Answers
Answer:
Explanation:
yesyesyesyesyesyesyesyesyes
Related Questions
A 3-column table with 5 rows. Column 1 is unlabeled with entries A, B, C, D, E. Column 2 is labeled Temperature in degrees Celsius with entries 2.4, 21.5, 39.6, 55.6, 71.2. Column 3 is labeled Volume in cubic centimeters with entries 5.8, 5.8, 6.7, 6.9, 7.4. A student collected the data shown above. Row may represent an error.
Answers
Since it reflects no change in volume – it is most likely that the data recorded in row B is erroneous.
How to solveIn order to pinpoint the row with a probable error, we will scrutinize the correlation between temperature and volume.
Typically, whenever there is an increase in temperature, there would be an accompanying escalation in the volume of a given substance.
Let's take a closer look at the values reflected in the table:
From A to B: Temperature rises but the volume remains stagnant.
From B to C: Both temperature and volume show an increment.
Similarly, from C to D, as well as from D to E, we see that for every rise in temperature, there is subsequently more volume illustrated.
Given the dissimilarity between rows A and B – where the former shows a temperature increase while the latter reflects no change in volume – it is most likely that the data recorded in row B is erroneous.
Read more about errors here:
https://brainly.com/question/30360094
#SPJ1
Which row in the table below is likely to represent an error in the student's data collection?
Temperature (°C) Volume (cm³)
A 2.4 5.8
B 21.5 5.8
C 39.6 6.7
D 55.6 6.9
E 71.2 7.4
Answer:
A student collected the data shown above. Row
✔ B
may represent an error.
Explanation:
edge 2023
The cyanide ion (CN-1) is highly toxic, but can be remediated in solution by redox reaction with the hypochlorite ion (the principal component of bleach). The balanced net ionic equation for this process is: 2 CN-1 + 5 ClO-1 + H2O ---> 5 Cl-1 + N2 + 2 HCO3 -1 A 3.5 x 106 L sample of water was found to be contaminated with cyanide ions at a concentration of 25mM. How many grams of cyanide were dissolved in the total sample?
Answers
Answer:
2.275x10⁶g of CN⁻¹ were dissolved in the sample
Explanation:
Molarity is defined as the ratio between moles of solute and liters of solution.
If a CN⁻¹ solution has a concentration of 25mM, there are 0.025 moles of CN⁻¹ per liter of solution.
If the sample has a colume of 3.5x10⁶L, moles of CN⁻¹ are:
3.5x10⁶L × (0.025moles / L) = 8.75x10⁴ moles of CN⁻¹ in the sample of water.
In grams (As molar mass of CN⁻¹ is 26g/mol):
8.75x10⁴ moles CN⁻¹ × (26g / mol) = 2.275x10⁶g of CN⁻¹ were dissolved in the sample
10 moles of hydrogen are allowded to react with 6 moles of oxygen .How much water molecules are obtained?
Answers
Answer:
Since 2H₂ + O₂ ---> 2H₂O
We need 2 moles of hydrogen for each mole of oxygen
So, we need 2(6) = 12 moles of H₂ but since we only have 10 moles,
H₂ is the limiting reagent
So since 2 moles of H₂ gives 2 moles of water,
10 moles of H₂ will give rise to 10 moles of Water with 1 mole of remaining oxygen
Which of these properties is the best one to use for indentification of an element
Answers
Answer:
you need to state the options
Assume you fill a balloon with 0.0500 mol of O2 that has a volume of 1.20 L on a boat where the temperature is 27°C and the pressure is 1.00 atm. You then tie a rock to the balloon and throw it in the ocean. As it sinks, the temperature decreases to 4°C and the volume decreases to 0.153 L. What is the pressure at this depth? (Assume no air can get out of the balloon so the moles stay the same)
Combined Gas Law Example:
________atm (Use 3 Sig Figs)
(50 points)
Answers
The temperature decreases to 4°C and the volume decreases to 0.153 L. Therefore, the pressure at this depth is 804.23 atm.
What do you mean by the term pressure ?The term pressure is defined as a way of measure out how much force is acting over an area. A strong forcing on a small area causes high pressure, while a weak push spread out over a large area creates only a little pressure.
PV = nRT
n = 0.0500 mol
P = ?
V = 1.53 L
T = 296 K
R = gas constant 8.314
By substituting this value in given equation we get,
P = 0.500 × 8.314 × 296 / 1.53
P = 804.23atm
Thus, the pressure at this depth is 804.23 atm.
