Of the choices below, which one is not an ionic compound?
A) PCl5
B) MoCl6
C) RbCl
D) PbCl2
E) NaCl
Answers
Answer: \(PCl_5\) is not an ionic compound.
Explanation:
An ionic compound is defined as the compound which is formed when electron gets transferred from one atom to another atom. The cation is formed by loss of electrons by metals and anions are formed by gain of electrons by non metals.
Thus \(MoCl_6\), \(RbCl\), \(PbCl_2\) and \(NaCl\) are all ionic compounds.
Covalent compound is defined as the compound which is formed by the sharing of electrons between the atoms forming a compound. These are usually formed when two non-metals react.
Thus \(PCl_5\) is a covalent compound as both Phosphorous and chlorine are non metals.
PCl₅ is not an ionic compound. Ionic or electrovalent compounds are formed
through the transfer of electrons in a chemical reaction. Metals and non-
metals mostly form ionic compounds.
Covalent bonding on the other involves the sharing of electrons between
two elements. Non-metals are mostly involved in this type of reaction.
PCl₅ possesses a covalent bond and not ionic as electrons are shared
between two non metals which are phosphorus and chlorine.
Read more on https://brainly.com/question/957239
Related Questions
reaction between carbon dioxide and water to create carbonic acid is _____
Answers
The reaction between carbon dioxide and water to create carbonic acid is reversible chemical reaction.
A reversible reaction is a chemical reaction in which the products can easily return to their original reactants. This means that the products of a reversible reaction are unstable and can easily react with each other to produce the original reactants. Carbon dioxide reacts with water to form carbonic acid, which is an unstable molecule that can break down into its reactants under certain conditions.
This is because the reaction is reversible, and the products can easily be converted back into the original reactants. Hence, the reaction between carbon dioxide and water to create carbonic acid is an example of a reversible chemical reaction.
To know more about reversible chemical reaction refer to:
https://brainly.com/question/29018235
#SPJ11
how much co(g) is required to completely react with 22.55 g fe2o3? if 15.32 g fe(s) are produced, what is the % yield?
Answers
11.87 g of CO(g) is required to completely react with 22.55 g Fe₂O₃(s) to produce 15.32 g Fe(s). The percent yield of the reaction is 97.15%.
The balanced equation for the reaction between CO(g) and Fe₂O₃(s) is:
Fe₂O₃(s) + 3 CO(g) -> 2 Fe(s) + 3 CO₂( g)
To determine how much CO is required to completely react with 22.55 g of Fe2O3, we need to use the stoichiometry of the reaction.
From the balanced equation, we know that for every 1 mole of Fe₂O₃(s) that reacts, 3 moles of CO(g) are consumed. To convert mass to moles, we use the molar mass of Fe₂O₃(s), which is 159.69 g/mol.
22.55 g Fe2O3 / 159.69 g/mol = 0.1412 moles of Fe2O3
From the balanced equation we know that for every 1 mole of Fe₂O₃(s), 3 moles of CO(g) is required, so we can calculate the moles of CO(g) needed for the reaction:
0.1412 moles Fe₂O₃(s) (3 moles of CO(g) / 1 mole of Fe₂O₃(s)) = 0.4236 moles of CO(g)
To get the mass of CO(g), we can convert the moles to grams using the molar mass of CO(g), which is 28.01 g/mol.
0.4236 moles of CO(g)(28.01 g/mol) = 11.8660 = 11.87 g of CO(g)
To find the % yield, we divide the actual yield (15.32 g Fe(s)) by the theoretical yield (mass of Fe(s) produced from all the Fe₂O₃(s) consumed) and multiply by 100.
Theoretical yield of Fe(s) = mass of Fe₂O₃(s) consumed(mass of Fe(s) produced from 1 mol Fe₂O₃(s) / mol of Fe₂O₃(s) consumed)
= 22.55 g Fe₂O₃(s) (55.85 g Fe(s) / mol Fe(s)) (2 mol Fe(s) / 1 mol Fe₂O₃(s)) (1 mol Fe₂O₃(s) / 159.69 g Fe₂O₃(s)) = 15.77 g Fe(s)
% yield = (15.32 g Fe(s) / 15.77 g Fe(s)) x 100 = 97.15%
So, 11.87 g of CO(g) is required to completely react with 22.55 g Fe₂O₃(s) to produce 15.32 g Fe(s) and the % yield of the reaction is 97.15%.
Learn more about percent yield here: https://brainly.com/question/27918618
#SPJ4
There are 55.0 g of neon gas in a 3.0 L cylinder, the pressure is 4.5 atm what is the temp of the gas
Answers
Answer:
60.9 Kelvin
Explanation: First, write out everything that you know. You are tring to find the temperature, so the temperature will be represented by x.
Pressure (P)= 4.5 atm
Volume (V)= 3L
Number of Moles (n)= ?
Gas Consant (R)= 0.0821, if the pressure is in atm, that means r is 0.0821
Temperature (T)= x
We don't have all the information we need to plug the values into the equation. We still need to know how many moles 55.0 grans of neon is.
Ne in Grams= 55
Atomic Mass of Ne= 20.1797
55/20.1797= 2.7
moles= 2.7
Now that we have all the information we need, plug everying into the equation. In case you don't know, the Ideal Gas Law Equation is PV= nRT.
(4.5)(3) = (2.7)(0.821)x
x= 60.9
Now you have your temperature! It is 60.9 in Kelvin.
Which compound do you expect to be miscible with octane (C8H18)?Which compound do you expect to be miscible with octane ?NH3CH3OHH2OCBr4
Answers
Out of the given compounds, the compound that is most likely to be miscible with octane is CH₃OH (methanol) due to its similar polarity.
Methanol has a polar hydroxyl group (-OH) that can participate in hydrogen bonding, but it also has a nonpolar methyl group that makes it partially nonpolar. This partial nonpolarity allows methanol to dissolve in nonpolar solvents like octane.
NH₃ (ammonia) is a polar compound due to its ability to form hydrogen bonds with water molecules. It is unlikely to be miscible with octane because the two compounds have very different polarities.
H₂O (water) is a highly polar compound due to its ability to form hydrogen bonds. It is unlikely to be miscible with octane because of their differences in polarity.
CBr₄ (carbon tetrabromide) is a nonpolar compound, but it is not likely to be miscible with octane due to the large size of its molecules and high molecular weight.
To know more about polarity here:
https://brainly.com/question/3184550#
#SPJ11
Under what conditions is the change in internal energy, δerxn , equal to the heat evolved in a chemical reaction?.
Answers
If there is no change in pressure or no change in volume of the system, then the heat of the reaction will be equal to the change in internal energy of the system.
According to the first law of thermodynamics,
The total change in internal energy of the system is equal to the sum of the heat added to the system and the work done by the system.
So, we can write,
ΔH = ΔQ + W
Where,
ΔH is the heat of the reaction,
ΔQ is the heat evolved or heat given,
W is the work done by the system,
We know work by the gas is,
W = VΔP or PΔV
Where V is the volume of the gas and P is pressure of the gas.
So, for the heat of the system to be equal to that of the hear evolved in the system.
W = 0
For W to be zero,
Either, ΔV or ΔP has to be zero,
So, we can conclude that if change in the pressure of the system of the change in volume of the system is zero.
To know more about First law of thermodynamics, visit,
https://brainly.com/question/1613201
#SPJ4
Suppose that you experimentally determine the molar extinction coefficient (ε) of a complex ion sample to be 8171 L/mol-cm at 510 nm. The literature value for the ε of the complex ion sample is reported as 8400 L/mol-cm. What is the percent purity of your experimental sample
Answers
The percent purity of your experimental sample is 97.3 %
The percentage purity % = impure value/pure value × 100 %.
Actual value of molar extinction coefficient = impure value = 8171 L/mol-cm, pure value = literature value = 8400 L/mol-cm.
Substituting the values of the variables into the equation, we have
% purity = 8171 L/mol-cm/8400 L/mol-cm × 100 %
% purity = 0.9727 × 100 %
% purity = 97.27 %
% purity ≅ 97.3 %
The percent purity of your experimental sample is 97.3 %
Learn more about percentage purity here:
https://brainly.com/question/17836636
How many atoms from the following molecule will be detected in a 13c nmr experiment? we are not asking for unique signals, just total number of atoms
Answers
The structure of the compound, there will be ten unique signals in the carbon 13 NMR of the compound.
What are NMR signals?The NMR signals shows the atoms in a compound that are in unique chemical environments. The atoms that are in the same chemical environment will only give one signal.
Based on the structure of the compound, there will be ten unique signals in the carbon 13 NMR of the compound.
Learn more about carbon 13 NMR :https://brainly.com/question/9442853
#SPJ12

is sugar made of tiny particles
Answers
Part G
Explain how the structure of ammonium lauryl sulfate, as described in parts E and F, produces the properties identified in part C. Write a short paragraph or two.
Answers
The molecule ammonium lauryl sulfate is a detergent because of its hydrophobic tail and a hydrophilic head which are responsible for its cleansing action.
Properties of ammonium lauryl sulfateThough not shown, I presume that the property labelled as C must have to do with the cleasing action of the molecule ammonium lauryl sulfate.
The substance has a hydrophobic tail and a hydrophilic head which enables it to form miscles and these are the responsible for the cleansing action of the ammonium lauryl sulfate molecule.
Learn more about ammonium lauryl sulfate: https://brainly.com/question/16483020
i’m marking brainiest

Answers
Atoms will combine with each in order to become more chemically stable. Compounds are formed between two atoms that have incomplete outer energy levels. The two types of compounds that can be formed between atoms are ionic and covalent. Ionic compounds are formed between two oppositely charged atoms. ionic compounds contain a metal ion and a non - metal ion:
Metals will lose electrons to form positive ions and nonmetals will gain elections to form negative ions. These oppositely charge ions are very attracted to each other and forms very strong bonds as a result.
Ionic compounds are characterized by crystalline structure.
High melting and boiling points, and are solids at room temperature.
Two non-metals will combine to form covalent compounds. Covalent compounds are formed when two atoms share one or more pairs of electrons The force of attraction between the main relatively weak. Covalent compounds are characterized by low melting and boiling points, and can be gases, liquids and soft solids at room temperature.
To learn about Chemical bonding
https://brainly.com/question/819068
#SPJ1
What volume of 0. 160 mli2s solution is required to completely react with 130 ml of 0. 160 mco(no3)2 ?.
Answers
For the complete reaction of 130 ml 0.160 M CO(NO3)2 , 130 ml of 0.16 Li2sis required.
The reaction of with will be:
Li2S + CO(NO3)2 ----------> 2LiNO3 + COS
Accordingly, 1 mole of Li2S completely reacts with 1 mole of CO(N03)2
Moles of CO(NO3)2 = Molarity * Volume (L)
Moles of CO(NO3)2 = 0.160 M * 0.13 L
Moles of CO(NO3)2 = 0.0208 moles.
The moles of Li2s reacts with CO(NO3)2 will be 0.0208 moles.
Volume of Li2S = moles / molarity
Volume of Li2S = 0.0208 / 0.160
Volume of Li2S = 0.13 L
Volume of Li2 = 130 ml.
For the complete reaction of 130 ml 0.160 M , 130 ml of 0.16 is required.
To know more about molarity click here:
https://brainly.com/question/8732513
#SPJ4
PLZ HELP ITS TIMED AND IM STUCK
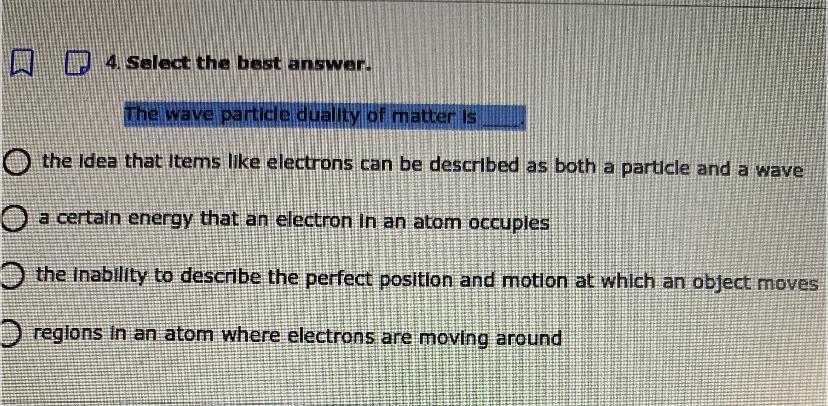
Answers
Answer:
I belive its A
Explanation:
A makes more sence then all of them
Using scanning tunneling microscopy, scientists at IBM wrote the initials of their company with 35 individual xenon atoms. Calculate the total mass of these letters in grams (mXe=131.29amu).
Express your answer using four significant figures.
Answers
Scientists at IBM used scanning tunnelling microscopy to write the initials of their corporation using 35 individual xenon atoms. The mass of the xenon atom is 7.631 x 10⁻²¹ g
The concept involved in the given problem is mass conversion. The mass conversion establishes connections between grams, moles and atoms. Mole can be used to relate mass of substance to the number of atoms.
Mole is the chemical unit to express the amount of a substance. One mole consists of Avogadro’s number of atoms that is
6.022 x 10²³ atoms
1 mol = 6.022 x 10²³ atoms
The Avogadro’s number acts as a conversion factor between the number of atoms and the number of moles.
The number of moles of a substance can be calculated using following formula:
Number of moles = Number of atoms divided by Avogadro number
The moles of a substance can be converted to grams of the substance using the following formula:
Grams = Moles x Atomic mass
Step: 1
Calculate the moles of Xe atoms by using the relation as follows:
Number of moles = Number of atoms divided by Avogadro number
Thus,
mol of Xe = number of atoms / Avogadro number
= 35/6.022x10²³
= 5.812 x 10⁻²³ mol
Explanation:
The Avogadro’s number acts as a conversion factor between number of atoms and moles. So, divide the number of atoms by Avogadro’s number to get the moles of Xe atoms.
Hint:
Convert the moles of Xe to grams.
Step: 2
Calculate the total mass of letters from the moles of Xe using the following relation:
Grams of Xe = (Moles of Xe) x (Atomic mass of Xe)
=(5.812 x 10⁻²³ mol) x (131.29 gmol⁻¹)
= 7.631 x 10⁻²¹ g Xe
Mole of a substance relates mass of substance to the number of atoms. Convert the number of atoms to moles of Xe using Avogadro’s number. Then convert moles to grams of Xe atoms by multiplying moles with atomic mass of Xe.
Learn more about Mass here:
https://brainly.com/question/5661976
#SPJ4
solid nacl is produced by the reaction of metallic sodium with chlorine gas. which equation shows this reaction?
Answers
Answer:
Na (s) + Cl2 (g) → NaCl (s)
Explanation:
Inspection of this equation, however, shows that, while there is one sodium atom on each side of the arrow, there are two chlorine atoms in the reactants and only one in the products.
How many moles of helium is required to blow up a balloon to 87.1 liters at 74 C and 3.5 atm?
R = 0.0821 (L*atm)/(mol*K) OR R = 8.31 (L*kPa)/(mol*K)
Answers
Moles of helium is required to blow up a balloon to 87.1 liters at 74 C and 3.5 atm is 021.65 mole
Mole is the unit of amount of substances of specified elementary entities
According to the ideal gas law he number of moles of a gas n can be calculated knowing the partial pressure of a gas p in a container with a volume V at an absolute temperature T from the equation
n =pV/RT
Here given data is volume = 87.1 liters
Temperature = 74 °C means 347.15 k
Pressure = 3.5 atm
R = 0.0821
Putting this value in ideal gas equation then
n =pV/RT
n = 3.5 atm×87.1 liters / 0.0821 ×347.15 k
n = 021.65 mole
Moles of helium is required to blow up a balloon to 87.1 liters at 74 C and 3.5 atm is 021.65 mole
Know more about mole of helium
https://brainly.com/question/23365554
#SPJ1
Write a nuclear equation for the beta decay of Promethium-165
Answers
Answer:
see your answer in pic
Explanation:

There can be emissions of radiations like gamma radiation. There can be emission of particles too like alpha particle. Therefore, the nuclear equation for the beta decay of Promethium-165 is
¹⁶⁵Pm\(\rightarrow\)⁰e₋₁ + ₆₂Sm¹⁶⁵
What is nuclear decay?Nuclear decay is process in which the radioactive element releases particles or radiations. Alpha particles is ⁴₂He. Alpha particle is nothing but helium particle. The kinetics that is related to nuclear decay is of first order.
The nuclear equation for the beta decay of Promethium-165 is
¹⁶⁵Pm\(\rightarrow\)⁰e₋₁ + ₆₂Sm¹⁶⁵
Beta decay of Promethium-165 is the emission of electron particle from the given isotope. the product is Samarium ₆₂Sm¹⁶⁵.
Therefore, the nuclear equation for the beta decay of Promethium-165 is
¹⁶⁵Pm\(\rightarrow\)⁰e₋₁ + ₆₂Sm¹⁶⁵
To know more about nuclear decay, here:
https://brainly.com/question/21114779
#SPJ2
PLEASE HELP ME WITH THIS

Answers
Answer:
I guess B
Explanation:
Answer:
The answer is D
Explanation:
How does density affect sinking and floating in water?
Answers
Answer:
Density is a measure of how heavy something is compared to its size.
If an object is more dense than water it will sink when placed in water, and if it is less dense than water it will float.
Density is a characteristic property of a substance and doesn’t depend on the amount of substance.
Explanation:
If you compared the weight of wood and an equal amount, or volume, of water the sample of wood would weigh less than the sample of water. This means that wood is less dense than water. Since wood is less dense than water, wood floats in water, no matter how big or small the piece of wood is.
A student might want to know why a boat made out of steel can float when steel is more dense than water. This is not an easy question and requires a different approach than what students have seen so far. We do not necessarily recommend the following explanation for 5th graders but here is the idea:
An object floats when it displaces a volume of water that has a mass equal to the mass of the object. So if a material like steel is shaped into a boat and made larger and larger, it will displace more and more water. When it is large enough to displace a volume of water that has a mass equal to the mass of the boat, the boat will float.
‼️‼️‼️‼️‼️‼️‼️‼️‼️‼️‼️‼️‼️‼️‼️‼️‼️‼️‼️‼️‼️
Is this right? If not which is it pls

Answers
Answer:
i believe it is b as well
Explanation:
Include one paragraph to explain the movement of energy during phase transitions.(solid, liquid gas)
just a quick 4 or 5 sentences about the 3 states of matter you wrote yourself please(i dpnt care how detailed it is)
Answers
The energy movement from solid liquid to gas is always going up and moving faster. A solid is a tight definite shape where the molecules are all stuck in one place and there’s barely energy and movement. The liquid has a more free space since it does not have a definite volume energy moves around quicker. The gas phase would have energy going everywhere Becuase it doesn’t have a definite shape and volume. As temperature goes up the kinetic energy also goes up
Suppose you mix red-tinted oil (density = 0.90 g/mL) with a liter of blue-tinted seawater (density =
1.025 g/mL). After sealing the container, you shake the mixture and then let it settle. What do you
see? Why?
Answers
The oil will separate from the seawater when the the mixture is left to settle.
A mixture is a combination of two or more substances that are not chemically combined together.
The red-tinted oil and blue-tinted seawater represents a mixture since the two substances do not combine chemically.
When the oil and seawater are mixed and the mixture is agitated, the oil appears to be all mixed up with the seawater.
After a while, the oil separates from the seawater and floats on top of the seawater because oil is less dense than seawater.
Learn more: https://brainly.com/question/14373997
A toothpaste factory situated on a large river disposes of a waste water containing villiamite (NaF) to the river on a continuous basis. The minimum flow in the river is 210 m3/s, and the discharge rate from the treatment plant is 12.5 m3/s. The river temperature averages 18
Answers
The river receives a wastewater discharge of 12.5 m³/s containing villiamite (NaF) from the toothpaste factory.
The toothpaste factory disposes of wastewater containing villiamite (NaF) into a large river. The discharge rate from the treatment plant is 12.5 m³/s, which is continuously released into the river. The river itself has a minimum flow of 210 m³/s. The average temperature of the river is 18°C. It is important to consider the potential environmental impact of this wastewater discharge on the river's ecosystem, as well as monitoring and complying with regulatory guidelines for wastewater management and pollutant levels in the river.
To learn more about temperature visit;
https://brainly.com/question/7510619
#SPJ11
Using the balanced equation for the next few questions: 4 Fe(s) + 30 (9) - 2Fe2O3(s)
How many moles of Iron (11) Oxide would be produced by complete reaction of 0.15
moles of iron? (Be sure to show your work including the mole ratio you used)
Answers
Answer:
0.075 moles of iron oxide would be produced by complete reaction of 0.15 moles of iron.
Explanation:
The balanced reaction is:
4 Fe + 3 O₂ → 2 Fe₂O₃
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Fe: 4 molesO₂: 3 molesFe₂O₃: 2 molesYou can apply the following rule of three: if by stoichiometry 4 moles of Fe produce 2 moles of Fe₂O₃, 0.15 moles of Fe produce how many moles of Fe₂O₃?
\(moles of Fe_{2} O_{3} =\frac{0.15 moles of Fe*2 moles of Fe_{2} O_{3} }{4 moles of Fe}\)
moles of Fe₂O₃= 0.075
0.075 moles of iron oxide would be produced by complete reaction of 0.15 moles of iron.
1. A 35. 3 g of element M is reacted with nitrogen to produce 43. 5 g of compo und M3N2. What is (i) the molar mass of the element and (ii) name of the element?
Answers
A 35. 3 g of element M is reacted with nitrogen to produce 43. 5 g of compo und M₃N₂ (i) The molar mass of element M is 24.0 g/mol. (ii) The name of the element is magnesium (Mg).
(i) To find the molar mass of element M, we need to use stoichiometry to relate the mass of M to the mass of M₃N₂. We can start by calculating the moles of M3N2 produced:
43.5 g M₃N₂ × 1 mol M₃N₂/100.9 g M₃N₂ = 0.43 mol M₃N₂
Since the molar ratio between M and M₃N₂ is 1:3, we can calculate the moles of M:
0.43 mol M₃N₂ × 1 mol M/3 mol M₃N₂ = 0.14 mol M
Finally, we can calculate the molar mass of M by dividing its mass (35.3 g) by the number of moles (0.14 mol):
molar mass of M = 35.3 g/0.14 mol = 253 g/mol
However, this value is much higher than the molar mass of any known element. We can recognize that the formula M₃N₂ implies that element M is a metal with a +2 charge, since each M atom forms 3 bonds with N atoms, and each N atom forms 2 bonds with M atoms.
(ii) Based on this information, we can identify element M as magnesium (Mg), which has a molar mass of 24.0 g/mol.
To know more about molar mass, refer here:
https://brainly.com/question/22997914#
#SPJ11
A compound contain only carbon, Hydrogen, Oxygen. Mass percentage of carbon is 52.2%, relative molecular mass of compound is 46, Find how many hydrogen atom present
Answers
Answer:
Six
Explanation:
Mᵣ = 46
Aᵣ(C) = 0.522 × 46 = 24
Difference = 22
Less 1 O = -16
Aᵣ(H) = 6
The relative atomic mass of the H atoms is 6.
The molecule contains six hydrogen atoms.
What gas will make a flame pop then go out
Answers
Answer:
Hydrogen Gas
Explanation:
The hydrogen gas now in the test tube is flammable. So when I hold the tube over the flame, the hydrogen gas ignites and creates a popping sound. It is like a tiny bomb or fireworks.
Hope this helped! :)
PLEASE HELP ME!!!!
Answers are below the sentence

Answers
Answer:
Mass
Explanation:
A gas has its pressure decrease from 760 torr to 320 torr at 225k. Was is the initial temperature of the gas?
Answers
Answer:
\( T_{1}= 94.75 K\)
Explanation:
Given the following data;
Final pressure, P2 = 760 Torr
Initial pressure, P1 = 320 Torr
Final temperature, T2 = 225K
To find the initial temperature, we would use Gay Lussac's law;
Gay Lussac law states that when the volume of an ideal gas is kept constant, the pressure of the gas is directly proportional to the absolute temperature of the gas.
Mathematically, Gay Lussac's law is given by;
\( PT = K\)
\( \frac{P1}{T1} = \frac{P_{2}}{T_{2}}\)
Making T1 as the subject formula, we have;
\( T_{1}= \frac{P_{1}}{P_{2}} * T_{2}\)
Substituting into the equation, we have;
\( T_{1}= \frac{320}{760} * 225 \)
\( T_{1}= 0.4211 * 225 \)
\( T_{1}= 94.75 K\)
Therefore, the initial temperature of the gas is 94.75 Kelvin.
what is the reduction half-reaction for the following overall galvanic cell reaction? co2 (aq) 2 ag(s) → co(s) 2 ag (aq)
Answers
The reduction half-reaction can be written as:
2 Ag⁺ (aq) + 2e⁻ → 2 Ag (s)
To determine the reduction half-reaction for the given overall galvanic cell reaction, we need to identify the species that undergoes reduction. In the reaction:
CO₂ (aq) + 2 Ag(s) → CO(s) + 2 Ag (aq)
We can see that Ag⁺ ions from the aqueous phase are being reduced to Ag atoms and gaining electrons. Therefore, the reduction half-reaction can be written as:
2 Ag⁺ (aq) + 2e⁻ → 2 Ag (s)
This half-reaction represents the reduction of Ag⁺ ions to Ag atoms, where two electrons (2e⁻) are gained by each Ag⁺ ion to form solid Ag.
Learn more about half-reaction from the link given below.
https://brainly.com/question/32677817
#SPJ4
Write the complete and net ionic equations for the following reactions (1)-(4). Be sure to indicate the states of the reaction products.
(1) K2SO4 (aq) + NaNO3 (aq) -->
(2) CaBr2 (aq) + Na2SO4 (aq) -->
(3) Pb(IO3)2 (aq) + NaOH (aq) -->
(4) K2SO4 (aq) + BaCl2 (aq) -->
Answers
Write complete and net ionic equations for the given reactions by identifying the states of the reaction products.
How to write complete and net ionic equations for the given reactions?
(1) Complete Ionic Equation:
2K⁺(aq) + SO₄²⁻(aq) + 2Na⁺(aq) + NO₃⁻(aq) → 2K⁺(aq) + 2NO₃⁻(aq) + Na⁺(aq) + SO₄²⁻(aq)
Net Ionic Equation:
2K⁺(aq) + 2NO₃⁻(aq) → 2K⁺(aq) + 2NO₃⁻(aq)
(2) Complete Ionic Equation:
Ca²⁺(aq) + 2Br⁻(aq) + 2Na⁺(aq) + SO₄²⁻(aq) → Ca²⁺(aq) + SO₄²⁻(aq) + 2Na⁺(aq) + 2Br⁻(aq)
Net Ionic Equation:
Ca²⁺(aq) + SO₄²⁻(aq) → Ca²⁺(aq) + SO₄²⁻(aq)
(3) Complete Ionic Equation:
Pb²⁺(aq) + 2IO₃⁻(aq) + Na⁺(aq) + OH⁻(aq) → Pb(IO₃)₂(s) + Na⁺(aq) + OH⁻(aq)
Net Ionic Equation:
Pb²⁺(aq) + 2IO₃⁻(aq) + 2OH⁻(aq) → Pb(IO₃)₂(s) + 2OH⁻(aq)
(4) Complete Ionic Equation:
2K⁺(aq) + SO₄²⁻(aq) + Ba²⁺(aq) + 2Cl⁻(aq) → 2K⁺(aq) + 2Cl⁻(aq) + BaSO₄(s)
Net Ionic Equation:
Ba²⁺(aq) + SO₄²⁻(aq) → BaSO₄(s)
Learn more about Ionic Equation
brainly.com/question/13887096
#SPJ11