One hundred students were surveyed about who they would vote for. What is
the best way to display the results? Explain your answer.
Answers
Related Questions
If 119.5 g of iron is mixed with 279.50 g of copper (II) nitrate, what is the limiting reagent for the reaction?
Answers
Answer:
Copper ii nitrate is the limiting reagent
Explanation:
The first thing to write here is the equation of reaction;
Fe + Cu(NO3)2 ——> Fe(NO3)2 + Cu
When we talk of limiting reagent, we mean the reactant that is totally consumed upon the completion of the chemical reaction
To get the limiting reagent, we can know this by calculating the mass of a specific product formed from each of the masses of the reactants
Now, we can use the amount of copper solid deposited by each of the reactants to know the limiting reagent here.
Since the mole ratio in all cases is 1 to 1, this will be easy
For the iron, the number of moles reacted is mass of iron/ atomic mass of iron
That would be 119.5/56 = 2.134 moles
Now since the mole ratio is 1 to 1, 2.134 moles of copper will be formed
Thus, the mass of copper produced from the iron will be number of moles * atomic mass of copper = 2.134 * 64 = 136.57g
Now from the nitrate, the number of moles is
mass mixed/ molar mass of copper nitrate
molar mass of copper nitrate is 188g/mol
number of moles is thus 279.5/188 = 1.487 moles
Since the mole ratio is 1 to 1, it means that the number of moles of copper produced too is 1.487 moles
The mass of copper produced from this is number of moles of copper * atomic mass of copper
That will be 1.487 * 64 = 95.15g
Now, since the copper nitrate produces less amount of copper solid, it is the the reagent to be consumed first and thus it is our limiting reagent
According to the graphs, by what factor will the ocean's acidity change from 2005 to 2100?
A) 0.2
B) 0.5
C) 1.6
D) 2.2
Answers
Answer: C) 1.6
Explanation: I got it right
Which statement(s) is/are FALSE?
SELECT ALL THAT APPLY
The number of protons determines what type of
а
element an atom is.
b
Protons and neutrons repel each other and
position themselves far apart inside the atom.
Neutral atoms are made of specific numbers of
C protons and electrons but the number of neutrons
they possess can vary a little bit.
d All atoms are about the same size.
Answers
Answer:
D
Explanation:
not all atoms would be the same size. As for they vary scaleing from left to right in the periodic table.
Engage in Argument A ball is pushed
stop and rolls 6 m in 2 s. Student A says
verage speed of the ball is 3 m/s. Student
the average speed of the ball is 1.5 m/s?.
student is correct? Explain your answer.
Answers
Answer:
student A
Explanation:
v = s ÷ t (speed = distance ÷ time)
v = 6m ÷ 2s
v = 3m/s
what is a galvanic cell made of?
A. two electrodes in electrolyte solution
B. two beakers connected by a tube
C. two ceramic plates in pure water
D.two medal strips surrounded by air
Answers
Explanation:
A because A galvanic cell consists of two half-cells, such that the electrode of one half-cell is composed of metal A, and the electrode of the other half-cell is composed of metal B; the redox reactions for the two separate half-cells are thus: An+ + ne− ⇌ A.
Answer:
A
Explanation:
Which is more likely to melt a cup of ice - a spoonful of boiling water, or a cup of room-temperature
Answers
Answer:
boiling water
Explanation:
its hotter
2. What were the independent, dependent, and control variables in your investigation?
Type your answer here:
3. Write a hypothesis based on observations and scientific principles.
Type your answer here:
Experimental Methods
1. What tools did you use to collect your data?
Type your answer here:
2. Describe the procedure that you followed to conduct your experiment.
Type your answer here:
Data and Observations
1. Record your observations.
Type your answer here:
Conclusions
1. What conclusions can you draw about whether a visual, auditory, or tactile stimulus resulted in the shortest reaction time? Write an evidence-based claim.
Type your answer here:
2. Write a testable question that could be used to further investigate the topic of stimulus response time.
Type your answer here:
60 points
Answers
The scientific method is used by biologists and other scientists to pose inquiries about the natural world.
The first step in the scientific method is an observation, which prompts a question from the scientist. They then develop a theory—a verifiable explanation—that responds to the query.
A controlled experiment is a scientific test that is carried out under predetermined circumstances, meaning that only one (or a small number of) variables are altered at a time while all other variables are held constant. The element that differs between the experimental and control groups—in this case, the volume of water—is referred to as the independent variable. The response tested in an experiment to establish if a treatment had an impact is known as the dependent variable.
