NEED HELP PLEASE
Part A:
In your own words, describe the process of a physical change.
Part B:
What is the difference between a physical change and a chemical change? Give an example of each.
Answers
B/ the different is that 1) chemical change is more quick to change rather than physical (ex: I’d there is rocks flying around and is wearing down another rock it would take longer rather than acid)
Answer:
Part A:
A physical change is a change of a substance from one form to another without changing its composition.
Part B:
A physical change is a change of a substance from one form to another without changing its composition. Example: A balloon deflating into its original form.
A chemical change occurs, one substance combines with another substance to make something completely new. Example: Making and baking a cake.
Related Questions
select the valid ways to make an ammonia/ammonium buffer for use in the laboratory (select all that apply). select the valid ways to make an ammonia/ammonium buffer for use in the laboratory (select all that apply). mix equal volumes of 1 m nh3 and 1 m hcl mix equal volumes of 1 m nh3 and 0.01 m nh4 mix equal volumes of 1 m nh3 and 1 m nh4 mix some volume of 1 m nh3 and half as much 1 m hcl
Answers
We should mix equal volumes of 1 M NH₃ and 1 M HCl or to mix equal volumes of 1 M NH₃ and 1 M NH₄+ to make an ammonia/ammonium buffer.
To make an ammonia/ammonium buffer for use in the laboratory, the valid ways are:
1. Mix equal volumes of 1 M NH₃ and 1 M HCl.
- This combination will react to form the ammonium (NH₄+) and chloride (Cl-) ions, creating an ammonia/ammonium buffer.
2. Mix equal volumes of 1 M NH₃ and 1 M NH₄+.
- By combining ammonia (NH₃) and ammonium (NH₄+) in equal volumes, you can create an ammonia/ammonium buffer.
Therefore, the valid ways to make an ammonia/ammonium buffer are to mix equal volumes of 1 M NH₃ and 1 M HCl or to mix equal volumes of 1 M NH₃ and 1 M NH₄+.
To know more about ammonia, visit:
https://brainly.com/question/29519032#
#SPJ11
overall balanced redox reaction for nitrite ion oxidizing iodide in acid to form molecular iodine, nitrogen monoxide and water.
Answers
The overall balanced redox reaction for nitrite ion oxidizing iodide in acid to form molecular iodine, nitrogen monoxide, and water can be represented as follows:
NO2^- (aq) + 2I^- (aq) + 4H^+ (aq) → I2 (s) + NO (g) + 2H2O (l)
In this reaction, nitrite ion (NO2^-) acts as an oxidizing agent, while iodide ion (I^-) acts as a reducing agent. The reaction takes place in an acidic medium, which provides the protons (H^+) necessary for the reaction to occur.
The products formed are molecular iodine (I2), nitrogen monoxide (NO), and water (H2O).
The reaction is balanced by ensuring that the number of atoms of each element is the same on both sides of the equation and that the charges are balanced.
To learn more about redox, refer below:
https://brainly.com/question/28300253
#SPJ11
Please help with these 10 questions!! Show all work!! Will give brainliest!! :)

Answers
Carbon Dioxide: CO2
Formula: C + O2
15.0 moles of CO2 contains (15.0 moles CO2) x (1 mole C / 1 mole CO2) = 15.0 moles of C
Carbon Dioxide: CO2
Formula: C + O2
15.0 moles of CO2 contains (15.0 moles CO2) x (2 moles O / 1 mole CO2) = 30.0 moles of O
Calcium Chloride: CaCl2
Formula: Ca + 2Cl
23.0 moles of CaCl2 contains (23.0 moles CaCl2) x (1 mole Cl / 2 moles Cl) = 11.5 moles of Cl
Sulfur Dioxide: SO2
Formula: S + O2
295.0 grams of SO2 contains (295.0 grams SO2) x (1 mole S / 64.066 grams) = 4.6 moles of S
Potassium Sulfate: K2SO4
Formula: 2K + S + 4O
295.0 grams of K2SO4 contains (295.0 grams K2SO4) x (1 mole S / 174.259 grams) = 1.69 moles of S
Iron: Fe
Formula: Fe
565.0 grams of Fe contains (565.0 grams Fe) x (1 mole Fe / 55.845 grams) = 10.15 moles of Fe
metallic elements
SO2 is a covalent compound. It consists of one sulfur atom and two oxygen atoms joined together by covalent bonds, which are strong chemical bonds where electrons are shared between atoms.
H
|
O-H
A water molecule is polar because the electrons are not shared equally between the oxygen and hydrogen atoms. Oxygen is more electronegative than hydrogen, so it pulls the electrons closer to itself, creating a partial negative charge on the oxygen side of the molecule and a partial positive charge on the hydrogen side. This causes the molecule to have a dipole moment and makes the molecule polar.
NH3 (Ammonia) - the molecule is a pyramidal shape, which means that the Nitrogen is at the center and the three hydrogen atoms are located at the three corners of a pyramid.
H2O (Water) - the molecule is a V-shape, this shape is determined by the angle between the two bonding pairs and the two non-bonding pairs of electrons.
CH4 (Methane) - the molecule is tetrahedral shape, the carbon is in the center, and the four hydrogen atoms surround the carbon in a tetrahedral arrangement.
A color change
The formation of a precipitate
The evolution of a gas
A change in temperature
Uday Tahlan
1. What is the molarity of a solution which contains 0.256 mol of a substance dissolved in 143 mL of solution.
2. What is the molarity of a solution containing 4.89 g of NaCl dissolved in 600. mL of solution?
3. If 895 g of KCl is dissolved in 950. mL of solution, what is the molarity of the solution?
4. How many formula units of solute are there in a 5.50 L of 0.460 M silver nitrate solution.?
5. How many grams of FeCl2 are needed to make 700. mL of a 1.4 M solution?
6. Find the mass required to prepare 2500 mL of a 0.250 M solution of strontium sulfate.
7. Calculate the molarity of a solution when 75.0 g of calcium nitrite is dissolved in 500.0 mL of solution.
8. How many molecules of glucose (C6H12O6) are contained in 150.0 mL of a 0.100 M solution?
9. What mass of potassium bromide is needed to prepare 1.00 L of 0.250 M solution?
10. If I have 350 mL of a 0.400 M NaBr solution, what will the concentration be if I add 250. mL more water to it?
11. If I dilute 270. mL of 0.250 M lithium acetate solution to a volume of 800 mL, what will the concentration of this solution be?
12. The molar mass of an unknown gas is to be determined. The gas at STP occupies 0.861 L and its mass is 3.22 g. With this information calculate the molar mass of the gas. Theo claims the gas to be a noble gas. Assuming Theo to be correct, what would the identity of the gas be?
13. What mass of sulfur dioxide gas at STP is needed to fill a 300.0 mL volumetric flask?
14. Suppose you fill up a 5.00 L scuba tank with oxygen at STP. How many molecules of oxygen are there in the tank?
15. The store manager at Party City weighs the helium tank before opening the store and then at the end of the day. The mass of the helium before and after the working day are entered in the table below. How many atoms of helium gas at STP were used to fill up balloons in that day?
HINT: 1 lb = 454 g
Mass of helium tank before opening the store 1565 lb
Mass of helium tank after closing the store 1329 lb
Answers
Answer:
The equation for molarity is moles/liter for the first question you would do 0.256/0.143 liters to get 1.790 mol/L
Explanation:
The second problem you would do need to find the moles of NaCl which you would do by doing 4.89 g/58.44g/mol= 0.08367 then do 0.08367/0.600= 0.139 mol/L
The third problem would be the same steps as the second one.
The fourth problem would be (0.460M)(5.50L)= 2.53 moles
The molarity of a solution which contains 0.256 mol of a substance dissolved in 143 mL of solution is 1.79.
What is Molarity?Molarity of a given solution is known as the total number of moles of solute per litre of the solution. A solution that is 1.00 molar (written 1.00 M) contains 1.00 mole of solute for every liter of solution.
Molarity = (No. of moles of solute ÷ Volume of solution in liters)
The unit of molarity is mol L⁻¹.
Molarity is temperature dependent because as temperature changes, volume of the solution also changes.
Given,
No. of moles = 0.256
Volume of solution = 143ml
Molarity = (0.256 ÷ 0.143) = 1.79
Therefore, The molarity of a solution which contains 0.256 mol of a substance dissolved in 143 mL of solution is 1.79.
Learn more about molarity, here:
https://brainly.com/question/8732513
#SPJ3
True or False: Placing a hydrophobic molecule into water disrupts some of the water-water hydrogen bonds.
Answers
The given statement, Placing a hydrophobic molecule into water disrupts some of the water-water hydrogen bonds is True.
Hydrophobic molecules are non-polar and do not have any charge, so they are not attracted to the partially charged regions of the water molecules. When hydrophobic molecules are placed in water, they try to reduce the amount of contact they have with the water molecules, which disrupts the water-water hydrogen bonds.
The disruption of water-water hydrogen bonds is called "hydrophobic effect". This effect is mainly due to the fact that the hydrophobic molecules take up space between the water molecules, so the water molecules are forced apart, reducing the attractive force between them.
Know more about hydrophobic molecule here
https://brainly.com/question/13829251#
#SPJ11
(15 points) Which element below has properties of both metals and nonmetals?
A. zinc
B. aluminum
C. copper
D. boron
Answers
Answer:
boron
Explanation:
boron is a metaloid meaning it has the property of a metal and a non metal located where metals and non metals meet on the periodic table
Answer:
The answer is boron, the second answer is chlorine
Explanation:
edge 2022, have a great day h0mies

In Part B of the Procedure and Analysis number 1, you record your exact mass as 17.850 g copper(II) sulfate. What will the molarity of your solution be after you dilute with water to 100 ml
Answers
After diluting the 17.850 g copper(II) sulfate solution with water to 100 mL, the molarity of the solution will be 0.715 M.
To calculate the molarity of the copper(II) sulfate solution after dilution, follow these steps:
Convert the mass of copper(II) sulfate (17.850 g) to moles by using its molar mass
Molar mass of CuSO₄•5H₂O = 63.55 + 32.07 + (4 x 16.00) + (5 x 18.02) = 249.68 g/mol
No. of moles = Given mass/Molar Mass
Moles= 17.850 g / 249.68 g/mol
moles= 0.0715 mol
Convert the final volume of the solution to liters:
100 mL = 0.1 L
Calculate the molarity of the diluted solution:
Molarity = moles / volume (L)
Molarity = 0.0715 mol / 0.1 L = 0.715 M
In Part B of Procedure and Analysis number 1, the molarity of the solution will be 0.715 M after diluting the 17.850 g copper(II) sulphate solution with water to 100 mL.
To learn more about molarity visit:
https://brainly.com/question/30404105
#SPJ11
An old refrigerator is rated at 500 W how many kilowatt hours of electric energy what does refrigerator use in 30 days assume the refrigerator is running 12 hours per day
Answers
The refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
To calculate the kilowatt-hours (kWh) of electric energy used by the refrigerator in 30 days, we need to multiply the power rating by the total running time.
Given:
Power rating of the refrigerator = 500 W
Running time per day = 12 hours
Number of days = 30
First, we need to convert the power rating from watts to kilowatts:
Power rating = 500 W / 1000 = 0.5 kW
Next, we calculate the total energy used in kilowatt-hours (kWh) over the 30-day period:
Energy used = Power rating × Running time × Number of days
Energy used = 0.5 kW × 12 hours/day × 30 days
Energy used = 180 kWh
Therefore, the refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
For more question on energy
https://brainly.com/question/29339318
#SPJ8
identify the conditions for a standard electrochemical cell. select one or more: pressure of 1 atm temperature of 298 k solution concentrations of 1 m pressure of 5 atm solute masses of 1 g temperature of 273 k
Answers
The conditions for a standard electrochemical cell. select one or more : pressure of 1 atm temperature of 298 k solution concentrations of 1 M.
The electrochemical cell is the cell that is capable of generating the electrical energy from the chemical reactions or by the use of the electrical energy to cause the chemical reaction. The conditions for a standard electrochemical cell. select one or more : pressure of 1 atm temperature of 298 k solution concentrations of 1 M.
There are the two types of the electrochemical cells is as follows : the galvanic called the electrolytic cells. the galvanic cell is also called as the voltaic cell.
To learn more about temperature here
https://brainly.com/question/14995282
#SPJ4
What type of molecule is shown below?
A. Ether
B. Alcohol
C. Aldehyde
D. Ketone
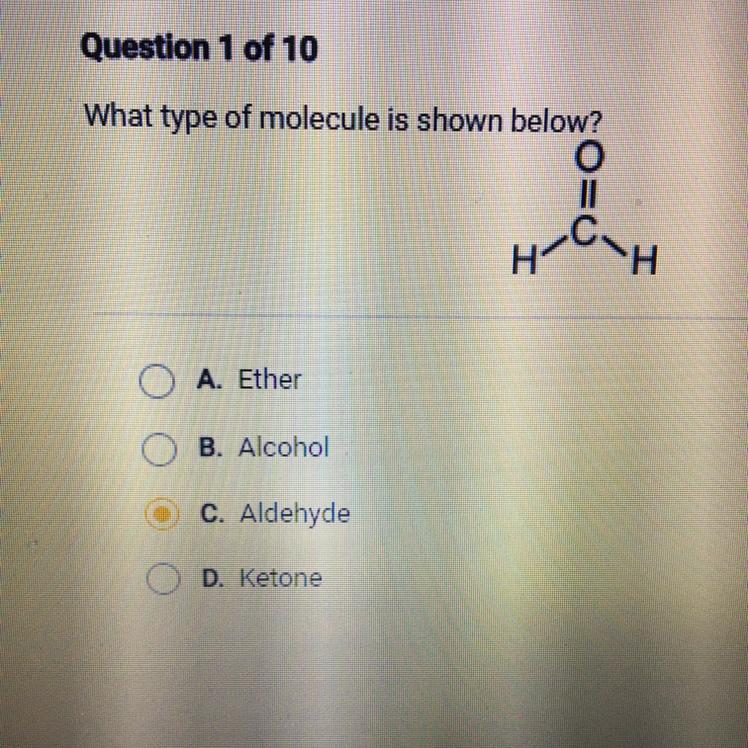
Answers
The given molecule is an aldehyde with the formula HCHO. It is the simplest and primary aldehyde . Hence, option c is correct.
What are aldehydes ?Aldehydes are a class of organic compounds that contain a carbonyl group (a carbon double-bonded to an oxygen) at the end of a carbon chain. The general formula for an aldehyde is RCHO, where R is a substituent or side chain attached to the carbonyl group.
Aldehydes are often characterized by their distinctive odor, which can be described as pungent and irritating. Some common aldehydes include formaldehyde (used in building materials and as a disinfectant), acetaldehyde (found in alcoholic beverages), and benzaldehyde (used in food flavorings).
Aldehydes are important intermediates in many chemical reactions, and they are used in a variety of industrial and laboratory applications. They can be synthesized by the oxidation of primary alcohols or by the partial oxidation of hydrocarbons. The given compound is formaldehyde HCHO.
Find more on aldehydes:
https://brainly.com/question/30902678
#SPJ7
Prussian Blue has a molar
mass of g/mol. Answer with four significant figures
Answers
The molar mass of Prussian Blue is 860 g / mole
Prussian BluePrussian blue is produced by the oxidation of ferrous ferrocyanide salts. The molecular formula is represented as follows:
Molecular mass
Molecular mass is a number equal to the sum of the atomic masses of the atoms in a molecule.
Therefore,
molecular mass of Prussian blue= 56 × 4 + 56 × 3 + 26 × 18
molecular mass of Prussian blue= 224 + 168 + 468
molecular mass of Prussian blue = 860 g / mole
learn more on molar mass here: https://brainly.com/question/12127540
A 25 g sample of compound Delta is mixed with a 25 g sample of compound Omicron. A reaction occurs in which a gas is produced. Once the reaction is complete, the final mixture has a mass of 35 g. (a) What is the mass of the gas? (b) What happened to the gas? (c) Does this violate the law of conservation of mass? Why or why not?
Answers
A 25 g sample of compound Delta is mixed with a 25 g sample of compound Omicron. A reaction occurs in which a gas is produced. Once the reaction is complete, the final mixture has a mass of 35 g.
(a) The mass of the gas can be calculated by finding the difference in mass before and after the reaction.
Initial total mass = Mass of compound Delta + Mass of compound Omicron = 25 g + 25 g = 50 g
Final total mass = Mass of the final mixture = 35 g
Mass of the gas produced = Final total mass - Initial total mass = 35 g - 50 g = -15 g
The mass of the gas is -15 g.
(b) The negative mass value indicates an inconsistency or error in the given information. In a chemical reaction, the mass cannot be negative. It is possible that there was a mistake in the measurements or calculations.
(c) Yes, this situation violates the law of conservation of mass. The law of conservation of mass states that the mass of the reactants should be equal to the mass of the products in a chemical reaction. In this case, the total mass after the reaction (35 g) is less than the total mass before the reaction (50 g). This violates the principle of mass conservation, suggesting that there may have been an error in the measurements or a loss of mass during the reaction that was not accounted for.
learn more about mass conservation here:
https://brainly.com/question/15442173
#SPJ11
Which is true if the weak base is stronger than the weak acid?

Answers
The option that describe if the weak base is stronger than the weak acid is option B which is the value of Ka<Kb.
Weak acid base explanation.
A weak acid is an acid that donates hydrogen ions (H+) to a solution only partially. For example, acetic acid (CH3COOH) is a weak acid that partially ionizes in water to produce H+ and acetate ions (CH3COO-). The equilibrium constant for this reaction (known as the acid dissociation constant or Ka) is relatively small, indicating that the reaction proceeds to a limited extent.
Similarly, a weak base is a base that only partially accepts hydrogen ions (H+) from a solution. For example, ammonia (NH3) is a weak base that partially ionizes in water to produce hydroxide ions (OH-) and ammonium ions (NH4+). The equilibrium constant for this reaction (known as the base dissociation constant or Kb) is relatively small, indicating that the reaction proceeds to a limited extent.
In general, weak acids and bases are less reactive than strong acids and bases because they do not dissociate completely into ions. However, they are still important in many chemical and biological processes, such as the buffering of solutions and the regulation of pH in the
Learn more about weak acid base below.
https://brainly.com/question/31075294
#SPJ1
what are the two ways in which the physical state of matter can be changed
Answers
The two ways in which the physical state of matter can be changed are melting and freezing.
Melting is the process by which a solid substance transitions to a liquid state. As a result, the energy added to the solid substance causes the molecules to vibrate at a higher rate. As a result, the heat breaks the bonds between the molecules, allowing them to flow freely.Freezing is the process by which a liquid substance transitions to a solid state. As a result, energy is removed from the liquid substance. The molecules in the substance are moving quickly, but when energy is removed, they slow down.Because of the decrease in energy, the molecules can no longer slide past one another and form a rigid structure, resulting in a solid state of matter.For such more questions on physical state
https://brainly.com/question/30214939
#SPJ8
The data found below measure the amounts of greenhouse gas emissions from three types of vehicles. The measurements are in tons per year, expressed as CO2 equivalents. Use a 0.025 significance level to test the claim that the different types of vehicle have the same mean amount of greenhouse gas emissions. Based on the results, does the type of vehicle appear to affect the amount of greenhouse gas emissions? Click the icon to view the data. What are the hypotheses for this test? A. H 0
:μ 1
=μ 2
=μ 3
H 1
: At least one of the means is different from the others. B. H 0
: At least one of the means is different from the others. H 1
:μ 1
=μ 2
=μ 3
C. H 0
:μ 1
=μ 2
=μ 3
H 1
:μ 1
=μ 2
=μ 3
D. H 0
:μ 1
=μ 2
=μ 3
H 1
:μ 1
=μ 2
=μ 3
Determine the test statistic. F (Round to two decimal places as needed.)
Answers
Answer: A. H 0 μ1 = μ2 = μ3
Ha μ1 ≠ 2μ ≠ μ3
2. Test Statistics is 95%
3. Critical F-Value is 3.76.
4. P-Value is 2.32.
5. Conclusion Reject the null hypothesis.
6. Type of vehicle does effect the amount of green house gas emissions.
The correct order of the steps of a hypothesis test is given below.
1. Determine the null and alternative hypothesis.
2. Select a sample and compute the critical value F-test for the sample mean.
3. Determine the probability at which you will conclude that the sample outcome is very unlikely.
4. Make a decision about the unknown population.
All steps are performed in the given sequence to test a hypothesis.
The null hypothesis is rejected or accepted on the basis of level of significance. When the p-value is greater than level of significance we fail to reject the null hypothesis and null hypothesis is then accepted. It is not necessary that all null hypothesis will be rejected at 95% level of significance. To determine the criteria for accepting or rejecting a null hypothesis we should also consider p-value.
For more details on hypothesis test follow the link:
brainly.com/question/10758924
Explanation:
3. Use the potential energy diagram to help you answer the questions below. Make sure to show ALL work for calculations and provide an explanation when directed for full credit. What is the activation energy of this reaction? Show your work ! b. What is the total change in enthalpy of this reaction? Show your work! . Is the slope positive or negative? d. Is this an endothermic or exothermic reaction? Explain.

Answers
The potential energy diagram is a common way of depicting the reaction profile. The following are true about the diagram;
The activation energy of the reaction is 35kJ. The enthalpy change of this reaction is 25kJ.The slope of the diagram is positive.The reaction is endothermic.What is a potential energy diagram?A potential energy diagram is a diagram that shows the conversion of reactants to products. It can tell us whether the reaction is endothermic or exothermic.
1) From the diagram, the activation energy of the reaction = 65 kJ - 30 kJ = 35 kJ
2) The enthalpy change of this reaction = 55kJ - 30 kJ = 25kJ.
3) The slope of the diagram is positive.
4) The reaction is endothermic.
Learn more about endothermic reaction:https://brainly.com/question/2192784
A bicycle tire holds 1.50 L of air at 5atm and 20.0 °C. How many moles of air is this?
If the average mass of air is 29.0 g/mol, what is the mass of air in the tire?
Answers
Answer:
9.05 g
Explanation:
PV=nRT
Use the ideal gas equation. Substitute values.
P = 5 atm
V = 1.50 L
n = ?
R (gas constant) = 0.08206 L-atm/mol-K
T = 20.0°C
*Always convert °C to K.
T = 20.0° + 273 = 293K
Substitute values.
(5 atm)(1.50 L) = n(0.08206 L-atm/mol-K)(293K)
n = (5 atm)(1.50 L) / (0.08206 L-atm/mol-K)(293K)
n = 0.3119335... mol
Convert to grams with the given average mass of air.
0.3119335... mol x (29.0 g/1 mol) = 9.05 g
Can you please help balance and solve the very last question?

Answers
Answer:
Explanation(i) The balanced chemical reaction is given as follows :
\(C_4H_{10}O(aq)\text{ +6O}_2\Rightarrow\text{ 5H}_2O(l)\text{ +4CO}_2(g)\text{ }\)Select the term that correctly describes a chemical reaction that strongly favors the formation of one stereoisomer over another.
Answers
As a result of the inherent reaction specificity, stereoselectivity describes the preferential formation of one merchandise stereoisomer (enantiomer or diastereomer) compared to another during a chemical reaction.
Provide an example of a stereoisomer?Stereoisomers, by definition, are isomers having a same composition (i.e., same parts), but distinct spatial orientations. Stereoisomers come in two main varieties: enantiomers and diastereomers.
What are some examples of stereoisomers?Several sets of molecules attached to a central core have varied relative orientations, which leads to the formation of stereoisomers. Stereoisomers include drugs like R- and S-thalidomide.
To know more about stereoisomer visit:
https://brainly.com/question/16986110
#SPJ4
A) For a volume of tissue, calculate the dose in mGy for a
fluence of 1 × 10^6 [cm−2] of an energy of 1 MeV.
B) what condition is necessary to make this calculation and
why?
Answers
For a volume of tissue, the Dose `D would be 1 × 10^6 [cm−2] × 0.0233 [cm2 g−1] × 1 [MeV] / 1.602 × 10^-13 [J MeV−1] × 1.0 [g] = 1.448 mGy]`. The condition that is necessary to make this calculation is to assume a tissue type because the value of the energy absorption coefficient varies with different tissue types.
A) Calculation of dose in mGy for a volume of tissue and fluence of 1 × 10^6 [cm−2] of an energy of 1 MeV
For this calculation, the required formula is:
`Dose = Fluence × Energy Absorption Coefficient`
The fluence given is:
1 × 10^6 [cm−2] of an energy of 1 MeV
The value of energy absorption coefficient varies according to the tissue. Therefore, we have to assume a tissue type.For example, for the tissue type of soft tissue, the value of the energy absorption coefficient is:
0.0233 [cm2 g−1]
Thus, Dose `D = 1 × 10^6 [cm−2] × 0.0233 [cm2 g−1] × 1 [MeV] / 1.602 × 10^-13 [J MeV−1] × 1.0 [g] = 1.448 mGy]`
B) Condition necessary to make this calculation and why?
The condition that is necessary to make this calculation is to assume a tissue type because the value of the energy absorption coefficient varies with different tissue types.
Learn more about energy absorption coefficient at https://brainly.com/app/profile/61424848
#SPJ11
The basic unit of electric current is the:
ohm.
ampere.
* conductivity.
volt.
Answer is ampere
Answers
Answer:
It's B
Explanation:
Edge2021
what is FGD /Flue gas desulfurization is used for?
Answers
Answer: Flue-gas desulfurization (FGD) material is a product of a process typically used for reducing SO2 emissions from the exhaust gas system of a coal-fired boiler.
Explanation:
Help me please thank you

Answers
Answer:
When nitric acid combine with sodium hydroxide the salt formed is called sodium nitrate. option B
Explanation:
It is the strong acid strong base reaction. When acid and base react with each other salt and water are formed.
In given reaction nitric acid combine with sodium hydroxide base and form sodium nitrate salt and water.
Chemical equation:
HNO₃(aq) + NaOH(aq) → NaNO₃(aq) + H₂O(l)
Ionic equation:
H⁺NO₃⁻(aq) + Na⁺OH⁻(aq) → Na⁺NO₃⁻(aq) + H₂O(l)
Net ionic equation:
H⁺(aq) + OH⁻(aq) → H₂O(l)
The Na⁺(aq) and NO₃⁻(aq) are spectator ions that's why these are not written in net ionic equation. The water can not be splitted into ions because it is present in liquid form.
Spectator ions:
These ions are same in both side of chemical reaction. These ions are cancel out. Their presence can not effect the equilibrium of reaction that's why these ions are omitted in net ionic equation.
Given the equation below, 12.35 grams of H2SO4, and excess Ca(OH)2, what mass of H2O can be produced? Round your answer to two digits after the decimal point.
H2SO4 + Ca(OH)2 à 2 H2O + CaSO4
Answers
To determine the mass of H₂O produced, one need to calculate the stoichiometry of the balanced chemical equation and use it to find the molar amounts involved. After solving the answer is the mass of H₂O that can be produced is approximately 4.53 grams.
The balanced chemical equation is:
H₂SO₄ + Ca(OH)₂ -> 2 H₂O + CaSO₄
the number of moles of H₂SO₄:
Given mass of H₂SO₄= 12.35 grams
Molar mass of H₂SO₄= 98.09 g/mol
Number of moles of H₂SO₄= Mass / Molar mass
= 12.35 g / 98.09 g/mol
≈ 0.1258 mol (rounded to four decimal places)
Since the stoichiometric ratio between H₂SO₄ and H₂O is 1:2, the number of moles of H₂O produced is twice the number of moles of H₂SO₄.
Number of moles of H₂O = 2 × Number of moles of H₂SO₄
= 2 × 0.1258 mol ≈ 0.2516 mol (rounded to four decimal places)
Molar mass of H₂O= 18.015 g/mol
Mass of H₂O= Number of moles of H₂O×Molar mass of H2O
= 0.2516 mol × 18.015 g/mol ≈ 4.53 grams (rounded to two decimal places)
Learn more about the molar mass here.
https://brainly.com/question/16340275
#SPJ1
Consider the reaction Cu + AgNO3 Ag + CUNO3 Which element is reduced? Which element is the oxidizing agent?
Answers
Answer:
Ag is both reduced and the oxidizing agent.
Explanation:
Ag is the answer for both
Cu + AgNO₃ → Ag + CuNO₃
Cu is a reducing agent, and AgNO3 is an oxidizing agent.
What is an oxidizing and a reducing agent?Oxidizing agent:In a chemical process, an oxidizing agent, also known as an oxidant, obtains electrons and becomes reduced. The oxidizing agent often referred to as the electron acceptor, is typically in one of its higher oxidation states since it will receive electrons and be reduced.
Reducing agent:In a chemical process, a reducing agent, also known as a reductant, loses electrons and is oxidized. The electron donor is a reducing substance, which is normally in one of its lower oxidation states. A reducing substance undergoes oxidation because of the redox reaction's electron loss.
Learn more about redox reactions here:
https://brainly.com/question/13978139
#SPJ2
To a reaction vessel, 21.5g of Li(s) and 21.3g of N2(g) are added. Given the reaction below, determine the maximum mass of Li3N that can be produced.
Li(s) + N2(g) --> Li3N
Answers
Answer:
Li₃N ( Lithium nitride)
The law of conservation of mass states that in a chemical reaction, the total mass of reactants is equal to the total mass of products.
mass of reactants there = 21.5 g if Li + 21.3 g of Nitrogen
= 42.8 g
hence the mass maximum mass of Li3N produced is 42.8 g
Which redox reaction would most likely occur if silver and zinc metal were added to a solution that contained silver and zinc?

Answers
The redox reaction which would most likely occur if silver and zinc metal were added to a solution that contained silver and zinc is Zn + Ag\(^+\) \(\rightarrow\)Zn\(^2+\) + 2 Ag .
What is redox reaction?Redox reactions comprise of two parts a reduced part and an oxidized part, which occur simultaneously . The part which is reduced gain electrons and hence there is a increase in oxidation state of the species.
While, the part which is oxidized looses electrons and hence there is a decrease in oxidation state of the species.During redox reactions, there is no net change in the number of electrons . Electrons which are given off in oxidation are used up in reduction.
The ion or molecule which accepts electrons is called as oxidizing agent while the ion or molecule which donates electrons is called as a reducing agent.
Learn more about redox reactions,here:
https://brainly.com/question/13293425
#SPJ1
Which type of plant would
MOST LIKELY have a taproot?
a
A. Monocot
B. Dicot
C. Cotyledon
D. Branched
Answers
Answer:
B
Explanation:
Dicots have a tap root system, while monocots have a fibrous root system and cotyledon produce taproots and Branched have taproot system for short time.
I hope it helps.
5.2 g of HCl are dissolved in 25 mL of solution. What is the molarity?
Answers
Answer:
The molarity is 5.7 moles per dm^3
Explanation:
Molarity is calculated in units of moles per dm^3 or simply moles per liter.
What this means is that to get the molarity, we simply find the number of moles and divide by the volume.
Since we are given the mass, we need to find the number of moles first.
Mathematically, the number of moles = mass/molar mass
The molar mass of HCl is 1 + 35.5 = 36.5 g/mol
The number of moles contained in 5.2g of HCl = 5.2/36.5 = 0.1425 moles
Now, we also need to convert the the volume to liters too.
1000 ml = 1L
25ml = xL
x * 1000 = 25
x = 25/1000 = 0.025 L
the molarity is thus 0.1425/0.025 = 5.7 mol/dm^3
Which of the following molecules contain a covalent bond?
A,CaO B,HCI C,CO2 D,SO2 E,Na2O F,PCL3 G,MgO H,NaH I,CH2
Answers
Answer:
B, C, D, F, I
Explanation:
A covalent bond is a chemical bond between two nonmetals (their electronegativity difference must be greater than 1.5-1.6)
.
A. Ca is a metal and O is nonmetal, so the bond in CaO is ionic
B. Both H and Cl are nonmetals, so the bond in HCl is covalent
C. Both C and O are nonmetals, so the bond in CO2 is covalent
D. Both S and O are nonmetals, so the bond in SO2 is covalent
E. Na is a metal and O is nonmetal, so the bond in Na2O is ionic
F. Both P and Cl are nonmetals, so the bond in PCl3 is covalent
G. Mg is a metal and O is nonmetal, so the bond in MgO is ionic
H. Na is a metal and H is nonmetal, so the bond in NaH is ionic
I. Both C and H are nonmetals, so the bond in CH2 is covalent