On the December solstice, the north end of Earth's axis points away from
the sun.*
True
False
Answers
Answer:
True
Explanation:
If you look at this sentences and your sentence so it means it is TRUE.
The December solstice is on December 21 or 22. The north end of Earth's axis is leaning farthest from the Sun.
Related Questions
2. Predict the shift in the reaction with each stress shift rt, shift left, or no
HEAT + Ti(s) + 2C1 (g)
a. CI, (g) is added to the system.
b. TiCk (g) is removed from the system.
TiCI (g)
c. The temperature of the container is decreased.
d. The pressure of the container is increased.,
e. Ti(s) is added to the system.
Answers
b. The removal of TiCl(k) from the system will shift the reaction right, towards the products, to counteract the decrease in the concentration of TiCl(k).
c. Decreasing the temperature of the container will shift the reaction to the right, towards the products, to counteract the decrease in kinetic energy of the system.
d. Increasing the pressure of the container will shift the reaction to the left, towards the reactants, to counteract the increase in pressure caused by the decrease in volume.
e. Adding Ti(s) to the system will not cause a shift in the reaction because Ti(s) is not involved in the reaction.
describe the symmetry of a cubic crystal?
Answers
Answer:
It is an imaginary plane which passes through the molecule and divides it into portions which are exactly the superimposable mirror images of each other.
Answer:
identifying repeatibg parts of molecules and simplifying both data collected and all calculations
Chemistry Give the IUPAC names for the following compounds. Use the abbreviations o, m, or p (no italics) for ortho, meta, or para if you choose to use these in your name. For positively charged species, name them as aryl cations. Example: ethyl cation. Be sure to specity stereochemistry when relevant. NO2 OH Ph ČI Name: Name: 1-choloro-4nitrobenzene
Answers
Using the given abbreviations, the name of NO2 OH Ph ČI is 1-chloro-4-nitrobenzene.
The International Union of Pure and Applied Chemistry (IUPAC) has established specific rules and guidelines that must be followed when naming a chemical compound with an IUPAC name. It is used to convey a chemical compound's molecular structure and composition as well as its distinctive identification.
The substance in the cited example is 1-chloro-4-nitrobenzene. The name adheres to the IUPAC guidelines for naming aromatic compounds, which include allocating the lowest numbers to the substituents for the carbons on the benzene ring. In this instance the benzene ring has two substituents a chlorine atom (Cl) and a nitro group (NO2).
The name 1-chloro-4-nitrobenzene comes from the fact that the chlorine atom is bonded to carbon 1 and the nitro group is bonded to carbon 4 respectively.
Learn more about IUPAC name at:
brainly.com/question/16631447
#SPJ4
What is the level of organization in the human body from the
least to the most complex?
Answers
The major levels of organization in the body, from the simplest to the most complex are: atoms, molecules, organelles, cells, tissues, organs, organ systems, and the human organism.
What is the most complex level of organization of the human body?As can be seen, there are many different degrees and approaches to studying anatomy. Understanding the order of these levels puts the complexity of the human body in context. The chemical level of organization is the most basic level of organization, but first-year chemistry students would disagree. Simple atoms interact to create relatively straightforward compounds at this level. For instance, water (H2O) is composed of two hydrogen atoms and one oxygen atom, and carbon dioxide (CO2) is composed of one carbon atom and two oxygen atoms. There are four main types of macromolecules in the human body: lipids (fats), proteins, nucleic acids, and carbohydrates (sugars). Macromolecules (macro: big) are larger and more complex.These four macromolecules serve as the foundation for the cellular level, the next level of organization.Learn more about Organization of the human body refer to :
https://brainly.com/question/17007898
#SPJ1
Given the balanced equation representing a reaction:
HCl + H2O H3O+ + Cl-
The water molecule acts as a base because it
Answers
Answer:
It accepted a proton from HCl
Explanation:
When properly written, the equation box the reaction is given as; HCl(aq) + H2O(l) ----> H3O+(aq) + Cl-(aq).
According to Brownstead-Lowry definition of acids and bases, an acid donates protons while a base accepts protons.
Water molecule acts as a base in the reaction because it accepted a proton from HCl in the reaction above.
What two elements were named after the United States?
Answers
How many cAMP molecules are necessary to activate protein kinase A?
4
Answers
One cAMP molecule is necessary to activate protein kinase A.
cAMP, or cyclic adenosine monophosphate, is a signaling molecule that is involved in many cellular processes, including the regulation of gene expression, metabolism, and muscle contraction. Protein kinase A, or PKA, is an enzyme that is activated by cAMP.
