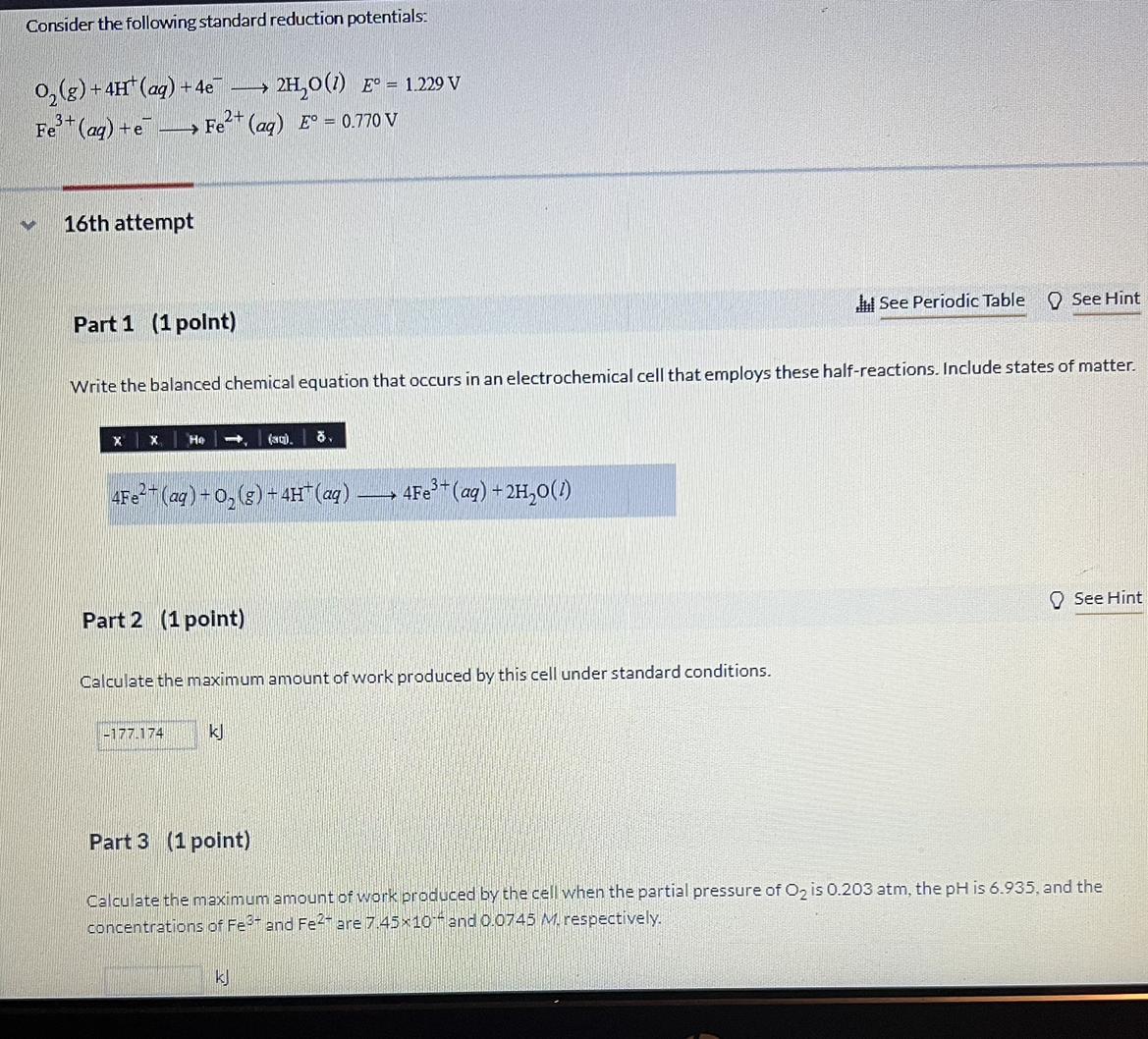
Answers
The maximum amount of work produced by the cell is 0.31 coulombs, or 310 joules.
What is work?Work in physics is the transfer of energy from one object to another. It can be described as a force acting on an object over a distance, resulting in a change of energy of the object. Work is typically measured in joules (J). Work can be done by an applied force either pushing or pulling on an object to cause a displacement, or by a force causing a rotation or a change in the object’s shape. Work can also be done by gravity, in which an object is moved along the direction of a gravitational field. In all cases, work results in the object experiencing a change in energy.
The maximum amount of work produced by the cell can be calculated using the Nernst equation. The Nernst equation is used to calculate the voltage of a cell, and the amount of work produced by the cell is equal to the voltage multiplied by the total charge that passes through the cell.
The Nernst equation is given by:
E = E° - (RT/nF) ln Q
Where E is the voltage of the cell, E° is the standard potential of the cell, R is the ideal gas constant, T is the temperature in Kelvin, n is the number of electrons transferred in the reaction, F is the Faraday constant and Q is the reaction quotient.
For the given reaction, the standard potential is 0.77 V, the number of electrons transferred is 2, and the reaction quotient is the product of the partial pressure of oxygen and the activity of Fe2+, which is given by:
Q = (0.203 atm)(0.0745 M) = 0.01514
Therefore, the voltage of the cell is given by:
E = 0.77 - (8.314 J/molK)(298 K)/(2)(96485 C/mol) ln 0.01514 = 0.31 V
The maximum amount of work produced by the cell can then be calculated as the voltage multiplied by the total charge that passes through the cell. Assuming that the total charge is 1 coulomb, the maximum amount of work produced by the cell is 0.31 coulombs, or 310 joules.
To learn more about work
https://brainly.com/question/26487364
#SPJ1
Related Questions
Which structure is the Lewis structure for ammonia (NH3)?
A.
A bond line structure of a compound has N H H H. The nitrogen has two dots at its bottom represents a lone pair of electrons.
B.
A bond line structure of a compound has H N H in the linear plane and hydrogen is branching upward, and the compound is H N (H) H.
C.
A bond line structure of a compound has H N H in linear plane and a hydrogen is branching upward, and the compound is H N (H) H. The nitrogen has two dots at its bottom represents a lone pair of electrons.
D.
A bond line structure of a compound has H N H H. The nitrogen has two dots on its top represents a lone pair of electrons.
Answers
Answer: **
H-N-H
|
H
Explanation:
Look at a periodic table to determine how many electrons you need to account for. Hydrogen (H) only has 1 electron, while Nitrogen (N) has 5. We have three Hydrogen atoms and one Nitrogen atom, so the total number of electrons will be 3 * 1 + 5 = 8 e-.
Now, place the center atom, which will be Nitrogen and place the three Hydrogens on three sides of it as above in the answer. You should use single bonds for this. Each single bond is a pair of electrons, so since we have three single bonds so far, we have accounted for 2 * 3 = 6 electrons. However, we need 2 more electrons for the total of 8. We put these electrons in as a lone pair above Nitrogen.
We check to see if everything follows the octet rule: Nitrogen has three single bonds, so that's 6 e-, as well as one lone pair, so that's another 2 e- for a total of 8 e-. Check. Now look at Hydrogen: H is the only element whose full orbital is 2 e-. Each H has a single bond with Nitrogen, so each does have 2 e-.
Thus, we know this is the correct diagram, and we are done.
Explanation:
A bond line structure of a compound has H N H in linear plane and a hydrogen is branching upward, and the compound is H N (H) H. The nitrogen has two dots at its bottom represents a lone pair of electrons. So ,the correct answer is option C.
The correct Lewis structure for ammonia (\(NH_3\)) is option C. It shows a bond line structure with three hydrogen atoms (H) bonded to a central nitrogen atom (N) in a linear plane.
One hydrogen atom branches upward from the plane. Additionally, the nitrogen atom in this structure has two dots at its bottom, indicating a lone pair of electrons. This arrangement follows the octet rule, as nitrogen has formed three covalent bonds with hydrogen, completing its valence shell. The lone pair on nitrogen gives ammonia its characteristic properties.
Thus, option C accurately represents the Lewis structure of ammonia, showing the bonding and lone pair arrangement of its atoms.
To know more about bond line structure:-
https://brainly.com/question/30639285
g You are given a 1.25 gram mixture of calcium nitrate and calcium chloride. You dissolve this mixture in 200.0 mL of water and add an excess of 0.300 M silver nitrate. You collect and dry the white precipitate which forms and find it has a mass of 0.535 grams. Calculate the percent calcium chloride by mass in the original mixture.
Answers
Answer:
16.51%
Explanation:
The reaction that takes place is
CaCl₂(aq) + 2AgNO₃(aq) → 2AgCl(s) + Ca(NO₃)₂(aq)Meaning that the white precipitate that formed is AgCl. Now we convert 0.535 g of AgCl into moles, using its molar mass:
0.535 g ÷ 143.43 g/mol = 0.00373 mol AgClThen we convert 0.00373 moles of AgCl into moles of CaCl₂, using the stoichiometric coefficients:
0.00373 mol AgCl * \(\frac{1molCaCl_2}{2molAgCl}\) = 0.00186 mol CaCl₂We convert moles of CaCl₂ into grams, using its molar mass:
0.00186 mol CaCl₂ * 110.98 g/mol = 0.206 gFinally we calculate the percent of CaCl₂ by mass in the original mixture:
0.206 g / 1.25 g * 100% = 16.51%The percent calcium chloride by mass in the original mixture is 16.4%
The equation of the reaction between calcium nitrate and silver chloride is:
\(\mathbf{CaCl_2 + 2AgNO_3 \to 2 AgCl+Ca(NO_3)_2}\)
Given that;
the weight mass of the white precipitate formed is = 0.535 gramsthe number of moles of the precipitated AgCl is:
= 0.535 g / 143.32 g/mol
= 0.0037 moles
From the above reaction, If 2 moles of AgCl are formed by 1 mole of CaCl2
Then, 0.0037 moles of AgCl will form (0.0037 × 1)/2 moles of CaCl2.
0.0037 moles of AgCl will form 0.00185 moles of CaCl2.
Now, we can say that the number of moles of CaCl2 present in the mixture is = 0.00185 moles
Mass amount of CaCl2 present = 0.00185 moles × 110.98 g/mol
Mass amount of CaCl2 present = 0.205 grams
Finally, the mass percentage \(\mathbf{=\dfrac{0.205}{1.25}\times 100\%}\)
= 16.4%
Therefore, we can conclude that the percent calcium chloride by mass in the original mixture is 16.4%
Learn more about mass percentage here:
https://brainly.com/question/23991850?referrer=searchResults
The table describes how some substances were formed. Substance Description P Formed by boiling pure water Q Formed by combining three hydrogen atoms to every nitrogen atom R Formed by adding 5 g of sugar to 1 L of water S Formed by compressing carbon under high pressure Based on the given descriptions, which substance is most likely a mixture? P Q R S Mark this and return
Answers
The combination that can be regarded as a mixture among the options is R.
What is a mixture?We can define a mixture as a combination of two or more substances that are not chemically combined together. Thus we know that the components that we can find in the mixture do not in any way have a chemical interaction that could lead to the alteration of the chemical properties of each of the substances that are combined.
Having said this, we can see that the combination of 5 g of sugar to 1 L of water is just a mixture since they can be separated by the use of evaporation of the solvent.
Learn more about mixture:https://brainly.com/question/24898889
#SPJ1
Which one of the following salts, when dissolved in water, produces the solution with a pH closest to 7.00?
Ba O
RbI
Na HSO4
NH4 Cl
Answers
Among all the salt , the one that ionizes to give strongest acid and strongest base, will have pH closest to 7 or neutral. RbI ionize in water to give strongest acid HI and strongest base Rubidium hydroxide.so RbI produces a neutral solution of pH closest to 7.(Option B)
The chemical difference between acids and bases is that acids produce hydrogen ions and bases absorb hydrogen ions. A base is a substance that neutralizes an acid. When bases are added to water, they decompose to form hydroxide ions. This is written OH-. A solution of water with a base added is called an alkaline solution.
Learn more about acid and base here:
https://brainly.com/question/9836972
#SPJ4
S-2-iodooctane lost his opticaly activity in soln on tratment wwithNaI. Explain
Answers
Answer:
Repeated SN2 reactions occur leading to the formation of a racemic mixture
Explanation:
S-2-iodooctane is a chiral alkyl halide with an asymmetric carbon atom. The presence of an asymmetric carbon atom implies that it can rotate plane polarized light and thus lead to optical isomerism. The two configurations of the compound are R/S according to the Cahn-Prelong-Ingold system.
However, when S-2-iodooctane is treated with sodium iodide in acetone, repeated SN2 reactions occur since the iodide ion is both a good nucleophile and a good leaving group. Hence a racemic modification is formed in the system with time hence we end up with (±)- Iodooctane.
Why does the moon and the sun have the same mass.
Answers
Why does the moon and the sun have the same mass?
Answer: The difference is that, on the moon, you are much lighter than on the Earth, but you still have the same mass
2. A restaurant offers a $19.99 three course meal special that lets you choose an appetizer,
an entree, and a dessert. There are 8 appetizers, 12 entrees, and 6 desserts from which
to choose. How many different meals are available?
Answers
There are 576 different ways to choose a meal.
Promotional offers. A promotional offer is a specific proposition to clients that specifies a reward and patron behavioral standards for earning a reward. the recognition and trouble of the praise are captured thru a retail transaction, purchaser order, rebate claim, rebate redemption, or different patron interplay. a discount is a difference between the unique charge and the lower charge it's far being bought at. a proposal is a deal wherein a product is normally bought at a reduction.
Calculation:-
There are 8 appetizers, 12 entrees, and 6 desserts.
Number of choices = 8 × 12 × 6
= 576
Learn more about different meals here:-https://brainly.com/question/8880115
#SPJ1
The figure below shows the rotation curves of several spiral galaxies, extending to the very edge of their visible disks. What do these curves tell us about the distribution of dark matter in these galaxies?
Group of answer choices
Most of the dark matter is in the bulge.
Most of the dark matter is in the galaxies’ outskirts.
Most of the dark matter is near stars.
Most of the dark matter is in clumps.
Answers
The thing that the curves tell us about the distribution of dark matter in these galaxies is option B: Most of the dark matter is in the galaxies’ outskirts.
Why do galaxy rotation curves suggest the existence of dark matter?Beyond the visible borders of galaxies, the velocity of stars remains relatively constant, suggesting that there must be more matter present than what we can see as stars and gas.
When the impact of DE became noticeable, the rotation curves are shown to dip at a specific distance. r. These flat, v = constant, rotation curve data suggest that the amount of dark matter is increasing linearly with r up to great distances from the spiral galaxies' centers.
From the graph you can see they all pointing or going one direction, hence we can deduce that it is option B.
Learn more about dark matter from
https://brainly.com/question/28256017
#SPJ1
How many moles of C2H2 are required to produce 0.60 mol of H2O?
Express your answer to two significant figures and include the appropriate units.
Answers
Answer:
0.60 moles
Explanation:
From the reaction:
\(2C_2H_2 + 5O_2 \to 4CO_2+2H_2O\)
From above, Only 2 moles of CH2CH2 are required to produce 2 moles of water (H_2O).
As such, 0.6 moles of H2O will require:
x (CH2CH2) × 2 moles of (H_2O) = 0.6 moles (CH2CH2) × 2 mole of (H_2O)
x mole of (CH2CH2) = 0.60 moles
∴
0.6 moles of H2O will require 0.60 moles of CH2CH2
calculate the frequency in hertz of electromagnetic radiation that has a wavelength of 685.0 nm.
Answers
\(\\ \sf\bull\longmapsto \lambda=\dfrac{c}{v}\)
\(\\ \sf\bull\longmapsto v=\dfrac{c}{\lambda}\)
\(\\ \sf\bull\longmapsto v=\dfrac{3\times 10^8}{685\times 10^{-9}m}\)
\(\\ \sf\bull\longmapsto v=0.00437\times 10^{17}Hz\)
\(\\ \sf\bull\longmapsto v=4.3\times 10^{14}Hz\)
Al^+3
protons =
electrons =
neutrons =
N^-3
protons =
electrons =
neutrons =
Answers
Explanation:
aluminium
proton= 13
electron=13
neutron=27
nitrogen
proton= 7
electron=7
neutron= 14
Bas +
PtF2 →
BaF2 +
Pts
Need to balance it

Answers
it is already balanced
REACTANTS
Barium sulfide (BaS) + platinum (Ii) fluoride
PRODUCT
Barium fluoride (BaF2) + Cooperite (PtS)
Hope this answer helps you dear! take care
A Hertzsprung-Russell diagram shows the relationship between star's luminosity and surface temperature. The Sun is shown as a 1 on the scale along the main axis. Which information can be inferred about the Sun according to the graph? Select ALL that apply. A) The Sun is the hottest star on record. B) The Sun is the brightest star in the galaxy. C) The Sun is the largest star in the universe. D) The Sun is a star with an intermediate temperature. E) Many stars are thousands or millions of times brighter than the Sun.
Answers
Answer:
Its D and E
Explanation:
i took the test and the other guy who answered gave u half of the answer
hope this helps
pls mark brainliest
The information which can be inferred about the Sun according to the graph include:
The Sun is a star with an intermediate temperature.Many stars are thousands or millions of times brighter than the Sun.What is a Graph?This is defined as a pictorial representation of data in an organized manner.
From the graph, we can infer that the Sun has an intermediate temperature and is less brighter than most stars.
Read more about Stars here https://brainly.com/question/21379923
If 12.3 g of Cu is deposited at the cathode of an electrolytic cell after 5.50 h, what was the current used?
Answers
Answer:
1.88 A
Explanation:
Let's consider the reduction of copper in an electrolytic cell.
Cu²⁺ + 2 e⁻ ⇒ Cu
We can calculate the charge used to deposit 12.3 g of Cu using the following relations.
The molar mass of Cu is 63.55 g/mol.1 mole of Cu is deposited when 2 moles of electrons circulate.1 mole of electrons has a charge of 96486 C (Faraday's constant).The charge used is:
\(12.3 g \times \frac{1 molCu}{63.55gCu} \times \frac{2molElectron}{1molCu} \times \frac{96486C}{1molElectron} = 3.73 \times 10^{4} C\)
We can convert 5.50 h to seconds using the conversion factor 1 h = 3600 s.
5.50 h × 3600 s/1 h = 1.98 × 10⁴ s
The current used is:
I = q/t = 3.73 × 10⁴ C/1.98 × 10⁴ s = 1.88 A
Aluminum undergoes a single-displacement reaction with copper (II) sulfate to form aluminum sulfate and _______________.
Answers
Ans cooper
what are thetypes of luminous flame
Answers
Types of luminous flames:
1. Yellow Luminous Flame
2. Smoky Luminous Flame
3. Orange Luminous Flame
4. Blue Luminous Flame
Luminous flames are characterized by their visible glow, which is caused by the incomplete combustion of fuel. The presence of soot particles in the flame causes the emission of light. There are different types of luminous flames, which can be classified based on their fuel composition and burning conditions. Here are some common types of luminous flames:
1. Yellow Luminous Flame: This is the most common type of luminous flame, often seen in open fires, candles, and gas stoves. It appears yellow due to the presence of soot particles in the flame. Yellow flames indicate incomplete combustion of hydrocarbon fuels, such as methane, propane, or natural gas. The high carbon content in these fuels leads to the formation of soot, which emits visible light.
2. Smoky Luminous Flame: This type of flame is characterized by a significant amount of black smoke and soot production. It is commonly observed in poorly adjusted or malfunctioning burners or engines. The excessive presence of unburned fuel in the flame results in incomplete combustion and the emission of dark smoke particles.
3. Orange Luminous Flame: An orange flame indicates a higher combustion temperature compared to a yellow flame. It is often seen in more efficient burners or when burning fuels with a higher carbon content, such as oil or diesel. The higher temperature helps in burning more of the carbon particles, reducing the amount of soot and making the flame appear less yellow.
4. Blue Luminous Flame: A blue flame is typically associated with complete combustion. It indicates efficient burning of fuel, resulting in minimal soot formation. Blue flames are commonly observed in gas burners or Bunsen burners. The blue color is a result of the combustion of gases, such as methane, in the presence of sufficient oxygen.
It's important to note that the luminosity of a flame can vary depending on factors such as fuel-air mixture, combustion temperature, and the presence of impurities. Achieving complete combustion and minimizing the production of soot is desirable for efficient and cleaner burning processes.
for more questions on luminous
https://brainly.com/question/27163038
#SPJ8
A gas occupying 50.0 ml volume in a confined space at 20.0 dc at 50.0 kpa is heated to 40.0 dc. What is the pressure exerted by the gas in the container?
Answers
Answer:The pressure exerted by the gas is 100kPa
Explanation:Let's apply the Charles Gay Lussac law, to solve the question.
At constant volume, the pressure varies proportionally with the temperature.
P initial / T° initial = P final / T° final
50kPa / 20°C = P final / 40°C
Temperature has increased the double, so the pressure will be increased, the double too.
100 kPa
If you need more help go to this link https://brainly.com/question/14378507
Iron reacts with chlorine to form iron(III) chloride.
2Fe + 3Cl2 → 2FeCl3
What mass (in grams) of chlorine gas is needed to react with 251 grams of iron?
Select one:
a.
71 grams
b.
392 grams
c.
479 grams
d.
622 grams
Answers
The mass (in grams) of chlorine gas is needed to react with 251 grams of iron is 479 grams. Option C.
To determine the mass of chlorine gas needed to react with 251 grams of iron, we need to use the stoichiometry of the balanced chemical equation:
2Fe + 3Cl2 → 2FeCl3
From the balanced equation, we can see that 2 moles of iron (Fe) react with 3 moles of chlorine gas (Cl2) to produce 2 moles of iron(III) chloride (FeCl3).
To calculate the mass of chlorine gas, we can follow these steps:
Step 1: Convert the given mass of iron (Fe) to moles.
Using the molar mass of iron (Fe), which is approximately 55.85 g/mol, we can calculate the number of moles of iron:
moles of Fe = mass of Fe / molar mass of Fe
moles of Fe = 251 g / 55.85 g/mol
moles of Fe ≈ 4.5 mol (rounded to one decimal place)
Step 2: Use the mole ratio from the balanced equation to find the moles of chlorine gas (Cl2) needed.
From the balanced equation, we know that 2 moles of Fe react with 3 moles of Cl2. Therefore, the moles of Cl2 can be calculated as:
moles of Cl2 = (moles of Fe / 2) * 3
moles of Cl2 = (4.5 mol / 2) * 3
moles of Cl2 ≈ 6.75 mol (rounded to two decimal places)
Step 3: Convert the moles of chlorine gas to grams.
Using the molar mass of chlorine gas (Cl2), which is approximately 70.90 g/mol, we can calculate the mass of chlorine gas:
mass of Cl2 = moles of Cl2 * molar mass of Cl2
mass of Cl2 = 6.75 mol * 70.90 g/mol
mass of Cl2 ≈ 479 grams (rounded to the nearest whole number) Option C is correct.
For more such question on mass. visit :
https://brainly.com/question/19385703
#SPJ8
It can be seen that the three methyl groups in camphor and isoborneol show up as separate peaks, whereas those in borneol overlap to where they almost appear as a single peak. Based on your knowledge of chemical shift factors, explain why this is so?
Answers
Answer:
isoborneol and camphor both have methyl groups that have different shielding zones with -OH and -C=O groups respectively.
Explanation:
Using the knowledge of chemical shift we can see that out of the three methyl groups in isoborneol, there are two methyl groups that are not influenced by the chemical Influence of the -OH functional group while one of the methyl groups is influenced by the -OH functional group.
For Camphor, two out of the three methyl groups are in shielding zones of the Carbonyl group, -C=O. While the last of the three methyl groups is out of the shielding zones of Carbonyl group, -C=O.
Describe how you would prepare exactly 100 mL of 0.109 M picolinate buffer, pH 5.61. Possible starting materials are pure picolinic acid (pyridine-2-carboxylic acid, FM 123.10), 1.0 M HCl, and 1.0 M NaOH. Place the given steps in order. Not all of the steps will be used.
Answers
Answer:
1.342g of picolinic acid and 6.743mL of 1.0M NaOH diluting the mixture to 100.0mL
Explanation:
The pKa of the picolinic acid is 5.4.
Using Henderson-Hasselbalch formula for picolinic-picolinate buffer:
pH = pKa + log [Picolinate] / [Picolinic]
Where [] could be taken as moles of each species
5.61 = 5.4 + log [Picolinate] / [Picolinic]
0.21 = log [Picolinate] / [Picolinic]
1.62181 = [Picolinate] / [Picolinic] (1)
Now, both picolinate and picolinic acid will be:
0.100L * (0.109mol / L) =
0.0109 moles = [Picolinate] + [Picolinic] (2)
First, as we will start with picolinic acid, we need add:
0.0109 moles picolinic acid * (123.10g/mol) = 1.342g of picolinic acid
Now, replacing (2) in (1):
1.62181 = 0.0109 moles - [Picolinic] / [Picolinic]
1.62181 [Picolinic] = 0.0109 moles - [Picolinic]
2.62181 [Picolinic] = 0.0109 moles
[Picolinic] = 4.157x10⁻³ moles
And:
[Picolinate] = 0.0109 - 4.157x10⁻³ moles =
6.743x10⁻³ molesTo obtain these moles of picolinate ion we need to make the reaction of the picolinic acid with NaOH:
Picolinic acid + NaOH → Picolinate + Water
That means to obtain 6.743x10⁻³ moles of picolinate ion we need to add 6.743x10⁻³ moles of NaOH
6.743x10⁻³ moles of NaOH that is 1.0M are, in mL:
6.743x10⁻³ moles * (1L / 1mol) = 6.743x10⁻³L * 1000 =
6.743mL of the 1.0M NaOH must be addedThus, we obtain the desire moles of picolinate and picolinic acid to obtain the buffer we want, the last step is:
Dilute the mixture to 100mL, the volume we need to prepareFollowing are the calculation to the mass of picolinic acid:
Let pKa of picolinic acid \(= 5.52\)
form the buffer: \(\bold{pH = pKa + \log(\frac{A-}{ HA})}\)
Acid concentration\(= 0.109\)
We will require the conjugate, which would be formed by interaction of picolinic acid and NaOH.
calcultion to the need:
\(pH = pKa + \log(\frac{A-}{ HA})\\\\5.61 = 5.52 + \log(\frac{A-}{ HA})\)
solve for A- by using antilog
\(0.09 = \log(\frac{A-}{ HA})\\\\1.230= \frac{A-}{HA}\)
When
\(HA = 0.109\\\\A- = 0.134\)
If \(V = 100 ml\)then
\(n A- = M\timesV = 0.134 \times 0.1 = 0.0134 \ mol\ of\ A-\)
needed
\(HA + NAOH \to H_2O + Na^+ \ and \ A-\)
therefore,
ratios =1:1
we need 0.0134 mol of NaOH
\(n = M\times V \\\\V = \frac{0.0134}{1} = 0.0134 \ liter \ of\ NaOH\)
but you also want \(0.109 M\) of free picolinic acid so
\(n = M\times V = 0.109\times 0.1 = 0.0109\ mol \ of \ Acid\)
Therefore:
\(n \ acid = 0.0109 + 0.0134\ mol = 0.0243 \ mol\ of \ acid\)
Preparation:
by add volume (19.07) in NaOH
M = 1.0 to the beaker, and by add water until the beaker marks 1 L
Then add 0.0243 mol of picolinic acid
\(\to m = 0.0243 \times 123 = 2.9889\ grams\) of picolinic acid
stir and pH will be that of buffer.
Learn more:
brainly.com/question/17156849
2. Explain why most synthesis reactions are exothermic. What does this imply about the energy needed to break the reactant bonds compared to energy released when the product bonds form? Draw an energy level diagram that could represent a synthesis
reaction.
GIVING BRAINLIEST
Answers
Answer: Most synthesis reactions are exothermic because the energy needed to break the reactant bonds is typically less than the energy released when the product bonds form. In other words, the reactant bonds have a lower energy level than the product bonds, so when the reactant bonds are broken and the product bonds are formed, energy is released in the form of heat.
This can be represented by an energy level diagram, in which the reactant bonds are shown at a lower energy level than the product bonds. When the reactant bonds are broken and the product bonds are formed, the energy level of the reaction decreases, indicating that energy is released.
Overall, the fact that most synthesis reactions are exothermic implies that the energy needed to break the reactant bonds is typically less than the energy released when the product bonds form. This is reflected in the energy level diagram, where the reactant bonds are shown at a lower energy level than the product bonds.
Explanation:
Answer:
Synthesis reactions are typically exothermic because the products of the reaction have lower energy than the reactants. This means that the energy needed to break the bonds in the reactants is less than the energy released when the product bonds form.
An energy level diagram can help illustrate this concept. In a synthesis reaction, the reactants are represented by two energy levels on the left side of the diagram, and the products are represented by a lower energy level on the right side. The energy difference between the reactants and the products is represented by the vertical distance between the two sets of energy levels. Since the product energy level is lower than the reactant energy levels, the reaction releases energy and is exothermic.
Here is an example of an energy level diagram for a synthesis reaction:
In this diagram, the vertical distance between the reactant and product energy levels represents the energy released by the reaction. This energy is typically in the form of heat, which is why most synthesis reactions are exothermic.


What are two ways to make a sugar cube dissolves faster in water
A. Chill the water after adding the sugar cube
B. Add Several sugar cubed instead of water
C. Warm the water before adding the sugar cube
D. Break The sugar cube in a smaller pieces
Answers
Answer:
c.warm water before adding the sugar cube
Answer:
C.Warm the warm the water before adding the sugar cube D. Break the sugar cube into smaller pieces
Explanation:
Heating. Speeds up particles causing them to bump and break, further exposing them to the solvent.
Increasing surface area. By crushing or breaking the solid, you are exposing it to more contact with the solute.
a. determine the formal charge of oxygen in the structure. if the atom is formally neutral, indicate a charge of zero.
Answers
The oxygen atom has no formal charge.
The total of its valence and inner shell electron counts equals 8, which is the atomic number of the substance.
Subtract the total number of valence electrons from the total number of non-bonding electrons and half the total number of bound electrons to determine the formal charge of an atom.
More potent oxidants than ozone, oxygen atoms cause chemiluminescence with a variety of analytes. However, this reagent's analytical use is very constrained.
The arrangement of atoms within molecules is referred to as chemical structure. The atoms of the molecule are shown in the Kekulé Formula or structural formula in the sequence in which they are bonded.
To know about charge
https://brainly.com/question/19886264
#SPJ4
If a neutral atom has 49 protons, how many electrons does it have?
Answers
So 49
Which two the following functional groups does the amino acid have according to the picture? ( worth 50 points <3)

Answers
The two functional groups that the aminoacid has according to the picture are amine and carboxyl.
What is a functional group?In chemistry and related areas, a functional group can be defined as a group of atoms bonded in a specific molecule that can affect the was the molecule reacts or the specific behavior of it.
In the case of the molecule presented, which is an amino acid, two functional groups can be identified:
An amine group: This includes the N atom bonded to the two hydrogens.A carboxyl group: This includes the terminal carbon linked to two oxygen atoms and a hydrogen atom.Learn more about functional groups in https://brainly.com/question/1356508
#SPJ1
Build the following atoms and provide their name An atom with 3 protons and 4 neutrons: b. An atom with 2 protons and 4 neutrons: An atom with 4 protons and 4 neutrons:
Answers
Lithium-7 has 3 protons and 4 neutrons, carbon-6 has 2 protons and 4 neutrons, while beryllium-8 has 4 protons and 4 neutrons.
What atom has three protons? three electrons, four neutrons What is the mass number and what is the atomic number of it?No matter how many neutrons or electrons are present, the nucleus of a lithium atom always has three protons. The atomic number for lithium is always 3, which is evident given that the lithium atom always has three protons. Yet, the mass number in the isotope with 3 neutrons is 6 and in the isotope with 4 neutrons is 7.
What exactly are electrons, neutrons, and protons?Negatively charged subatomic particles include electrons. A category of subatomic particle having a positive charge is the proton. The strong nuclear force holds the protons together in the atom's nucleus. A subatomic particle with no charge is called a neutron (they are neutral).
To learn more about protons and neutrons visit:
brainly.com/question/29248303
#SPJ9
Which of the following molecules would be most favorable to undergo an E2 reaction rather than an SN2 reaction with NaOH
Answers
2-bromopropane molecules would be most favorable to undergo an E2 reaction rather than an SN2 reaction with NaOH. Here option B is the correct answer.
An E2 reaction occurs when a strong base removes a proton from a beta-carbon adjacent to a leaving group, leading to the formation of a double bond and the departure of the leaving group. An SN2 reaction, on the other hand, involves a nucleophile attacking the carbon bearing the leaving group while the leaving group departs.
The relative favorability of E2 and SN2 reactions depends on the strength of the nucleophile and the base, the steric hindrance around the carbon bearing the leaving group, and the nature of the leaving group.
In this case, NaOH is a strong base, and the molecule that is most favorable to undergo an E2 reaction is the one with the least steric hindrance around the carbon bearing the leaving group, which is 2-bromopropane. The other molecules have more steric hindrance around the carbon bearing the leaving group, making them less favorable to undergo E2 reactions.
To learn more about bromopropane molecules
https://brainly.com/question/489491
#SPJ4
Complete question:
Which of the following molecules would be most favorable to undergo an E2 reaction rather than an SN2 reaction with NaOH?
a) 1-bromopropane
b) 2-bromopropane
c) 1-chloropropane
d) 2-chloropropane
Check all the statements that describe applications and properties of substances that result from intermolecular forces.
Answers
Answer:
The ability for water to form bubbles
the formation of a meniscus in a graduated cylinder with water
the inability of water to mix with oil
the melting point of a substance
the ability of detergents to clean clothes
Explanation:
Answer:these are the answers
Explanation:
edge

Explain the oxygen cycle. I’m in the 6th grade I need a answer please
Answers
Answer:
Oxygen cycle, along with the carbon cycle and nitrogen cycle plays an essential role in the existence of life on the earth. The oxygen cycle is a biological process which helps in maintaining the oxygen level by moving through three main spheres of the earth which are:
Atmosphere Lithosphere BiosphereExplanation:
The atmosphere is the layer of gases presents above the earth’s surface. The sum of Earth’s ecosystems makes a biosphere. Lithosphere is the solid outer section along with the earth’s crust and it is the largest reservoir of oxygen.
Answer:
Oxygen cycle, circulation of oxygen in various forms through nature. ... Free in the air and dissolved in water, oxygen is second only to nitrogen in abundance among uncombined elements in the atmosphere. Plants and animals use oxygen to respire and return it to the air and water as carbon dioxide (CO2).
Explanation:


Please help with this chemistry question

Answers
the cell potential for the given galvanic cell is +3.16 V at 25°C. This means that the reaction is strongly favored to occur spontaneously in the direction written, from left to right.
To calculate the cell potential for the given galvanic cell, we need to use the standard reduction potentials of the two half-reactions involved in the cell reaction. From the table of standard reduction potentials, we can find the following half-reactions:
Al³+ (aq) + 3e⁻ → Al(s) E°red = -1.66 V
Au³+ (aq) + 3e⁻ → Au(s) E°red = +1.50 V
To obtain the overall cell potential, we can use the equation:
E°cell = E°reduction (cathode) - E°reduction (anode)
where the cathode is the half-cell where reduction occurs and the anode is the half-cell where oxidation occurs. In this case, we can see that Au³+ is reduced to Au(s) (cathode) and Al(s) is oxidized to Al³+ (anode).
Therefore, we can calculate the cell potential as follows:
E°cell = E°red (Au³+ → Au) - E°red (Al → Al³+)
= (+1.50 V) - (-1.66 V)
= +3.16 V
Thus, the cell potential for the given galvanic cell is +3.16 V at 25°C. This means that the reaction is strongly favored to occur spontaneously in the direction written, from left to right.
To know more about reduction potentials, visit:
https://brainly.com/question/23881200
#SPJ1