To learn more about the pressure, follow the link;
brainly.com/question/15175692
#SPJ1
Experiment 4: A chemist mixes aqueous solutions of sodium hydroxide and aluminum chloride in a double-displacement reaction, which forms a white solid precipitate and a clear solution. Write the complete, balanced molecular equation for the reaction. Include physical states.
balanced equation:
Answers
The balanced molecular equation for the reaction between sodium hydroxide (NaOH) and aluminum chloride (\(AlCl_3\)) in aqueous solution can be written as follows: 2NaOH(aq) + 3\(AlCl_3\)(aq) → 3NaCl(aq) + \(Al(OH)_3\)(s)
In this reaction, sodium hydroxide (NaOH) reacts with aluminum chloride (\(AlCl_3\)) to form sodium chloride (NaCl) and aluminum hydroxide (\(Al(OH)_3\)). The coefficients in the balanced equation indicate the stoichiometric ratio between the reactants and products.
The physical states of the substances are indicated by the symbols (aq) for aqueous solutions and (s) for the solid precipitate.
The reaction is a double-displacement reaction, also known as a precipitation reaction. Double-displacement reactions involve the exchange of ions between two compounds, resulting in the formation of a precipitate.
In this case, sodium hydroxide and aluminum chloride react to form sodium chloride and aluminum hydroxide, with aluminum hydroxide being the white solid precipitate.
It's worth noting that the actual reaction might involve hydrated forms of the compounds, such as NaOH·x\(H_2O\) and \(AlCl_3\)·y\(H_2O\). However, for simplicity, these hydrated forms are not included in the balanced equation.
Overall, the balanced equation represents the chemical reaction between sodium hydroxide and aluminum chloride, showing the reactants, products, and their stoichiometric ratios.
For more such question on balanced molecular equation visit:
https://brainly.com/question/11904811
#SPJ8
What would the unit of measurement be for the density of a regular solid?
Answers
Answer:
SI unit: kg/m³
but g/cm³ is commonly used
Explanation:
At a fixed volume, a four-fold increase in the temperature of a gas will lead to _______ in pressure.
Question 2 options:
A)
no change
B)
a two-fold decrease
C)
a four-fold decrease
D)
a four-fold increase
Answers
Answer:
D) a four-fold increase
Explanation:
According to Gay-Lussac's law, which states that the pressure of a given amount of gas is directly proportional to the temperature at a constant volume, the pressure increases with an increase in temperature.
According to this question, at a fixed volume, a four-fold increase in the temperature of a gas will lead to a four-fold increase in the pressure as well.
please help thanks
Balance the following chemical equation (if necessary):
c6h6(l) + o2(g) → h2o(g) + co2(g)
Answers
The balanced equation is:
2C₆H₆ + 15O₂ —> 6H₂O + 12CO₂Chemical equation is simply a representation of chemical reaction with symbols and formula of the reactants and products involved in the reaction.
The balancing of chemical equation is guided by the law of conservation of matter which states that matter can neither be created nor destroyed during a chemical reaction but can be transferred from one for to the other.
Thus, an unbalanced equation simply means that matter has been created or destroyed.
Now, we shall balance the equation given in the question as follow:
C₆H₆ + O₂ —> H₂O + CO₂
There are 6 atoms of H on the left side and 2 atoms on the right side. It can be balance by writing 3 before H₂O as shown below:
C₆H₆ + O₂ —> 3H₂O + CO₂
There are 6 atoms of C on the left side and 1 atom on the right side. It can be balance by writing 6 before CO₂ as shown below:
C₆H₆ + O₂ —> 3H₂O + 6CO₂
There are 2 atoms of O on the left side and a total of 15 atoms on the right side. It can be balance by writing \(\frac{15}{2}\) before O₂ as shown below:
C₆H₆ + \(\frac{15}{2}\)O₂ —> 3H₂O + 6CO₂
Multiply through by 2 to clear the fraction.
2C₆H₆ + 15O₂ —> 6H₂O + 12CO₂Now, the equation is balanced.
Learn more: https://brainly.com/question/6685771
Taking into account the Law of Conservation of Mass, the balanced reaction is:
2 C₆H₆ + 15 O₂ → 6 H₂O + 12 CO₂
In first place, you have to know that the Law of Conservation of Mass postulates that "mass is neither created nor destroyed, it only transforms."
This means that the reagents interact with each other and form new products with different physical and chemical properties than the reagents.
But the amount of matter or mass before and after the chemical reaction is always the same, that is, the amounts of the masses involved in a given reaction must be constant at all times, not changing in their proportions when the reaction ends.
So, since the mass of the reactants must be equal to the mass of the products, the number of atoms present in the reactants must be kept in the products. Therefore, the equation must be balanced.
To balance the chemical equation, you must first look at the subscripts next to each atom to find the number of atoms in the equation. If the same atom appears in more than one molecule, you must add its amounts.
The coefficients located in front of each molecule indicate the amount of each molecule for the reaction. This coefficient can be modified to balance the equation, just as you should never alter the subscripts.
By multiplying the coefficient mentioned by the subscript, you get the amount of each element present in the reaction.
Then, taking into account all of the above, in this case you can determine the amount of elements on each side of the equation:
Left side: 6 C, 6 H and 2 O
Right side: 1 C, 2 H and 3 O
In first place, you can balanced the C:
C₆H₆ + O₂ → H₂O + 6 CO₂
Then, you have:
Left side: 6 C, 6 H and 2 O
Right side: 6 C, 2 H and 13 O
Now, balanced the H:
C₆H₆ + O₂ → 3 H₂O + 6 CO₂
Then, you have:
Left side: 6 C, 6 H and 2 O
Right side: 6 C, 6 H and 15 O
Finally, balanced the O:
C₆H₆ + \(\frac{15}{2}\) O₂ → 3 H₂O + 6 CO₂
Then, you have:
Left side: 6 C, 6 H and 15 O
Right side: 6 C, 6 H and 15 O
The reaction is balanced, but you can't balance a chemical equation with fractions, so multiplying all the stoichiometric coefficients by 2 finally obtains:
2 C₆H₆ + 15 O₂ → 6 H₂O + 12 CO₂
Finally, the balanced reaction is:
2 C₆H₆ + 15 O₂ → 6 H₂O + 12 CO₂
Learn more: https://brainly.com/question/14236219?referrer=searchResults
Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCl would have to be present to form 34.52 g of MgCl₂?
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
Answers
Approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
To determine the number of molecules of HCl required to form 34.52 g of MgCl₂, we need to use the molar mass and stoichiometry of the balanced equation:
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
The molar mass of MgCl₂ is 95.21 g/mol.
First, we need to calculate the number of moles of MgCl₂ formed:
Moles of MgCl₂ = mass of MgCl₂ / molar mass of MgCl₂
Moles of MgCl₂ = 34.52 g / 95.21 g/mol
Moles of MgCl₂ = 0.363 mol
According to the balanced equation, the stoichiometric ratio between HCl and MgCl₂ is 2:1. Therefore, the moles of HCl required can be calculated as follows:
Moles of HCl = 2 * Moles of MgCl₂
Moles of HCl = 2 * 0.363 mol
Moles of HCl = 0.726 mol
To calculate the number of molecules, we need to use Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules of HCl = Moles of HCl * Avogadro's number
Number of molecules of HCl = 0.726 mol * 6.022 x 10^23 molecules/mol
Number of molecules of HCl = 4.37 x 10^23 molecules
Therefore, approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
For more such questions on molecules
https://brainly.com/question/1351818
#SPJ8
what happens to particles when they are heated?
A. STop moving
B.Slow down and compress
C.Move closer together and speed up
D.Speed up and spread out
Answers
Answer:
D. Speed up and spread out
Select the correct answer.
Which phrase correctly describes temperature?
A.
average rotational kinetic energy of the particles in an object
B.
average energy of the particles in an object
C.
average translational kinetic energy of the particles in an object
D.
all energy possessed by the particles in an object
Answers
Answer:
C. average transnational kinetic energy of the particles in an object.
Explanation:
Temperature measures the average kinetic energy of the particles in a substance. Thermal energy measures the total kinetic energy of the particles in a substance. The greater the motion of particles, the higher a substance's temperature and thermal energy. Therefore we could say that the average kinetic energy of a gas particle is directly proportional to the temperature. Hence an increase in temperature increases the speed in which the gas molecules move. All gases at a given temperature have the same average kinetic energy.A student made a sketch of a potential energy diagram to represent an exothermic reaction. Explain, using complete sentences, why the diagram made by the student is correct or incorrect. Be sure to also explain what the values of X and Y represent. (10 points)

Answers
The values of X and Y in the diagram represent the progress of the reaction along the x-axis and the potential energy along the y-axis, respectively.
To determine whether the student's sketch of the potential energy diagram for an exothermic reaction is correct or incorrect, we need to understand the characteristics of such a reaction. In an exothermic reaction, the reactants have a higher energy level than the products, and energy is released during the reaction.The potential energy diagram should have the reactants positioned at a higher energy level (y-axis) than the products. As the reaction progresses from reactants to products, the potential energy should decrease, indicating the release of energy. This decrease in energy should be evident in the form of a downward slope or a curve.If the student's sketch accurately depicts these characteristics, with the reactants at a higher energy level than the products and a downward slope or curve indicating energy release, then the diagram is correct. However, if the student's sketch shows the reactants at a lower energy level or depicts an upward slope, it would be incorrect.The x-axis typically represents the reaction coordinate, which can be the extent of the reaction or the progression of time.
The y-axis represents the potential energy, which can be measured in joules or any other energy unit. The specific values on the axes will depend on the reaction and the units chosen.
for such more questions on energy
https://brainly.com/question/5650115
#SPJ8
For the following questions, use a periodic table and your atomic calculations to find the unknown information about each isotope:
You have a Helium Isotope with 2 neutrons. What is the mass number?
2
4
4.0026
6
Answers
Answer:
Option B is correct.
4
Explanation:
We know that an atom consist of electron, protons and neutrons. Protons and neutrons are present with in nucleus while the electrons are present out side the nucleus.
All these three subatomic particles construct an atom. A neutral atom have equal number of proton and electron. In other words we can say that negative and positive charges are equal in magnitude and cancel the each other. For example, if neutral atom has 6 protons than it must have 6 electrons. The sum of neutrons and protons is the mass number of an atom while the number of protons are number of electrons is the atomic number of an atom.
In given problem we are given with 2 neutrons of helium. We know that the atomic number of He is 2. Thus Mass number of He is,
Number of neutrons + number of proton
2 + 2 = 4
Thus, option B is correct.
In each of the following equations identify the acid and the base for the reactants
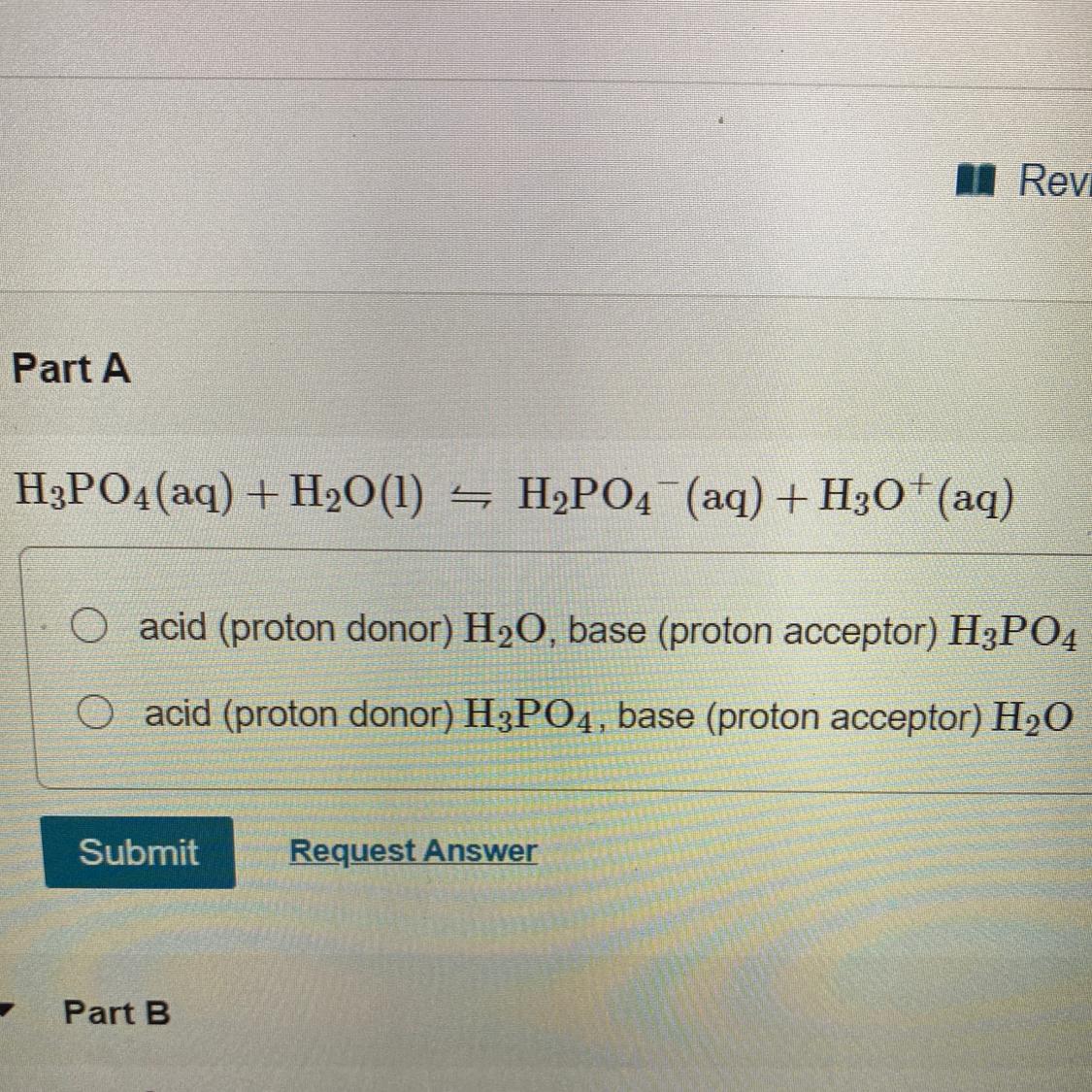
Answers
Answer:
B
Acid H3PO4 And base H2O
Explanation:
Starting with 2.50 mol of N2 gas (assumed to be ideal) in a cylinder at 1.00 atm and 20.0C, a chemist first heats the gas at constant volume, adding 1.36 * 104 J of heat, then continues heating and allows the gas to expand at constant pressure to twice its original volume. Calculate (a) the final temperature of the gas; (b) the amount of work done by the gas; (c) the amount of heat added to the gas while it was expanding; (d) the change in internal energy of the gas for the whole process.
Answers
Answer:
a) \(T_b=590.775k\)
b) \(W_t=1.08*10^4J\)
d) \(Q=3.778*10^4J\)
d) \(\triangle V=4.058*10^4J\)
Explanation:
From the question we are told that:
Moles of N2 \(n=2.50\)
Atmospheric pressure \(P=100atm\)
Temperature \(t=20 \textdegree C\)
\(t = 20+273\)
\(t = 293k\)
Initial heat \(Q=1.36 * 10^4 J\)
a)
Generally the equation for change in temperature is mathematically given by
\(\triangle T=\frac{Q}{N*C_v}\)
Where
\(C_v=Heat\ Capacity \approx 20.76 J/mol/K\)
\(T_b-T_a=\frac{1.36 * 10^4 J}{2.5*20.76 }\)
\(T_b-293k=297.775\)
\(T_b=590.775k\)
b)
Generally the equation for ideal gas is mathematically given by
\(PV=nRT\)
For v double
\(T_c=2*590.775k\)
\(T_c=1181.55k\)
Therefore
\(PV=Wbc\)
\(Wbc=(2.20)(8.314)(1181_590.778)\)
\(Wbc=10805.7J\)
Total Work-done \(W_t\)
\(W_t=Wab+Wbc\)
\(W_t=0+1.08*10^4\)
\(W_t=1.08*10^4J\)
c)
Generally the equation for amount of heat added is mathematically given by
\(Q=nC_p\triangle T\)
\(Q=2.20*2907*(1181.55-590.775)\\\)
\(Q=3.778*10^4J\)
d)
Generally the equation for change in internal energy of the gas is mathematically given by
\(\triangle V=nC_v \triangle T\)
\(\triangle V=2.20*20.76*(1181.55-293)k\)
\(\triangle V=4.058*10^4J\)
The pressure inside a tire is measured as 28.0 pounds/inches^2. What is its pressure in newtons/centimeters^2
Answers
The pressure inside the tire is approximately 1.970796 newtons per square centimeter (N/cm²) when measured in those units.
To convert the pressure from pounds per square inch (psi) to newtons per square centimeter (N/cm²), we need to use the conversion factors between these units.
First, let's convert pounds to newtons:
1 pound = 0.45359237 kilograms
1 kilogram = 9.80665 newtons
Next, let's convert square inches to square centimeters:
1 square inch = 6.4516 square centimeters
Now, we can perform the conversion:
1 psi = (0.45359237 kg) × (9.80665 N/kg) / (6.4516 cm²)
≈ 0.070307 N/cm²
Therefore, the pressure inside the tire of 28.0 psi is approximately equal to 28.0 × 0.070307 N/cm², which is approximately 1.970796 N/cm².
For more question on pressure
https://brainly.com/question/28012687
#SPJ8
the inner part of the ovary that contains an egg is the ovule
true or false
Answers
Answer:
The answer is true
Explanation:
the ovary is a tubular structure and contains ovules
12. Which two elements have chemical properties that are most
similar?
A.C and N
B. Cl and Ar
C.K and Ca
D.Li and Na
Answers
Li and Na two elements have chemical properties that are most similar
The element that have the most similar chemical properties are those in the same group or column of the periodic table and one such group includes lithium sodium potassium these element are all shiny and conduct heat and electricity well and have similar chemical properties and the element in a group have the same number of electron in the outermost shell hence they have similar chemical properties
Know more about element
https://brainly.com/question/491273
#SPJ1
1. During an investigation, a student measured the amount of space various objects take up. Which property of matter was the student investigating?
volume
mass
weight
density
Answers
Consider the reaction below P4(g) + 6 Cl2(g) → 4 PCl3(g) If 0.351 moles of P4 is mixed with 1.17 moles of Cl2 in a 1.12-L rigid reaction vessel, what is the final pressure in the vessel after the reaction goes to completion? The final temperature is 299. °C. Assume 100% yield.
Answers
The pressure of the gas is now obtained as 32.7 atm.
What is the final pressure?We know that the reaction has been shown in the question and we have been asked to be able to obtain the final pressure of the system. We need to obtain the limiting reactant so that we can be able to get the amount of the product that is formed.
If 1 mole of phosphorus reacts with 6 moles of chlorine
0.351 moles of phosphorus reacts with 0.351 moles * 6 moles/1 mole
= 2.1 moles
Thus chlorine is the limiting reactant.
6 moles of chlorine produces 4 moles of phosphorus trichloride
1.17 moles of chlorine produces 1.17 moles * 4 moles/6 moles
= 0.78 moles
We would now apply the ideal gas equation;
PV = nRT
P = pressure
V = volume
n = Number of moles
R = gas constant
T = temperature
Then
P = nRT/V
P = 0.78 * 0.082 * 572/1.12
P = 32.7 atm
Learn more about pressure:https://brainly.com/question/18124975
#SPJ1
what is an example of reactivity?
Answers
What is the pH of a 0.045 M H2SO4 solution?
Answers
Answer:
pH = 1.05
Explanation:
A solution of sulfuric acid, H₂SO₄, dissociates in water as follows:
H₂SO₄ → 2H⁺ + SO₄²⁻
That means per mole of H₂SO₄ you will have 2 moles of H⁺
pH = -log [H⁺]
As [H₂SO₄] = 0.045M and [H⁺] = 2×[H₂SO₄]
[H⁺] = 0.090M
pH = -log [0.090M]
pH = 1.05Question on the image. PLS HELP

Answers
Answer:
water
Explanation:
specific heat means the quantity of heat required to raise the temperature of one gram of a substance by one Celsius degree.
Discuss the large-scale environmental impacts of soil pollution caused by industrial wastes.
Answers
Answer: Industrial processes including mining and manufacturing historically have been leading causes of soil pollution. Industrial areas typically have much higher levels of trace elements and organic contaminants. This is due to intentional and unintentional releases from industrial processes directly into the environment, including to the soil, adjacent water bodies, and the atmosphere.
Explanation:
Where in the laboratory is long, unrestrained hair most likely to be a safety concern? near an open flame close to a fume hood next to a concentrated sodium chloride solution by a cabinet that contains flammable liquids
Answers
Answer:
near an open flame
Explanation:
if your hair is not tied back near open flames in combination with most hair care products it would turn you into a living torch
Long, unrestrained hair is most likely to be a safety concern near an open flame in the laboratory and the correct option is option 1.
The open flame poses a direct risk of igniting loose hair, potentially causing burns or fires. In a laboratory setting, there are often various sources of open flames, such as Bunsen burners, gas stoves, or torches.
The heat and proximity of these flames increase the likelihood of hair coming into contact and catching fire. To prevent accidents, it is essential to follow proper safety protocols and secure long hair by tying it back or using hair restraints when working in areas with open flames. This precaution helps minimize the risk of personal injury and maintains a safe working environment.
Thus, the ideal selection is option 1.
Learn more about Laboratory safety, here:
https://brainly.com/question/30450948
#SPJ6
A student makes a model of a mineral crystal. The scale is 1:24. The crystal in the model is 5 cm long. How long Is the real-life crystal. A. 120cm B. 0.96cm C. 4.8cm D. 29cm
Answers
The length of the real-life crystal is 120 cm which is 24 times longer than the length of the crystal in the model.
How long Is the real-life mineral crystal?Given that, a student makes a model of a mineral crystal.
The scale is 1:24. The crystal in the model is 5 cm long.
We are given a scale of 1:24, which means that every unit in the model represents 24 units in real life.
To find the length of the real-life crystal, we need to use the scale factor of 24.
We can set up a proportion to solve for the real-life length of the crystal:
1 unit in the model : 24 units in real life
5 cm in the model : x cm in real life
Using cross-multiplication, we get:
1 × x = 24 × 5
x = 24 × 5
x = 120cm
Therefore, the real-life crystal measures 120cm.
Option A) 120cm is the correct answer.
Learn more about scale factor here: https://brainly.com/question/29464385
#SPJ1
Benzene can be converted to 1,3,5-tribromobenzene in five reaction steps and four intermediate compounds. Select the appropriate reagent from the followings.
Br2, R2O2
CH3Cl, AlCl3
CH3COCl, AlCl3
NaNO2, HCl
HNO3, H2SO4
H3PO2
H3PO4
KMnO4
Answers
Answer:
The appropriate reagent is: H3PO2.
Explanation:
H3PO2 is in charge of eliminating the amino group by diazotization, remember that the amino group had previously achieved bromination at positions m; that is to say that it achieved in the beginning that the three bromine atoms of 1,2,4 tribromobenzene were introduced in the meta positions among themselves, which finally corresponds as part of the last reaction to the 1,3,5-tribromobenzene position.
Calculate the mole fraction of Ba Cl 2 in an aqueous solution prepared by dissolving 0.400 moles of Ba Cl 2 in 850.0 g of water.
Answers
Answer:
0.0084
Explanation:
The mole fraction of BaCl₂ (X) is calculated as follows:
X = moles BaCl₂/total moles of solution
Given:
moles of BaCl₂ = 0.400 moles
mass of water = 850.0 g
We have to convert the mass of water to moles, by using the molecular weight of water (Mw):
Mw of water (H₂O) = (2 x 1 g/mol)+ 16 g/mol = 18 g/mol
moles of water = mass of water/Mw of water = 850.0 g/(18 g/mol) = 47.2 mol
The total moles of the solution is given by the addition of the moles of solute (BaCl₂) and the moles of solvent (water):
total moles of solution = moles of BaCl₂ + moles of water = 0.400 + 47.2 mol = 47.6 mol
Finally, we calculate the mole fraction:
X = 0.400 mol/47.6 mol = 0.0084
A physical change produces a new element, a chemical change results from breaking bonds between atoms. A physical change results in a new compound being formed; a chemical change results in the formation of a new element. A physical change results in a new compound being formed; a chemical change results in the formation of a new element. A physical change results in a new substance being formed, which is different than what results from a chemical change. A physical change results in a new substance being formed, which is different than what results from a chemical change. A physical change is a change from one state of matter to another; a chemical change results in the formation of a new substance.
Answers
A physical change is a change from one state of matter to another; a chemical change results in the formation of a new substance.
What is Physical and Chemical change ?Examples of physical changes include changes in the composition or size of materials. Physical changes include changes from one state to another, for as going from a solid to a liquid or a liquid to a gas. Some of the processes that result in physical changes are cutting, bending, dissolving, freezing, boiling, and melting.
Chemical changes are those in which one or more new compounds are created. A chemical reaction is another name for a chemical change. Examples include the burning of any substance and the corrosion of iron.Chemical synthesis, or, alternatively, chemical breakdown into two or more separate molecules, occurs when one material reacts with another to create a new substance. These processes are referred to as chemical reactions, and they are typically irreversible barring additional chemical reactions.Learn more about Physical and Chemical change here:
https://brainly.com/question/17166994
#SPJ9