When cAMP levels increase within a cell, it binds to and activates PKA, which then carries out its enzymatic function. PKA acts as a kinase, meaning that it transfers a phosphate group from ATP to target proteins, which changes their activity and function.
The activation of PKA by cAMP is a highly specific process, and it has been found that only one cAMP molecule is necessary to activate PKA. This allows for precise regulation of PKA activity in response to changes in cAMP levels.
To learn more about molecules, visit:
https://brainly.com/question/19556990#
#SPJ11
How many moles of O2 are used to produce 4 moles of NO?
Answers
The number of moles of O₂ used to produce 4 moles of NO is 2 moles
How do I determine the mole of O₂ used?First, shall write the balanced equation. This is given below:
N₂ + O₂ -> 2NO
From the balanced equation above,
2 moles of NO were obtained from 1 mole of O₂
With the above information, we can determine the number of moles of O₂ used to produce 4 moles of NO. This can be obtained as follow:
From the balanced equation above,
2 moles of NO were obtained from 1 mole of O₂
Therefore,
4 moles of NO will be obtain from = (4 × 1) / 2 = 2 moles of O₂
Thus, number of mole of O₂ used is 2 moles
Learn more about number of mole:
https://brainly.com/question/23350512
#SPJ1
What is the molarity of 0.25 moles of FeCl3 dissolved in 120 ml of solution?
Answers
Answer:
Molarity = 2.0833 M (Approx)
Explanation:
Given:
Number of moles (n) = 0.25
Volume of solution = 120 ml = 0.12 L
Find:
Molarity
Computation:
Molarity = Number of moles (n) / Volume of solution.
Molarity = 0.25 / 0.12
Molarity = 2.0833 M (Approx)
avogadro's law states that under constant temperature and pressure, the volume of a gas is directly proportional to the number of moles of gas. what is the relationship consistent with this statement?
Answers
According to Avogadro`s law, the volume of gas is directly proportional to number of moles of gas.
This law mathematically can be written as:
V ∝ n
V = K n
V/n = K
Where V represents the volume of gas
n represents the number of moles of gas
k is proportionality constant at a given temperature and pressure
This law explains that how equal volumes of all gases contain the same number of molecules when the temperature and pressure are the same. Similarly, this law can be used to compare the same substances under two different sets of circumstances.
V₁ / n₁ = V₂ / n₂
This equation shows that the volume of the gas increases proportionally to the amount of number of moles of gas. Similarly, if the amount of gas decreases result in decrease in number of moles. As a result, neither the size of the molecules nor the molar mass of the gas affects how many atoms or molecules there are in a given volume of an ideal gas.
To learn more about Avogadro`s law click here
brainly.com/question/4133756
#SPJ4
Please help me with chemistry questions!

Answers
5 *10^-6 = 0.00005
When the power is positive, you move the decimal to the right. When it is negative, you move the decimal left.
When comparing a mole of oxygen and a mole of sulfur, how many atoms does each have?
Answers
Answer:
both contains 6.02 x 1023 atoms
Explanation:
Pls, choose me as brainliest!
Precipitate-forming reactions would be most appropriate for identifying the presence of?
Answers
Precipitate-forming reactions would be most appropriate for identifying the presence of whether an element is present in the solution.
What is precipitate forming reaction?In a precipitation reaction, dissolved chemicals will get combined to form one or more solid products. These kinds of reactions, which are also known as double displacement, double replacement, or metathesis reactions which are frequently taken place through the aqueous solutions and involved in the exchange of ions between ionic compounds.
Through the precipitation reactions, the presence of element will be confirmed and analyzed. The presence of lead in water sources could be checked by adding the chemical and looking for the appearance of a precipitation whether it should be at instant , and the chemical combines with lead to generate a precipitate.
To learn more about precipitate forming reaction,
brainly.com/question/9052471
#SPJ4
The isomerization of cyclopropane to propylene is a first-order process with a half-life of 19 minutes at 500 °C. The time it takes for the partial pressure of cyclopropane to decrease from 1.0 atmosphere to 0.125 atmospheres at 500 °C is closest to... (A) 38 minutes (B) 57 minutes (C) 76 minutes (D) 152 minutes (E) 190 minutes
Answers
t = 57 min., Therefore, we can say that it takes 57 minutes for cyclopropane's partial pressure to drop from 1 atm to 0.125 atm at 500°C.
Is the first-order isomerization of cyclopropane to propylene?Propene (CH3CH=CH2) is produced by the isomerization of cyclopropane, which is a first-order process. A sample of cyclopropane converts to propene in 79 minutes at 760 K.
Isomerization: first-order reaction or not?The reaction is a first-order reaction because it is an isomerization reaction, and its rate constant is expressed in terms of minutes. The sum of the powers or exponents that the concentration terms are raised in the rate law expression can be used to define the order of a reaction.
to know more about isomerization here:
brainly.com/question/2226351
#SPJ4
cars run on gasoline, where octane (c8h18) is the principle component. this combustion reaction is responsible for generating enough energy to move a vehicle, or do other work. how much co2 and h2o (in grams) are produced in the combustion of 0.87 gallons of octane? (density of octane
Answers
The combustion of 0.87 gallons of octane produces approximately 6.98 kg of CO₂ and 3.21 kg of H₂O.
To calculate the amount of CO₂ and H2O produced in the combustion of octane, we need to first convert the volume of octane from gallons to moles using its density and molar mass.
The density of octane is around 0.703 g/mL and its molar mass is 114.23 g/mol. One gallon is approximately 3.785 liters.
So, the amount of moles of octane in 0.87 gallons is:
moles of octane = (0.87 gallons) x (3.785 L/gallon) x (0.703 g/mL) / (114.23 g/mol) = 19.8 moles
The balanced chemical equation for the combustion of octane is:
2 C₈H₁₈ + 25 O₂ → 16 CO₂ + 18 H₂O
From this equation, we see that 2 moles of octane reacts with 25 moles of oxygen to produce 16 moles of CO₂ and 18 moles of H₂O.
Using stoichiometry, we can calculate the amount of CO₂ and H₂O produced from 19.8 moles of octane:
moles of CO₂ produced = 16/2 x 19.8 moles = 158.4 molesmoles of H₂O produced = 18/2 x 19.8 moles = 178.2 molesTo convert moles to grams, we can use the molar mass of each compound:
mass of CO₂ produced = 158.4 moles x 44.01 g/mol = 6,979 g or 6.98 kg (rounded to 2 decimal places)mass of H₂O produced = 178.2 moles x 18.02 g/mol = 3,209 g or 3.21 kg (rounded to 2 decimal places)Therefore, the combustion of 0.87 gallons of octane produces approximately 6.98 kg of CO₂ and 3.21 kg of H₂O.
Learn more about combustion on:
https://brainly.com/question/10458605
#SPJ11
answer correct
urgent

Answers
Answer:
i don't understand let us know which one you want us to answer
What happens in a neutralization reaction?
A. Hydrogen ions and hydroxide ions react to form a salt.
B. A neutral solution breaks up to form an acid and a base.
C. An acid reacts with a base to produce a neutral solution.
D. An acid reacts with a salt to produce a basic solution.
Answers
Answer:
i think its either A or D so if one of them doesn't work maybe try the other if you can. i hope that helps
Explanation:
In a neutralization reaction an acid reacts with a base to produce a neutral solution. Hence, the option C is correct.
In a neutralization reaction, an acid and a base react with each other to form a salt and water. The hydrogen ions (H⁺) from the acid combine with the hydroxide ions (OH⁻) from the base to form water (H₂O). The remaining ions combine to form a salt.
The general chemical equation for a neutralization reaction is:
Acid + Base → Salt + Water
For example, in the neutralization of hydrochloric acid (HCl) with sodium hydroxide (NaOH), the reaction can be represented as:
HCl + NaOH → NaCl + H₂O
Here, hydrogen ions (H⁺) from HCl combine with hydroxide ions (OH⁻) from NaOH to form water (H₂O), and the remaining sodium ion (Na⁺) and chloride ion (Cl⁻) combine to form sodium chloride (NaCl), which is the salt.
The net effect of the neutralization reaction is to produce a solution that is neither acidic nor basic but neutral. The pH of the resulting solution will be close to 7, depending on the strength of the acid and base used in the reaction.
Learn more about neutralization from the link given below.
https://brainly.com/question/15395418
#SPJ6
what is the bond order for a second-period diatomic particle containing five electrons in antibonding molecular orbitals and eight electrons in bonding molecular orbitals?
Answers
The bond order for a second-period diatomic particle containing five electrons in antibonding molecular orbitals and eight electrons in bonding molecular orbitals is 1.5
Bond order is defined as the number of electrons in bonding molecular orbitals minus the number of electrons in antibonding molecular orbitals divided by two. As a result, we may determine the bond order of this diatomic particle by the formula: Bond order = (number of bonding electrons - number of antibonding electrons) / 2
Bond order = (8 - 5) / 2
Bond order = 1.5.
This diatomic molecule, according to the bond order, is a stable molecule since the bond order is greater than 1, indicating that it is a double bond. The molecule has an overall bond strength that is greater than a single bond, but not as strong as a triple bond. So therefore he bond order for a second-period diatomic particle containing five electrons in antibonding molecular orbitals and eight electrons in bonding molecular orbitals is 1.5
Learn more about bond order at:
https://brainly.com/question/30641030
#SPJ11
If 25.0g NO are produced, how many grams of nitrogen gas are used?
N2 + O2 ---> NO
How do I answer this question?
Answers
According to the stoichiometry of the chemical equation, 29.46 grams of nitrogen gas are used.
Stoichiometry is defined as the determination of proportions of elements or compounds in a chemical reaction. The related relations are based on the concepts of law of conservation of mass and concept of law of combining weights and volumes.
The concept of stoichiometry is used in quantitative analysis for measuring the concentrations of substances which are present in the sample.
As per the equation 28 g nitrogen gives 33 g NO thus 25 g nitrogen gives 25×33/28=29.46 g.
Learn more about stoichiometry,here:
https://brainly.com/question/28780091
#SPJ1
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
Other than color, what evidence can you use to identify chemical changes?
Answers
Explanation:
There are several pieces of evidence that can be used to identify chemical changes, other than a change in color. Some of these include the production of a gas, the formation of a precipitate, a change in temperature, and a change in the state of matter (e.g. from a solid to a liquid or vice versa). For example, if a solid substance is added to a liquid and bubbles of gas are produced, this is evidence of a chemical change. Similarly, if two clear solutions are mixed together and a solid precipitate forms, this is also evidence of a chemical change. Additionally, if a substance is heated and it changes from a solid to a liquid or from a liquid to a gas, this is also evidence of a chemical change.
Hey besties can y'all help me out
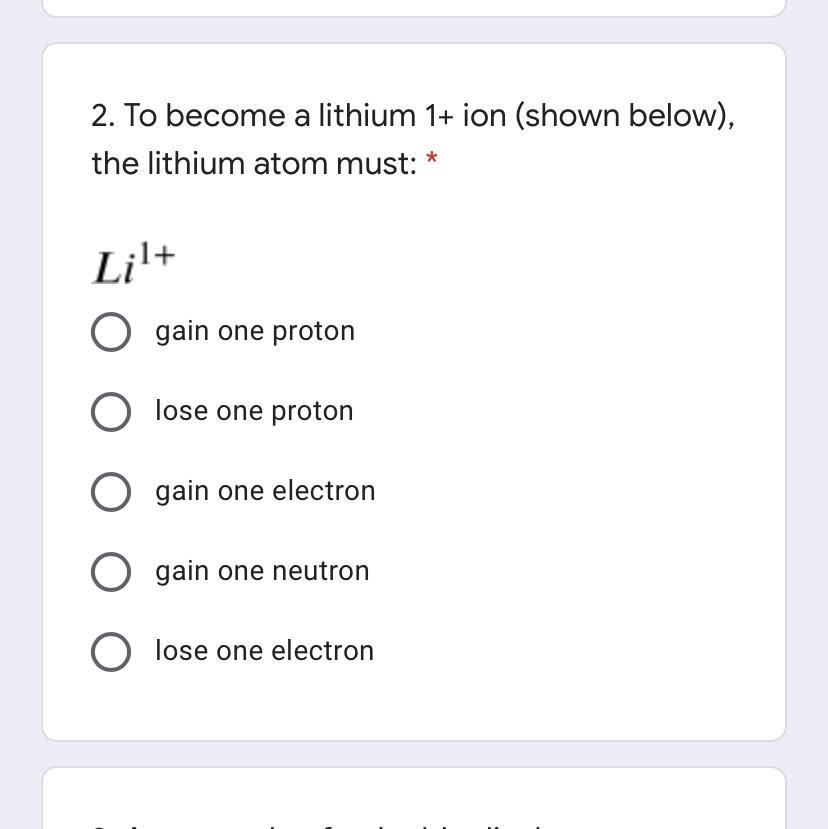
Answers
you need to lose one electron
Charlotte's car averages 25 miles per gallon of gas. How many dal would she need to travel 3,241 hm?
Answers
Answer:
Around 129 galons
Explanation:
if you divied 3,241 by 4 you get 129 gallons but if you times that by 25 you get 3,225 so a little bit off but close.
In Rutherford’s gold foil experiment, most alpha particles passed through the gold foil without deflection and were detected on the screen. What caused the particles to pass through without any deflection? A. a large area of positively charged particles present in the gold foil B. the extremely high speed of the alpha particles passing through the gold foil C. a small area of negatively charged particles present in the gold foil D. empty space in the atoms present in the gold foil
Answers
Answer:
empty space in the atoms present in the gold foil
Answer:
D.) empty space in the atoms present in the gold foil.
Explanation:
for plato users :)
Suppose that a substance in a beaker is heated over a burner in a science lab. Which observation would most likely indicate that a chemical change has occurred in the substance?
A. If the substance is a liquid or solid, an increase in temperature would indicate a
chemical change.
B. If the substance is a liquid, a change of some of the liquid to a gaseous form would indicate a chemical change.
C. If the substance is a solid, a change of some of the solid to liquid form would indicate a chemical change.
D. If the substance is a liquid or solid, production of an odor would indicate a chemical change.
Answers
Answer: the answer is D
Explanation:
4. (a) Draw resonating structure of Phenol. 5. What happens when (write the reactions involved) (a) Cyclohexanol reacts with concentrated sulfuric acid and resulting product is ozonolyzed (b) Phenol is heated with CH3COCl (c) Propyne reacts with hydrogeniodide in presence of benzene peroxide (d) Propoxypropane is reacted with access of NH3
Answers
(a) Draw resonating structure of PhenolPhenol is a common organic molecule. It consists of a phenyl group (C6H5) attached to a hydroxyl group (OH). The hydroxyl group is connected to the benzene ring at the para position, denoted as p-phenol.
The two main resonating structures of Phenol are shown in the figure below: This reaction takes place by cleaving the double bond of Cyclohexene using ozone, followed by a reductive workup step.
(b) Phenol is heated with CH3COCl:When Phenol is heated with Acetyl Chloride, it forms Acetophenone. The reaction is as follows:Phenol reacts with Acetyl Chloride to form Acetophenone, with the elimination of HCl as a by-product.(c) Propyne reacts with hydrogeniodide in the presence of benzene peroxide:Propyne reacts with hydrogen iodide in the presence of benzene peroxide to form 2-Iodopropane.
The reaction proceeds via a radical mechanism, as shown below:The chain initiation step:This step involves the homolytic cleavage of the benzene peroxide bond to generate benzene and two free radicals. These free radicals then interact with hydrogen iodide to form iodine radicals.The chain propagation step:The chain propagation steps involve the following sequence of reactions:The chain termination step:
This reaction involves the formation of 2-Iodopropane.(d) Propoxypropane is reacted with access of NH3:Propoxypropane is reacted with excess of NH3 to form Propylamine. The reaction is as follows:Propoxypropane undergoes nucleophilic substitution with ammonia, followed by deprotonation, to form the corresponding amine. Excess ammonia is required to drive the reaction to completion.
To know more about organic molecule visit:-
https://brainly.com/question/31574152
#SPJ11
The two types of market exchanges are: Group of answer choices functional and strategic relationships. solo exchanges and functional relationships. tactical exchanges and profit relationships. tactical and behavioral exchanges. one-sided relationships and bipartisan exchanges.
Answers
The two types of market exchanges are functional relationships and strategic relationships.
Market exchanges refer to the interactions and transactions that take place between buyers and sellers in a market. The two primary types of market exchanges are functional relationships and strategic relationships.
Functional relationships focus on the basic exchange of goods or services between buyers and sellers. These exchanges are often driven by immediate needs and involve straightforward transactions. In functional relationships, the emphasis is on the efficiency and effectiveness of the exchange process, with both parties seeking to fulfill their specific requirements.
On the other hand, strategic relationships go beyond the basic exchange and involve a more long-term and collaborative approach. Strategic relationships are characterized by mutual trust, cooperation, and shared goals. These exchanges often involve strategic partnerships, alliances, or contracts that aim to create value and achieve competitive advantages for both parties involved.
Learn more about Market exchanges here:
https://brainly.com/question/31810324
#SPJ11
Match each of the following carboxylic acids to their appropriate PK a values (4.8, 0.2 and 3.2): (A) ICH2COOH (B) CH3COOH (C) CF3 COOH
Answers
The pKa values for the given carboxylic acids are ICH₂COOH (pKa) = 4.8, CH₃COOH (pKa) = 4.8 and CF₃COOH (pKa) = 0.2
The quantitative behavior of acids and bases in solution can be understood only if their pKa values are known. Ka denotes the acid dissociation constant. It measures how completely an acid dissociates in an aqueous solution.
The larger the value of Ka, the stronger the acid as acid largely dissociates into its ions.
pKa is the negative base -10 log of the acid equilibrium constant (Ka) of a solution.
Learn more about pKa, here:
https://brainly.com/question/30655117
#SPJ4
Please answer the questions in the picture below





Answers
Answer b
Explanation:
PLEASE HELP!! :((((((((((((((((((((((((

Answers
Where is the word problems? Like the problems that you have to put it in.
Explanation: