Answers
Answer:
\(ns^2\)
Explanation:
Related Questions
The pH of a solution of Ca(OH)2 is 8.57. Find the [Ca(OH)2]. Be careful, the fact that this base produces 2 OH- is important!
Answers
The concentration of Ca(OH)2 in the solution is approximately 1.33 x 10^(-6) M.
To find the concentration of Ca(OH)2 in a solution with a pH of 8.57, we need to use the concept of pOH, which is the negative logarithm of the hydroxide ion concentration ([OH-]). The pOH can be calculated by subtracting the pH from 14, which gives us 14 - 8.57 = 5.43.
Since Ca(OH)2 produces two OH- ions for every molecule of Ca(OH)2 that dissolves, the concentration of OH- ions will be twice the concentration of Ca(OH)2. Thus, we have [OH-] = 2x, where x represents the concentration of Ca(OH)2.
Taking the antilogarithm of the pOH, we find that [OH-] = 10^(-pOH) = 10^(-5.43).
Since [OH-] = 2x, we can write 2x = 10^(-5.43) and solve for x.
x = (10^(-5.43))/2 ≈ 1.33 x 10^(-6) M
For more such questions on Ca(OH)2
https://brainly.com/question/31035177
#SPJ8
Which of the following regulates food labels? HACCP FSIS FDA EPA
Answers
Answer:
FDA
Explanation:
Answer:
FDA
Explanation: it right ;)
how do atoms show both unity and diversity
Answers
Answer: As one atomic dipole nears another atom it will affect the electron density distribution, so for example if the slightly positive end of the atom is located next to another atom, it will attract the electron(s) in the other atom. A covalent bond happens when atoms share an electron between them. The other kind of bond is called ionic, and it happens when one atom gives an electron to another atom. These bonds are held together with electric forces. Two atoms of the same element can be different if their electrons are in different states. If one copper atom has an electron in an excited state and another copper atom has all of its electrons in the ground state, then the two atoms are different.
Answer:
there are relatively few atoms but they can make many molecules
Explanation:
Which ball has the most energy?
A ball held at 2 meters.
A ball held at 3 meters.
A ball held at 1 meter.
A ball resting on the ground.
tory
Answers
A ball held at 3 metres
Explanation:
Because it is in motion.
1.
Which description accurately compares mass and weight?
A Mass indicates the density of an object, while weight indicates its volume.
B. O Mass indicates the volume of an object, while weight indicates the pull of gravity on an object.
Mass indicates how much matter an object has, while weight indicates the pull of gravity on a
Mass indicates the pull of gravity on an object, while weight indicates the amount of matter ar
D
Answers
Answer:
B
Explanation:
how many chloride ions (cl-1) are needed to balance the positive charge of a barium ion (ba+2)?
Answers
To balance the positive charge of a barium ion (Ba+2), 2 chloride ions (Cl-1) are needed.
In order to balance the positive charge of a barium ion (Ba+2), we need to determine the number of chloride ions (Cl-1) required to neutralize the overall charge. Barium ion (Ba+2) has a positive charge of +2, while a chloride ion (Cl-1) has a negative charge of -1. To balance the charges, we can use the following equation:
(Number of Cl-1 ions) x (Charge of Cl-1 ion) = (Charge of Ba+2 ion)
Let's plug in the values and solve for the number of chloride ions:
(Number of Cl-1 ions) x (-1) = +2
To find the number of chloride ions needed, simply divide the charge of the barium ion by the absolute value of the charge of a chloride ion:
Number of Cl-1 ions = (+2) / (|-1|)
Number of Cl-1 ions = 2
To learn more about barium, visit:
https://brainly.com/question/11040331
#SPJ11
Calculate the chemical shift in ppm of a proton if it's resonance frequency is 147 Hz downfield from TMS in an NMR operating at 80 MHz.
Answers
The chemical shift of the proton, given its resonance frequency of 147 Hz downfield from TMS in an NMR operating at 80 MHz, is approximately -999,998.16 ppm.
To calculate the chemical shift in parts per million (ppm) of a proton, given its resonance frequency downfield from TMS (Tetramethylsilane) in an NMR operating at 80 MHz, we can use the following formula:
Chemical Shift (in ppm) = (Resonance Frequency - Reference Frequency) / Reference Frequency
In this case, the resonance frequency is given as 147 Hz downfield from TMS, and the reference frequency is 80 MHz.
