atoms of same element having different atomic masses are called isotopes.
Explanation:
Plz mark brainliest thanks
Some of the brine is encapsulated within ice crystals, but most is trapped in the spaces between neighboring crystals. When air temperature falls below 0°C, the brine migrates downward, toward the higher water temperatures below. Eventually, the high-density brine drains into the water beneath the ice. In the process, the sea ice freshens while the salinity of the underlying water. and becomes
a increases less dense
b
increases
more dense
c. decreases less dense
d. decreases
more dense
Answers
The high-density brine drains into the water beneath the ice and in the process, the sea ice freshens while the salinity of the underlying water decreases, becoming less dense (Option C).
Sea ice is usually less salty than the ocean water it freezes from. During the process of ice formation, salt in the ocean water is expelled from the ice as it grows; most of the salt is ejected into the ocean but some remain trapped inside pockets of brine within the ice. When the air temperature falls below the freezing point of seawater (usually around -1.8 °C), water molecules start to form ice crystals, which grow and aggregate into a solid sheet of ice.
During this process, the salt rejected by the growing ice also accumulates, causing the salinity of the remaining brine to increase. Some of the brine is encapsulated within ice crystals, but most are trapped in the spaces between neighboring crystals.
Thus, the correct option is C.
Learn more about brine: https://brainly.com/question/14667236
#SPJ11
I need help, plz help me with this problem

Answers
Answer:
It's b
Explanation:
I had the same exact question
write a balanced equation for the precipitation reaction that occurs when aqueous solutions of manganese(ii) chloride and potassium carbonate are combined.
Answers
The balanced chemical equation for the precipitation reaction that occurs when aqueous solutions of manganese(ii) chloride and potassium carbonate are combined is equals to the 2Cr³⁺ (aq) + 3 CO₃²⁻ → Cr₂(CO₃)₃(s).
Balanced Chemical Equation : If the species present in the reactants side and the products side separated by a right arrow contains equal number of atoms of all the type is known as the balanced chemical equation. The precipitation reaction that occurs when aqueous solutions of chromium (III) bromide and potassium carbonate are combined. First, we will write the molecular chemical reaction:
CrBr₃ (aq) + K₂CO₃(aq) → Cr₂(CO₃)₃(s) + KBr(aq)
Balanced chemical equation :
2CrBr₃ (aq) + 3K₂CO₃(aq) → Cr₂(CO₃)₃(s) + 6KBr(aq)
Balanced ionic chemical equation :
2Cr³⁺ (aq) + 6 Br⁻(aq) + 6 K⁺(aq) + 3 CO₃²⁻ → Cr₂(CO₃)₃(s) + 6K⁺(aq) + 6Br⁻(aq)
Net ionic equation:
2Cr³⁺ (aq) + 3 CO₃²⁻ → Cr₂(CO₃)₃(s)
Hence, the required balanced equation is 2Cr³⁺ (aq) + 3 CO₃²⁻ → Cr₂(CO₃)₃(s).
To learn more about balanced chemical equation, refer :
https://brainly.com/question/26694427
#SPJ4
In 20 moles of copper (II) phosphate, there are _____ moles of copper ions and _____ moles of oxygen atoms.
(a) 20, 60
(b) 20, 80
(c) 40, 80
(d) 60, 120
(e) 60, 160
Answers
The answer is (e) 60, 160: there are 60 moles of copper ions and 160 moles of oxygen atoms in 20 moles of copper (II) phosphate.
The formula for copper(II) phosphate is Cu3(PO4)2.
To find the number of moles of copper ions in 20 moles of copper (II) phosphate, we must first find the number of moles of copper in one mole of copper (II) phosphate.
We have 3 moles of copper in one mole of copper (II) phosphate.
Therefore, we have:3 x 20 = 60 moles of copper ions
To find the number of moles of oxygen atoms in 20 moles of copper (II) phosphate, we first need to find the total number of oxygen atoms in 20 moles of copper (II) phosphate.
In one mole of copper (II) phosphate, there are 8 oxygen atoms (2 from each phosphate ion).
We have:8 x 20 = 160 oxygen atoms.
So, the answer is (e) 60, 160: there are 60 moles of copper ions and 160 moles of oxygen atoms in 20 moles of copper (II) phosphate.
learn more about moles here:
https://brainly.com/question/30885025
#SPJ11
when considering precise mass, which particle has the greatest mass?
Answers
When considering the precise mass, the particle has the greatest mass is the neutrons.
The Neutron is the heaviest among all the subatomic particles with the mass of the 1.0087 amu, and the proton has the mass of the 1.0073 amu. The Positron and the electron both have the mass of the 0.00055 amu.
The difference in between the mass of the neutron and the proton is very small. This is the reason that they are both have the relative mass of the one atomic mass unit. The mass of the neutron in the grams is the 1.674 × 10⁻²⁴ grams.
To learn more about mass here
https://brainly.com/question/28907859
#SPJ4
What is known about a reaction with a positive enthalpy?
Answers
Answer:
"Endothermic reaction: In an endothermic reaction, the products are higher in energy than the reactants. Therefore, the change in enthalpy is positive, and heat is absorbed from the surroundings of the reaction."
Explanation:
When enthalpy is positive and delta H is greater than zero, this means that a system absorbed heat. This is called an endothermic reaction. When enthalpy is negative and delta H is less than zero, this means that a system released heat. This is called an exothermic reaction.
What does a high positive enthalpy mean?
The sign of q for an endothermic process is positive because the system is gaining heat. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Because the surroundings are gaining heat from the system, the temperature of the surroundings increases.
Learn more about the endothermic reaction here: brainly.com/question/6506846
#SPJ2
Which two planets are called "ice giants"?

Answers
Answer:
Uranus and neptune
Explanation:
Answer:
B, Neptune and Uranus
Explanation:
The “ice giants” Uranus and Neptune are made primarily of heavier stuff, probably the next most abundant elements in the Sun – oxygen, carbon, nitrogen, and sulfur. For each giant planet the core is the “seed” around which it accreted nebular gas.
temperature to the nearest degree

Answers
Answer:
102 C
Explanation:
Can someone balance these equations?
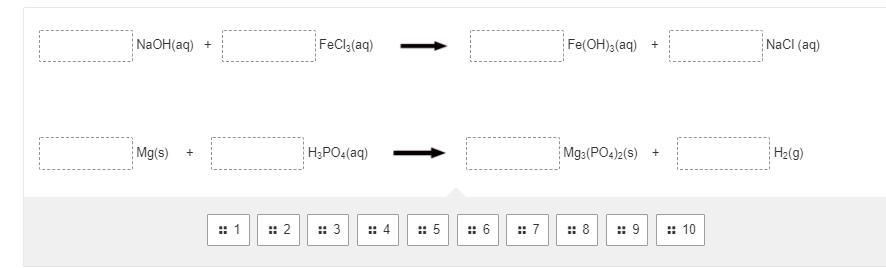
Answers
This is mind numbing, but I got you.
3NaOH + 1FeCl3 = 1Fe(OH)3 + 3NaCl
3Mg + 2H3(PO)4 = 1Mg(PO4)2 + 3H2
help does anyone know this

Answers
A piece of iron at 75.0oC is dropped into 50.0 g of water at 20.0oC. The final temperature of the water is 28.3oC. The specific heat of iron is 0.450 j/goC. Determine the mass of the piece of iron.
Answers
Answer:
13Explanation:
be critical thinkers
how many pounds are in 14.8 grams
Answers
Answer:
0.03262841
I tried, hope this helps :)
Answer:
0.032 lbs
Explanation:
Divide the mass value by 454.
hich of the following statement(s) is/are correct? i) the energy change when is (hypothetically) formed from 8 protons and 8 neutrons is known as the energy defect. ii) the splitting of a heavier nucleus into two nuclei with smaller mass numbers is known as nuclear fission. iii) the first example of nuclear fission involved bombarding with nuclei
Answers
Nuclear fission is used to produce electric power in nuclear reactors. The correct option is (ii).
Nuclear fission is the basis for how nuclear reactors operate. Nuclear fission is sustained in a nuclear reactor by regulating the rate of fission and collecting the released energy. Steam is produced using the heat produced by this controlled fission reaction. The steam powers turbines, which produce electricity.
This method is widely used in nuclear power plants all over the world to produce a sizable portion of the electricity used in society. A large amount of energy is released when an atom's nucleus splits into two smaller nuclei, a process known as nuclear fission. Utilizing this power will enable nuclear reactors to produce electricity. The correct option is (ii).
To learn more about Nuclear follow the link:
https://brainly.com/question/13090058
#SPJ4
Concentrated nitric acid has a molarity of 15.8 M. Determine the volume of nitric acid required to completely react with 1.00 g of copper.
Answers
The volume of 15.8 M concentrated nitric acid required to completely react with 1.00 g of copper would be 0.004 L or 4 mL.
Stoichiometric problemConcentrated nitric acid and copper react to produce hydrogen gas, nitrogen dioxide gas, and copper (II) nitrate according to the following equation:
\(Cu + 4HNO_3 --- > Cu(NO_3)_2 + 2H_2 +2NO_2\)
The mole ratio of copper the nitric acid is 1:4.
I g of copper is equivalent to:
mole = mass/molar mass
= 1/64
= 0.016 mol
The equivalent mol of the nitric acid would be: 0.016 x 4 = 0.064 mol
Recall that molarity is the ratio of the number of moles of solutes and the volume of the solution in liters.
Thus, volume = mole/molarity.
Volume of 0.064 mol nitric acid = 0.064/15.8
= 0.004 liters
0.004 liters = 4 mL
Thus, the volume of the concentrated nitric acid that will be required to completely react with 1.00 grams of copper would be 0.004 liters or 4 mL.
More on stoichiometric problems can be found here: https://brainly.com/question/14465605
#SPJ1
iodine-131 undergoes beta emission with a decay constant of 0.0864 1/days. if you start with 50.0 mg of the i-131, how many days will it take for the amount of i-131 to drop to 17.5 mg?
Answers
It will take approximately 8.26 days for the amount of iodine-131 to drop from 50.0 mg to 17.5 mg.
To determine the number of days it will take for the amount of iodine-131 (I-131) to drop from 50.0 mg to 17.5 mg, we can use the radioactive decay formula:
Amount(t) = Amount(0) * e^(-λt)
Where:
- Amount(t) is the amount of I-131 at time t.
- Amount(0) is the initial amount of I-131.
- λ (lambda) is the decay constant.
- t is the time elapsed.
We can rearrange the formula to solve for t:
t = (1/λ) * ln(Amount(0) / Amount(t))
Substituting the given values:
- Amount(0) = 50.0 mg
- Amount(t) = 17.5 mg
- λ = 0.0864 1/days
t = (1/0.0864) * ln(50.0 / 17.5)
Using a calculator, we can compute the value:
t ≈ 8.26 days
Therefore, it will take approximately 8.26 days for the amount of iodine-131 to drop from 50.0 mg to 17.5 mg.
learn more about iodine here:
https://brainly.com/question/30957837
#SPJ11
write the correct representation of an element x which contains 12 electrons and 16 neutrons
Answers
Answer:
²⁸Mg
Explanation:
12 electrons means it is a Mg;
A typical Mg has 12 neutrons, but this has 16 hence it must be an isotope with 4 extra neutrons.
Determine the ph of a solution made by dissolving 400.0 of sodium acetate dissolved in 100.0 of 0.100 acetic acid.
Answers
The pH of a solution made by dissolving 400.0 g of sodium acetate in 100.0 mL of 0.100 M acetic acid is determined by the equilibrium between acetic acid and its conjugate base. With the pKa value for acetic acid (4.76), we can substitute these values into the Henderson-Hasselbalch equation to find the pH of the solution.
The pH can be calculated using the Henderson-Hasselbalch equation, which is pH = pKa + log([A-]/[HA]), where [A-] represents the concentration of the conjugate base (sodium acetate) and [HA] represents the concentration of the acid (acetic acid). In this case, acetic acid is in excess, so it acts as the limiting reagent. Using the given information, we can calculate the concentration of acetic acid ([HA]) as 0.100 M and the concentration of sodium acetate ([A-]) as 0.400 M (after converting grams to moles and mL to liters). With the pKa value for acetic acid (4.76), we can substitute these values into the Henderson-Hasselbalch equation to find the pH of the solution.
To learn more about pH:
https://brainly.com/question/2288405
#SPJ11
balancing chemical equation helpp me H3PO4+KOH---> K3PO4+H2O some one balance this please help me
Answers
Answer:
H3PO4 + 3KOH -> K3PO4 + 3H20
Answer:
hope its helpful to uh....

Explain why Hydrogen-1 has a binding energy
of zero.
Answers
answers: therefore, binding energy equal to zero
Explanation:in this case of a hydrogen atom, it has only one proton and zero neutrons. in this case the proton is already seperated from other nucleons. so energy is not released in this case.
The H?X bond length is 105 pm and the H?Y bond length is 119 pm. What is true about the bonding in H?X and H?Y? Select the correct answer below:
Question 18 options:
There is greater orbital overlap between H and Y than between H and X
There is greater orbital overlap between H and X than between H and Y
H?Y is a stronger bond than H?X
The H?Y bond energy is greater than the H?X bond energy.
Answers
There is greater orbital overlap between H and X than between H and Y is the correct option according to the given Bond length.
As the average distance between the nuclei of two bonded atoms in a molecule, bond length or bond distance is known. It is a transferable quality of a bond between atoms of fixed kinds that is comparatively independent from the other components of the molecule. The distance between the nuclei of two chemically connected atoms in a molecule is measured as bond length. In relation to the two bound atoms' covalent radii, it is about equal to their sum.
When several orbitals on nearby atoms are concentrated in the same areas of space, it is known as an orbital overlap. Bond formation is facilitated by orbital overlap. Due of space constraints, atomic orbitals overlap. An electron is most likely to be found in an atomic orbital, which is a location in space.
learn more about bond length here
https://brainly.com/question/14924352
#SPJ4
what would be the effect of bringing a negatively charged metal ball near an iron bar
Answers
When the negatively charged metal ball comes in contact with the iron bar, the positive charges of the bar aligns near to the negative charges and they electrostatically attracts results in a static electricity.
What is static electricity?When a negatively charged body is comes in contact with an conductor or an insulator, the random charges gets polarized, where the positive charges of the second object align with the negative charges and there occurs a flow of electron from the negatively charged object. This is called static electricity.
A metal in its neutral state contains a sea of positive ions and free electrons. Here, the when the negatively charged metal ball comes near the iron bar, the iron bar gets polarized.
The positive charges of the iron bar gets polarized near the negative charge of the ball and there occurs an electrostatic force of attraction. Electrons from the negative charged ball tends to flow to the conductive iron bar and leads to a static electricity.
To find more on static electricity, refer here:
https://brainly.com/question/12791045
#SPJ6
Balancing Quesrion AP chem. How much do I add in each to balance?

Answers
a) We have a Fe ion with a positive charge +2, therefore we will also have a Fe ion with a +3 charge.
The charge difference is +1, this means that the Fe3+ ion gained an electron, therefore 1 must be put in front.
\(Fe^{+2}\rightarrow1Fe^{+3}+1e^-\)b) Now, in the second reaction we have 4 oxygens in the reactants and one in the products, so we put the coefficient 4 in front of H2O and thus we will have 4 oxygens in the products.
Now it would be necessary to balance the hydrogens, we have 8 hydrogens in the products and 1 in the reactants, so we put the coefficient of 8 in front of the hydrogen
Now the Mn, there is an atom of Mn in the reactants, the coefficient 1 is placed in front of the Mn+2.
So far the balanced reaction will go like this:
\(Mn_{}O^-_{4^{}}+8H^++ne^-\rightarrow1Mn^{+2}+4H_2O\)We need to balance the electrons. For that, we see what is the oxidation state of Mn in the molecule MnO4-. Oxygen has an oxidation state of -2.:
\(Mn^{+7}\lbrack O^{-2}_4\rbrack^{-8}\)The oxidation state in the MnO4 molecule is +7, therefore it must gain 5 electrons to be left with a +2 charge.
\(Mn_{}O^-_{4^{}}+8H^++5e^-\rightarrow1Mn^{+2}+4H_2O\)And so we have the balanced equation.
If Jupiter’s gravity is 10 times stronger than the Earth’s, why aren’t you being pulled toward Jupiter?
A.The distance is too great
B.Your mass is too small
C.Because Jupiter is made of gas
D.Gravity doesn’t exist on Jupiter
Answers
Answer:
I think the answer is A. The distance is to great.
How can we be more careful with how we use natural resources?
Answers
calclulate the mass of one molecule of nitrogen in gram
Answers
Answer:
for its one mole it is of 28gram. according to mole concept in one mole, there is 6.022*10^23 moles are present. so mass of 1 molecule of nitogen gas is 28/(6.022*10^23)
= 1.6605779 x 10^-24 mols
Molar mass of nitrogen: 14.01 g / mol
Mass = mols x molar mass
Mass = (1.6605779 x 10^-24 mols) x (14.01 g/mol)
Mass = 2.326 x 10^-24 g