Learn more about biologists:
https://brainly.com/question/28447833
#SPJ4
suppose you have an alkaline buffer consisting of .2m aqueous ammonia and .1 ammonsium chlorisde what is the ph of the solution
Answers
The pH of the alkaline buffer consisting of 0.2 M aqueous ammonia and 0.1 M ammonium chloride is 9.55.
We must utilize the ammonium ion's (\({NH_4}^+\)) \(pK_a\) value of 9.25 to determine the pH of an alkaline buffer made up of 0.2 M aqueous ammonia (\(NH_3\)) and 0.1 M ammonium chloride (\(NH_4Cl\)). The following reaction takes place in this buffer solution:
\(NH_3 + H_2O\) ↔ \({NH_4}^+ + OH^-\)
The ammonium chloride will dissociate to form ammonium ion and chloride ion, but the ammonium ion will react with the hydroxide ion (\(OH^-\)) produced by the reaction above to form ammonia and water, effectively preventing any significant increase in the pH of the solution.
To calculate the pH of the buffer, we can use the Henderson-Hasselbalch equation:
\(pH = pK_a + log(\frac{base}{acid})\)
Where [base] is the concentration of the base (\(NH_3\)) and [acid] is the concentration of the acid (\({NH_4}^+\)).
Putting in the values, we get:
\(pH = 9.25 + log(\frac{0.2}{0.1})\)
⇒ \(pH = 9.25 + log(2)\)
⇒ \(pH = 9.25 + 0.301\)
⇒ \(pH = 9.55\)
To know more about pH
brainly.com/question/29766400
#SPJ4
What is forensic
science?
Answers
Answer:
"Forensic science is a critical element of the criminal justice system. Forensic scientists examine and analyze evidence from crime scenes and elsewhere to develop objective findings that can assist in the investigation and prosecution of perpetrators of crime or absolve an innocent person from suspicion."
Explanation:
Strictly from: https://www.justice.gov/olp/forensic-science#:~:text=Forensic%20science%20is%20a%20critical,an%20innocent%20person%20from%20suspicion.
Forensic science is any science used to aid in a criminal/legal investigation
what alkane, with molecular formula c7h16 , forms seven monochlorinated products (disregarding stereoisomers) when heated with cl2 ?
Answers
2,2-dimethylpentane is the alkane that produces four isomeric monochloro derivatives. There are four different sorts of carbon atoms present, resulting in four different isomeric products.
Alkane:
Alkanes are organic substances that only include single-bonded hydrogen and carbon atoms and do not contain any other functional groups. Alkanes can be broken down into the following three groups: linear straight-chain alkanes, branched alkanes, and cycloalkanes. Alkanes have the general formula CnH2n+2. Saturated hydrocarbons also include alkanes. The only components of alkanes are carbon and hydrogen, making them the most basic and least reactive hydrocarbon type.
Isomers:Isomerism is a phenomena where a substance has a similar chemical formula but a different structure. Isomers are the particular chemicals that displayed this resemblance.
The 4 different isomeric products are shown in the picture.
To know more about Isomers, checkout:
https://brainly.com/question/18474884
#SPJ4

Explain the process of how James Chadwick
found the neutron.
Answers
Answer:
In 1932, the physicist James Chadwick conducted an experiment in which he bombarded Beryllium with alpha particles from the natural radioactive decay of Polonium. The resulting radiation showed high penetration through a lead shield, which could not be explained via the particles known at that time.
Explanation:
how many kilojoules is 1,500,000 calories
Answers
Answer:
1 cal = 0.004187 kJ
1,500,000 cal = 6280.5 kJ
what do hot subsurface waters contain when they are identified as hydrothermal solutions?
Answers
Hydrothermal solutions are hot subsurface waters that are rich in minerals and gases. These waters are formed when groundwater or seawater is heated by the Earth's mantle and rises to the surface through cracks in the crust. When the hot water comes in contact with rocks and minerals, it dissolves them and becomes enriched with minerals and gases.
The minerals found in hydrothermal solutions include sulfur, copper, gold, silver, and zinc, among others. These minerals are often deposited around the vent or opening through which the water exits, creating mineral deposits known as hydrothermal vents. Hydrothermal solutions are also known to contain gases such as hydrogen sulfide, carbon dioxide, and methane.
These gases can create unique ecosystems around hydrothermal vents, providing energy and nutrients to organisms that live there. Overall, hydrothermal solutions are fascinating geological phenomena that have significant economic and ecological importance.
For more information on Hydrothermal solutions visit:
brainly.com/question/9008281
#SPJ11
MULTIPLE CHOICE QUESTION
Substances that would be described as an Element (such as Argon gas Ar,
fluorine gas F2, pure metal like gold Au ) have an oxidation state of what
number?
mentary
2
3
-1
1
O
Answers
Answer:
the oxidation number of those elements is 2 because some of them are molecules
The balanced equation for the reaction in this lab
is:
2Mn04- (aq) + 5H202(aq) + 6H* (aq) El 2Mn2*(aq) +
502(g) + 8H20(I)
A) Calculate the grams of H202 that were present in 1.00 mL of hydrogen peroxide solution.
B) Assume the density of the H202 solution to be 1.00 g/mL, calculate the percentage of H202 in solution.
C) The accepted percentage of hydrogen peroxide in solution is 3.00%. Calculate the percent error.

Answers
5.75 liters of 3% hydrogen peroxide solution are included by the company in the container.
What is percentage?A measurement used to determine a number's value out of 100 is the percentage.
Due to that,
Hydrogen peroxide solutions in various containers have concentrations of 35% and 3%.
Since the corporation wants an 8-liter container with a 12% hydrogen peroxide concentration.
Use the allegation and combination properties,
35 3 12
9 : 23
Total quantity equals 23x + 9x = 32x.
Since the business requests 8 liters,
Consequently, 32x = 8 x = 1/4.
The concentration of 3% hydrogen peroxide will be equal to = 23x = 23/4 = 5.75.
5.75 liters of 3% hydrogen peroxide solution are needed.
To learn more about hydrogen peroxide refer
https://brainly.com/question/29302613
#SPJ1
Which of the following represent a mole ratio between silver nitrate and appper(II) nitrate in the following reaction: 2AgNO3 + Cu --> Cu(NO3)2 + 2Af
Answers
There is no direct involvement of \(Cu(NO_3)_2\) in the mole ratio calculation as it is not a reactant with \(AgNO_3.\)
The balanced chemical equation for the given reaction is:
\(2AgNO_3 + Cu -- > Cu(NO_3)_2 + 2Ag\)
According to this equation, the mole ratio between \(AgNO_3\) and Cu is 2:1, which means that for every 2 moles of \(AgNO_3\) used, 1 mole of Cu is consumed.
There is no direct mole ratio between \(AgNO_3\) and \(Cu(NO_3)_2\) or between \(AgNO_3\) and Ag. However, we can calculate the mole ratio between \(AgNO_3\) and Ag using the stoichiometric coefficients in the balanced equation.
For every 2 moles of \(AgNO_3\) used, 2 moles of Ag are produced. Therefore, the mole ratio between \(AgNO_3\) and Ag is 2:2 or simply 1:1.
To know more about reaction here
https://brainly.com/question/11231920
#SPJ1
SOMEBODY PLEASE HELP ME ASAP. WILL GIVE BRAINLIEST TO BEST ANSWER.

Answers
Answer:
135.80 g of Ag
Explanation:
\(40gCu *\frac{1mol Cu}{63.546g Cu} *\frac{2mol Ag}{1molCu} *\frac{107.868g Ag}{1mol Ag}\)
~ 135.80 g of Ag
2. Which one of the following statements is true about halogens?
They're never found as individual elements in nature because they are not reactive.
They're commonly found by themselves in nature.
None of the answers are true.
They're never found as individual elements in nature because they are so reactive.
Answers
Answer:
They're never found as individual elements in nature because they are so reactive.
Explanation:
Halogens are a highly reactive group of elements. They are usually found combined in nature.
Because each of the elements has seven electrons in the outer shells of their atoms the elements are all univalent and also acceptors of electrons. They are all non-metals and oxidizing agents. This makes the elements in this group highly reactive and combine readily with other species in nature.• 1. An object has a mass of 570g and a volume of 2280ml. Calculate its
density. 250 kg/m3
Answers
Answer:
Density=mass/volume
= 570/2280
=0.25g/ml
A chemical company has just employed you to solve their financial dilemma. The company has an overabundance of silver nitrate solution and a huge debt that it must settle or announce bankruptcy.
Evaluate the following data and suggest a chemistry based plan for the company that may just prevent it from going bankrupt. (Hint: Think about a type of reaction and how it might be used to make the company money. The answer should include the reaction and an explanation. Use the information below.)
Answers
To solve the financial dilemma, the chemical company can consider using the excess silver nitrate solution to synthesize silver nanoparticles, which have various applications in industries.
What is Silver nanoparticles?Silver nanoparticles can be synthesized by reducing silver ions with a reducing agent, and silver nitrate can serve as a source of silver ions.
One possible reaction for the synthesis of silver nanoparticles using silver nitrate is the reduction of silver ions with sodium borohydride (NaBH4). The reaction can be represented as:
AgNO3 + NaBH4 → Ag nanoparticles + NaNO3 + B2H6
In this reaction, silver nitrate is the oxidizing agent, which accepts electrons, while sodium borohydride is the reducing agent, which donates electrons to reduce the silver ions. The reaction also produces sodium nitrate and borane gas as byproducts.
The synthesized silver nanoparticles can be sold to various industries, generating revenue for the company and potentially reducing their debt. The company can also consider optimizing the synthesis process to increase the yield and purity of the silver nanoparticles, which can increase their market value.
Learn more about silver nanoparticles here: https://brainly.com/question/16055011
#SPJ1
explane how chemical equation shows that the reactants change and form the products
Answers
Chemical equation shows that the reactants change and form the products as a result of the reactants and products being rearranged during the reaction which result in different combinations in the products.
What is a Chemical equation?This is referred to as a written representation of the process that occurs during a chemical reaction and contains the reactants and products involved and an example is
H₂ + O₂ ⇒ H₂O
It shows that reactants change and form the products because the reactants and products are rearranged during the reaction and there is also a different combinations in the products.
Read more about Chemical equation here https://brainly.com/question/26694427
#SPJ1
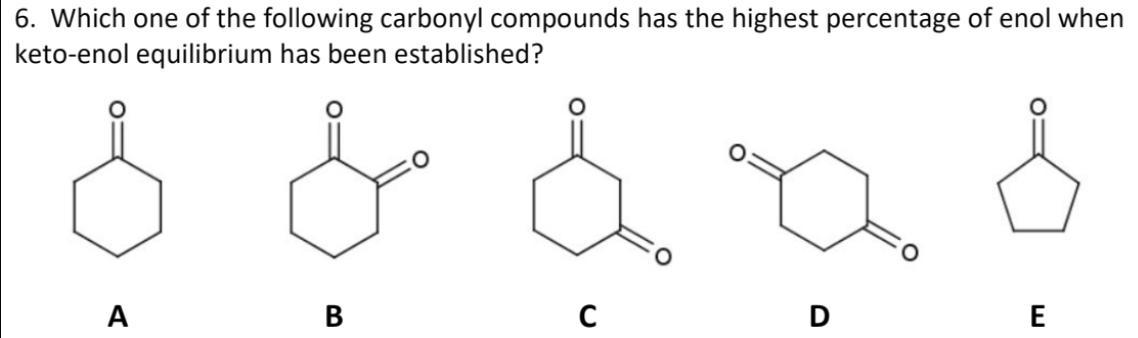
which one of the following carbonyl compounds has the highest percentage of enol when keto-enol equilibrium has been established?
Answers
When keto-enol equilibrium has been reached, the compound with the largest percentage of enol is 1,2 cyclo hexanedione.
1,2-cyclohexanedioneThe chemical compound 1,2-cyclohexanedione has the formula (CH2)4(CO)2. It is one of three cyclohexanediones that are isomeric.
It is a substance with no colour that dissolves in a number of organic solvents. It can be made by using selenium dioxide to oxidise cyclohexanone.From this di ketone, numerous diimine and dioxime ligands have been created. In order to produce diaza heterocycles, it condenses with 1,2-diamines.chemical formula: C6H8O21.1305 g/cm3 is the density.Molecular weight: 112.128 g/molCyclohexane-1,2-dione hydrolase, a novel tool to degrade alicyclic molecules, is studied using 1,2-cyclohexanedione as a substrate. Additionally, it serves as a particular reagent for arginine residues.learn more about keto-enol equilibrium here
https://brainly.com/question/4476334
#SPJ4
Using the periodic table predict which three elements will have similar chemical properties or reactivity

Answers
oxygen, sulfur and selenium as they are all in the same group of the periodic table - they are all chalcogens
The response of a pH electrode can be modeled as a first order or second order passive low pass filter (i.e. two RC circuits in series). A limitation of commercial pH electrodes is their slow response time, which is typically 2 seconds (i.e. = 2 s).
Analytically, find the transfer function, H(s), of this series electrodes (two RC circuits in serie both are have the same values). This transfer function is defined as the measured pH (output) divided by the actual pH (input).
Obtain the analytical expression of the magnitude response of the system and plot the Bode plot of the system using MATLAB.
Obtain the analytical expression for h(t) and plot the impulse response of the electrode using MATLAB.
Obtain the analytical expression for the step response and plot it for the electrode using MATLAB.
All of this considering the RC series which contains the same values.
Please someone can help me with this questions. Thank you
Answers
In order to analytically find the transfer function, H(s), of the pH electrode, we can model it as a second-order passive low-pass filter consisting of two RC circuits in series.
The transfer function can be obtained by determining the ratio of the output voltage to the input voltage in the frequency domain.
Let's denote the Laplace transform variable as 's'.
The transfer function H(s) can be expressed as: H(s) = Vout(s) / Vin(s)
For a second-order passive low-pass filter, the transfer function can be written as:
H(s) = 1 / (s + s(R₁C₁ + R₂C₂) + R₁R₂C₁C₂)
Where R₁, R₂ are the resistances in the two RC circuits, and C₁ C₂ are the corresponding capacitances.
Now, let's assume the time constant
τ = R₁C₁ =R₂C₂ = 2 seconds (as given),
we can substitute this into the transfer function:
H(s) = 1 / (s² + 4s + 4)
Simplifying the transfer function further, we can factorize the denominator:
H(s) = 1 / ((s + 2)²)
So, the transfer function of the pH electrode, H(s), is:
H(s) = 1 / ((s + 2)²)
This transfer function represents the relationship between the measured pH (output) and the actual pH (input) of the electrode.
To know more about pH electrode here
brainly.com/question/28855246
#SPJ4
The complete question should be
The response of a pH electrode can be modeled as a first order or second order passive low pass filter (i.e. two RC circuits in series). A limitation of commercial pH electrodes is their slow response time, which is typically 2 seconds (i.e. = 2 s).
Analytically, find the transfer function, H(s), of this electrode. This transfer function is defined as the measured pH (output) divided by the actual pH (input).
in metal IE 1 < IE 2 < IE 3. why?
Answers
Answer:
Explanation:
in metal IE 1 < IE 2 < IE 3. why
Sugar could be classified as which of the following?Question 5 options:compoundsolution (homogenous)elementmechanical mixture (heterogenous)
Answers
Compound, according to our last session.
In the experiment by yoshida and kinosita, fluorescently labeled actin was attached to atp synthase. No atp was added to one preparation, which showed no change over the course of the experiment. Atp was added to a second preparation and the movement of the actin was recorded. Explain what can be concluded from this experiment.
Answers
With this experiment, we can conclude that when ATP synthase binds to ATP and the ATP synthase rotates.
What role does ATP synthase play?Definition of ATP Synthase Adenosine triphosphate (ATP) is produced by the enzyme ATP synthase during the process of cellular respiration. The primary source of energy used by cells is ATP.
What is the name of the enzyme responsible for ATP synthesis?a catalytic enzyme that uses inorganic phosphate and ADP to create ATP The big protein enzyme known as ATP synthase, which produces energy for the cell, is the location to which angiostatin binds.
Learn more about enzymes here:-
https://brainly.com/question/2015607
#SPJ4
why oxygen is more active than
sulphur?
Answers
It's bcoz due to the small atomic size oxygen atom which is more electronegative
What happens to the central bright fringe on a single-slit diffraction experiment when the width of the slit through which the light passes is reduced?
Answers
The central bright fringe on a single-slit diffraction experiment refers to the bright spot in the middle of the diffraction pattern. When the width of the slit through which the light passes is reduced, the central bright fringe becomes narrower and more intense.
1. In a single-slit diffraction experiment, light waves pass through a narrow slit and diffract, or spread out, creating a pattern of alternating bright and dark fringes.The central bright fringe corresponds to the region where the waves from different parts of the slit constructively interfere, resulting in a bright spot.
3. The width of the central bright fringe is determined by the width of the slit. If the slit is wider, the central bright fringe will be wider.When the width of the slit is reduced, the central bright fringe becomes narrower because there is less diffraction occurring. The narrower slit allows less room for the waves to spread out, leading to a narrower bright spot.
To know more about that bright VISIT:
https://brainly.com/question/12500676
#SPJ11
Which of these equations is balanced, and which is not balanced? Explain how you can tell.
a) 2C2H6+ 7O2 → 4CO2+ 6H2O
b) 2MgCO3 + H2SO4 → 2MgSO4 + H2O + 2CO2
Answers
Answer:
a is balanced
Explanation:
a) reactant/ product
(2x2)=4c / 4c
(2x6)=12h / 12 h
14o / (8o+6o)=14 o