First, we need to convert the reference frequency from MHz to Hz:
Reference Frequency = 80 MHz * 10^6 Hz/MHz
Reference Frequency = 80,000,000 Hz
Now we can calculate the chemical shift in ppm:
Chemical Shift = (147 Hz - 80,000,000 Hz) / 80,000,000 Hz
Chemical Shift = -79,999,853 Hz / 80,000,000 Hz
Chemical Shift ≈ -0.99999816
Finally, we can convert the chemical shift to ppm by multiplying by 10^6:
Chemical Shift (in ppm) = -0.99999816 * 10^6
Chemical Shift (in ppm) ≈ -999,998.16 ppm
Therefore, the chemical shift of the proton is approximately -999,998.16 ppm.
The chemical shift of the proton, given its resonance frequency of 147 Hz downfield from TMS in an NMR operating at 80 MHz, is approximately -999,998.16 ppm.
To know more about chemical shift, visit:
https://brainly.com/question/8171972
#SPJ11
The fuel used to power the booster rockets on space shuttles is a mixture of aluminum metal and ammonium perchlorate. the following balanced equation represents the reaction. 3al + 3nh4clo4 → al2o3 + alcl3 + 3no + 6h2o what is the mole ratio of al to al2o3?al:al2o3 = 3:
Answers
Pour trouver le nombre de moles d'aluminium, nous devons d'abord trouver le nombre d'atomes d'aluminium. Nous le faisons en multipliant le nombre d'atomes d'aluminium par l'équation chimique donnée. 3al * 1 = 3 atomes d'aluminium.
Nous pouvons maintenant trouver le nombre de moles d'aluminium en divisant le nombre d'atomes d'aluminium par le nombre d'Avogadro (6,02 x 10^23). 3atomes d'aluminium/6,02x10^23= 5,00 x 10^-23 moles d'aluminium.
Nous devons maintenant trouver le nombre de moles d'oxyde d'aluminium. Pour ce faire, nous devons trouver le nombre d'atomes d'oxygène et le multiplier par 2 pour obtenir le nombre d'atomes d'oxyde d'aluminium. Nous pouvons le faire en multipliant le nombre d'atomes de perchlorate d'ammonium par l'équation chimique donnée. 3NH4ClO4 * 4 = 12 atomes d'oxygène
Nous pouvons maintenant trouver le nombre de moles d'oxyde d'aluminium en divisant le nombre d'atomes d'oxygène par le nombre d'Avogadro (6,02 x 10^23). 12atomes d'oxygène/6,02x10^23= 2,00 x 10^-22 moles d'oxyde d'aluminium.
Maintenant que nous avons trouvé le nombre de moles d'aluminium et d'oxyde d'aluminium, nous pouvons trouver le rapport molaire de l'aluminium à l'oxyde d'aluminium. Nous le faisons en divisant le nombre de moles d'aluminium par le nombre de moles d'oxyde d'aluminium. 5,00 x 10^-23/2,00 x 10^-22= 2,50 x 10^-1
Le rapport molaire de l'aluminium à l'oxyde d'aluminium est donc de 2,50 x 10^-1.
Explanation:
Answer: Al:Al2O3 = 3: 1
Explanation:
the answer is 1
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11
On a hot day, a student places a glass of cold lemonade on a table outdoors. After a few minutes, water droplets have formed on the outside of the glass.
Is energy absorbed or released by the cold lemonade? Explain your answer.
Compare average kinetic energy for the air molecules and lemonade molecules when the student first places the lemonade outdoors. Explain your answer.
Explain how and why the water droplets form on the outside of the glass.
Answers
Answer:
ye but sdfajkd tryng to play me
Explanation:
Answer:
Vapor is released out of the lemonade because when you place it in a hot it will increase.
Explanation:
What nucleophile could be used to react with butyl iodide to prepare the compound shown?.
Answers
The nucleophile used to react with butyl iodide to form te compound below is :
CH₃CH₂O⁻What is a NucleophileA Nucleophile is a molecule in a chemical reaction that seeks for a positive center of the reaction ( i.e. nucleus ) and this is because of the presence of a electron pair in the Nucleophile.
Given the compound expressed in the question the chemical reaction that takes place between the nucleophile and butyl iodide is called Nucleophilic substitution reaction.
Hence we can conclude that the nucleophile that could be used to react with butyl iodide is CH₃CH₂O⁻
Learn more about Nucleophile : https://brainly.com/question/9126498
Attached below is the complete question
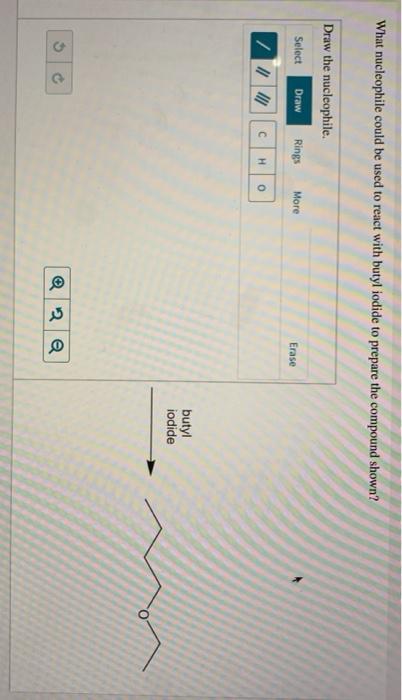
I need help with this question

Answers
Answer:
Liquid; melting.
Explanation:
So what is being shown is a phase change diagram. Because we start at an atm of 1.5 (staying steady means that it doesn't drop so we always stay at 1.5 atm) and at a temperature of -150, the original state of matter is a solid. Next, we look at what happens when the temperature goes to 300 degrees, yet still maintaining the 1.5 atm. This means that it now turns into a liquid (based on placement on diagram). This means the matter after the temperature change was a liquid. And going from solid to liquid would be called melting.
Here's an example phase diagram so that you can rely on it in the future problems!

Jen collected 1.05 g Na2CO3 by decomposing 2.00 g NaHCO3 but she should have collected more. What is the percent yield of Jens experiment ?
Answers
The percent yield of Jen's experiment is 100%.
To calculate the percent yield of Jen's experiment, we need to compare the actual yield (the amount of Na2CO3 she collected) to the theoretical yield (the amount of Na2CO3 that should have been produced based on the starting amount of NaHCO3).
The balanced equation for the decomposition of NaHCO3 is:
2 NaHCO3 -> Na2CO3 + H2O + CO2
According to the equation, 2 moles of NaHCO3 should produce 1 mole of Na2CO3. We can use the molar mass of NaHCO3 (84.01 g/mol) and Na2CO3 (105.99 g/mol) to calculate the theoretical yield.
The theoretical yield of Na2CO3 can be calculated as:
Theoretical yield = (mass of NaHCO3) x (1 mol Na2CO3 / 2 mol NaHCO3) x (molar mass of Na2CO3)
Theoretical yield = (2.00 g) x (1 mol Na2CO3 / 2 mol NaHCO3) x (105.99 g/mol Na2CO3)
Theoretical yield = 1.05 g
Since the actual yield is also 1.05 g, the percent yield can be calculated as:
Percent yield = (actual yield / theoretical yield) x 100
Percent yield = (1.05 g / 1.05 g) x 100
Percent yield = 100%
Therefore, the percent yield of Jen's experiment is 100%.
To know more about percent yield click this link -
brainly.com/question/17042787
#SPJ11
4) How much of the world population lives in countries where there isn't enough water, or
the quality has been compromised?
Answers
Answer:
The answer is 1/3 of the population.
Fill in the table with the correct number of each subatomic particle for the elements given the isotope mass number. (12 pts)
Answers
You can fill in the table for other elements and their respective isotopes by determining the appropriate number of protons, neutrons, and electrons based on the given isotope mass number and atomic number.
To accurately fill in the table with the correct number of subatomic particles for the elements given the isotope mass number, we need to consider the composition of atoms and their respective subatomic particles. Atoms are composed of protons, neutrons, and electrons.
The number of protons in an atom is equivalent to its atomic number, which uniquely identifies the element. The number of neutrons can be determined by subtracting the atomic number from the isotope mass number. Electrons in a neutral atom are equal to the number of protons.
Let's take an example using the isotope mass number:
Isotope: Carbon-14 (mass number = 14)
Element: Carbon (atomic number = 6)Based on the atomic number and isotope mass number, we can determine the number of subatomic particles as follows:
Protons: 6 (same as the atomic number)
Neutrons: 14 - 6 = 8
Electrons: 6 (same as the number of protons in a neutral atom)
For morew such questions on elements visit:
https://brainly.com/question/18096867
#SPJ8
6. Define What is a
planetesimal?
Answers
brine (saturated aqueous nacl solution) is often used in extraction procedures. the concentration of a saturated brine at room temperature is 358 g of nacl in 1.00 l of water. calculate the molarity and density of saturated brine.
Answers
The molarity and the density of the saturated Brine solution is 6.12M and 1.358Kg/L respectively.
The brine solution is composed of water and NaCl.
The volume of water is 1 liter and 358 grams is the mass of NaCl.
We know,
Molarity = Moles/volume
Moles of NaCl = Mass of Nacl/Molar mass of NaCl
Mass of NaCl = 358
Molar mass of NaCl = 58.44 g/mol
Moles of NaCl = 358/58.44
Moles of NaCl = 6.12
Molarity of NaCl = 6.12/1
Molarity of NaCl = 6.12M.
We know, the mass of one liter water is one Kg.
Hence, total mass of the solution = 1+0.358
Mass of solution = 1.358 kg.
The volume of the solution is 1 leiter.
Density = mass/volume
Density = 1.385/1
Density = 1.358kg/l
Hence, the density of the solution is 1.358 kg/L and molarity of the solution is 6.12 M.
To know more about Molarity, visit,
https://brainly.com/question/14469428
#SPJ4
3. Which of the following elements is more reactive than potassium, K?
a. Sodium, Na
b. Copper, Cu
C. Vanadium, V
d. Caesium, Cs
Answers
Answer:
d. Caesium
Explanation:
Actually Rubidium is more reactive than potassium but since it is not in the options given, Caesium is the next most reactive
Can someone please help me with the last column!! ASAP

Answers
The ratio of the volume and temperature of the gas in the given table is as follows:
0.72/276 = 0.002620.78/294 = 0.002650.84/313 = 0.002680.87/330 = 0.002630.93/355 = 0.002620.98/371 = 0.00264What is the relationship between the volume and temperature of a gas?Charles's law, also known as the law of volumes, describes the relationship between the volume and temperature of a gas at a constant pressure. According to this law, the volume of a gas is directly proportional to its absolute temperature (measured in Kelvin) when the pressure is constant.
In other words, as the temperature of a gas increases, its volume will also increase proportionally, and vice versa. Mathematically, Charles's law can be expressed as:
V/T = k
where V is the volume of the gas, T is its temperature in Kelvin, and k is a constant of proportionality.
Learn more about the volume and temperature of a gas at: https://brainly.com/question/17100204
#SPJ1
electron affinity and ionization energy do not rigorously adhere effective nuclear charge arguments because:
Answers
The capacity of an atom to absorb electrons can be indicated by its electron affinity. An atom gains electrons more readily the lower its first electron affinity. The ability of an atom to gain electrons is weaker the higher the electron affinity. The ionization energy demonstrates an atom's capacity to lose electrons.
What is effective nuclear charge ?
The attractive positive charge of nuclear protons acting on valence electrons is known as effective nuclear charge. Due to the shielding effect, the effective nuclear charge is always smaller than the total amount of protons in a nucleus.
To know about effective nuclear charge from the link
https://brainly.com/question/13647434
#SPJ4
summarize the first step in drawing lewis dot structures, including deciding which atom will be in the central position,
Answers
Most electronegative atom will always lie in the central position.
What are the steps in drawing Lewis dot structures:Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram.Step 2. Place least electronegative element in given compound, in center and draw single bonds from the central atom to other atoms.Step 3. Determine how many electrons must be added to central element.Step 4. Add double or triple bonds to central atom until it has a full octetStep 5. Add electrons to outer elements until they have full octets.
For example: Ammonia (NH3)
Learn more about some more Lewis dot structure here: https://brainly.com/question/1407731
#SPJ4

Substance A reacts spontaneously with substance B at room temperature. Substance A reacts with substance C at room temperature only in the presence of a catalyst. Which statement best explains this difference?
Answers
Between A and B
The reaction is spontaneousMeans reaction is happening by itself.So randomness of A and B is increasing .The highest probability is that A and B are solids and they are forming liquid or gases.
Between A and C
The reaction is non spontaneousRandomness of particles is decreasing.Highest probability is that A and C are gases and they are turning into liquid or solid
40 points pls help me
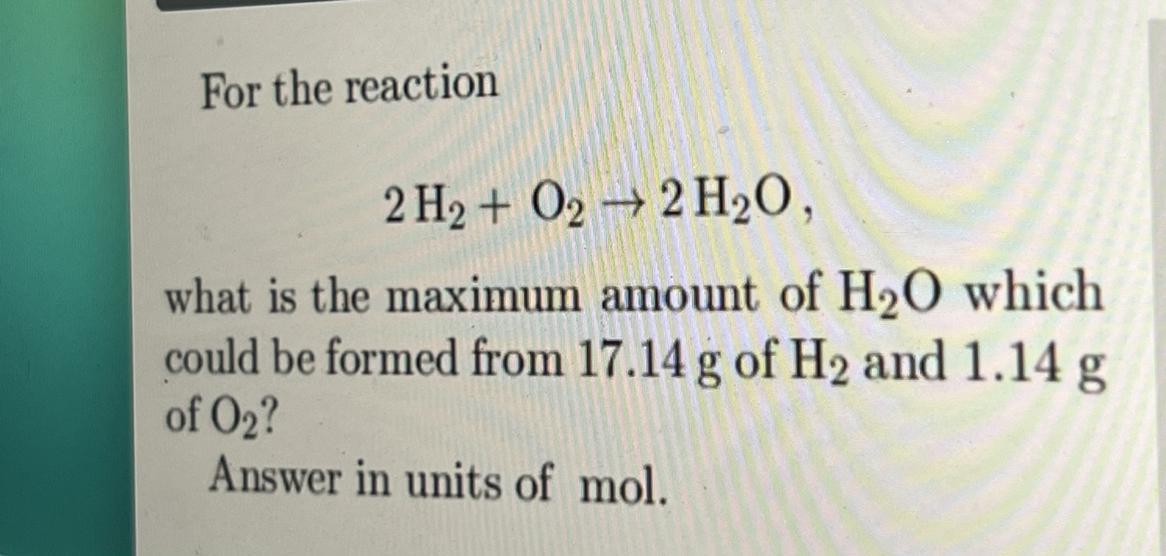
Answers
Answer:
H2O 2
Explanation:
H2O because 2 H2 + O2--- 2 H2O. 17.14 g of H2 and 1.14 g of O2= H2O
Kg/L ? Determine the mass of salt in the tank after tmin. Mass =kg When will the concentration of salt in the tank reach 0. 03 kg/L ? The concentration of salt in the tank will reach 0. 03 kg/L after minutes. (Round to two decimal places as needed. ) A cold drink initially at 35
∘
F warms up to 38
∘
F in 3 min while sitting in a room of temperature 72
∘
F. How warm will the drink be if left out for 30 min? If the drink is left out for 30 min, it will be about
∘
F. (Round to the nearest tenth as neede
Answers
The mass of salt in the tank after t minutes can be determined using the formula: Mass of salt = concentration of salt × volume of solution.
To find the mass of salt in the tank after t minutes, we need to multiply the concentration of salt by the volume of the solution. The concentration of salt is given as 0.03 kg/L, and the volume of the solution is given as kg. Therefore, the mass of salt can be calculated by multiplying 0.03 kg/L by kg.
The concentration of salt in the tank will reach 0.03 kg/L after minutes. This means that at this time, the amount of salt in the tank will be such that if it is divided by the volume of the solution, it will equal 0.03 kg/L. To calculate the time it takes for the concentration of salt to reach 0.03 kg/L, we need to divide the mass of salt by the volume of the solution and then multiply it by 1/L. This will give us the time in minutes.
In the second part of the question, a cold drink initially at 35°F warms up to 38°F in 3 minutes while sitting in a room of temperature 72°F. To find how warm the drink will be if left out for 30 minutes, we need to consider the rate at which the drink warms up and the time it is left out.
learn more about mass of salt
https://brainly.com/question/8896558
#SPJ11
what happens when there is more kinetic energy?
Answers
Energy that a moving object has due to its motion is Kinetic Energy. Kinetic Energy: ... The faster an object moves, the more kinetic energy it has. The more mass an object has, the more kinetic energy it has.
Answer:
when there is more kinetic energy the objects moves fast...
Which of the following contains the same number of electrons as an atom of neon?
Cl-
Li
Li+
02
Answers
Answer:
D. \( O^{2-}\)
Explanation:
In Chemistry, electrons can be defined as subatomic particles that are negatively charged and as such has a magnitude of -1.
Valence electrons can be defined as the number of electrons present in the outermost shell of an atom. Valence electrons are used to determine whether an atom or group of elements found in a periodic table can bond with others. Thus, this property is typically used to determine the chemical properties of elements.
Oxygen has a total number of eight (8) electrons and as such the \( O^{2-}\) is able to gain (receive) two (2) more electrons in order to have the same electron arrangements as the noble gas i.e an atom of neon that has a total number of ten (10) electrons.
Hence, \( O^{2-}\) contains the same number of electrons as an atom of neon.
X
What is the electron configuration for an atom of polonium (Po-209) at ground state?
A
[Xe]6s25d106p4
B [Xe]6s24f¹45d¹06p4
[Xe]6s26f146d106p4
[Xe]6s²6d¹06p4
Answers
The ground state electron configuration of ground state gaseous neutral polonium is [Xe]. 4f14. 5d10.
The electron configuration of an element describes how electrons are disbursed in its atomic orbitals. Electron configurations of atoms observe a well-known notation wherein all electron-containing atomic subshells are positioned in a sequence.
Eighty four electrons successively occupy to be had electron shells (rings). Polonium is a radioactive metalloid in organization 16, length 6, and the p-block of the periodic table. Discovered in 1898 through the Curies, it turned into named after Poland. Polonium-209 has a half-lifestyles of 103 years.
The symbols used for writing the electron configuration begin with the shell wide variety (n) observed through the kind of orbital and in the end the superscript suggests what number of electrons are withinside the orbital. For example: Looking on the periodic table, you may see that Oxygen has eight electrons.
Learn more about electronic configuration here https://brainly.com/question/26084288
#SPJ9
Which correctly lists three forms of frozen water?
dew, frost, rain
frost, hail, sleet
hail, rain, sleet
rain, dew, frost
Answers
Answer:
frost, hail, sleet
Explanation:
Answer:
its the second answer
Explanation:
Find an equation for the perpendicular bisector of the line segment whose endpoints are (9,3) and (−3,−9)
Answers
The equation for the perpendicular bisector of the line segment whose endpoints are (9,3) and (−3,−9) = y = -x.
To find an equation for the perpendicular bisector of the line segment whose endpoints are (9,3) and (−3,−9), we will first find the midpoint of the line segment and then the slope of the perpendicular bisector.
The midpoint of the line segment can be found using the formula:
Midpoint = [(x₁ + x₂)/2 , (y₁ + y₂)/2]
= [(9 + (-3))/2 , (3 + (-9))/2]
= [(6)/2 , (-6)/2]
= (3 , -3)
The slope of the line segment can be found using the formula:
Slope = (y₂ - y₁)/(x₂ - x₁)
= ((-9) - 3)/((-3) - 9)
= (-12)/(-12)
= 1
The slope of the perpendicular bisector will be the negative reciprocal of the slope of the line segment, which is -1.
Using the point-slope form of a line, the equation for the perpendicular bisector can be found:
y - y₁ = m(x - x₁)
y - (-3) = -1(x - 3)
y + 3 = -x + 3
y = -x
Therefore, the equation for the perpendicular bisector of the line segment whose endpoints are (9,3) and (−3,−9) is y = -x.
Learn more about perpendicular bisector here: https://brainly.com/question/11541769
#SPJ11
Explain The process of a pot of boiling water left for to long drying up.
Answers
Answer:Evaporation
Explanation:
