Answers
2.Lithium Bromide
3.Beryllium Sulfide
4.Lithium Fluoride
5. Potassium hydroselenide
6. Strontium phosphide
7.Barium Chloride
8.Iron Oxide
9.Iron
10.?
11.Copper Nitride
Related Questions
Which statement is true about the speed of light? (2 points) Light travels relatively slowly. Distance in space is measured using the speed of light. Time in space is measured using the speed of light. The speed of light is unknown.
Answers
Distance in space is measured using the speed of light.
Answer:
Distance in space is measured using the speed of light.
Explanation:
I took the test
What seems to be the basic model for how groups are arranged
Answers
The concept VSEPR theory is mainly based on the arrangement of electron groups. This theory is generally used to predict the geometry of the molecules.
What is VSEPR theory?The Valence shell electron pair repulsion theory is based on the assumption that valence shell electron pairs repel each other and are oriented in space as far apart as possible to minimize mutual repulsion.
Each group around the central atom is designated as a bonding pair or non-bonding pair. For example BeF₂ is a two electron group in which the number of bond pairs on the central 'Be' atom is two.
Similarly the molecule BCl₃ is a three electron group, the number of electron pairs on the 'Be' atom is 3.
To know more about electron groups, visit;
https://brainly.com/question/29104023
#SPJ1
A functional group introduces heteroatoms into a carbon chain to increaseQuestion 5 options: reactivity. molecular mass. polarity. chain length.
Answers
The question asks us to specify the effect of introducing a functional group into a carbon chain.
A simple carbon chain, with only carbons and hydrogens, doesn't show great reactivity because the carbon-to-carbon and carbon-to-hydrogen bonds are strong and the charge of the electrons from theses bonds is spread relatively even over the bonded atoms. Therefore, chains that contain only these two atoms, specially those with only single bonds, are not very reactive.
On the other side, if one or more weaker bonds are introduced into the chain, with the charge of electrons unequally spread over the bond, the reactivity of the molecule tends to increase. When an heteroatom is introduced, for example, the electronegativity difference between atoms will make the electrons more drawn to one side of the bond than the other.
For that reason, we can say that a functional group introduces heteroatoms into a carbon chain to increase the reactivity of this chain (option A).
What is the gaseous state of a green bean casserole?
Answers
Answer:
Green Bean Casserole is a classic.
The family recipe has passed down from one great-aunt to another.
Now it's causing gas and bloating across multiple generations of the family.
Explanation:
suppose you measure the absorbance of a yellow dye solution in a 1.00 cm cuvette. The absorbance of the solution at 427 nm is 0.20. If the molar absorptivity of yellow dye at 427 nm is 27400 M-1cm-1, what is the concentration of the solution?
Answers
The concentration of the solution is obtained as 7.3 * 10^-6 M.
What is the Beer Lamberts law?We know that one of the ways by which we are able to obtain the concentration of substances especially those that are colored is by the use of spectrophotometry. In a spectrophotometer, there is a wavelength of maximum absorption that could be used to measure the concentration of the solution as we can see in the question.
By the use of the Beer Lambert's law we have;
A = εcl
A = Absorption
ε = molar absorptivity
c = concentration
l = path length
Thus we would have;
0.2 = 27400 M-1cm-1 * c * 1.00 cm
c = 0.2/27400 M-1cm-1 * 1.00 cm
c = 7.3 * 10^-6 M
We would have from the calculation that we have a solution whose concentration is 7.3 * 10^-6 M.
Learn more about absorptivity:https://brainly.com/question/8831959
#SPJ1
Draw the products formed when both cis- and trans-but-2-ene are treated with OsO4, followed by hydrolysis with NaHSO3 H2O. Explain how these reactions illustrate that syn dihydroxylation is stereospecific.
Answers
Answer:
See explanation
Explanation:
The reaction with \(OsO_4\) and \(NaHSO_3\) will add two "OH" groups to the molecule. In the reaction (figure 1) we can see the different structures for the alkenes. In the Z alkene (cis) we have the 2 methyl groups on the same side. In the E alkene (trans) the 2 methyl groups are placed on opposite sides. In the products, all the "OH" groups are placed at the top. This indicates that the addition of the hydroxyl groups is "syn". In the syn reactions, all the groups are bonded on the same side therefore we will have a stereospecific reaction.
I hope it helps!

Select the TWO statements that are true about the amount and types of energy that are visible when each of the light bulbs are shining.
A. The incandescent light bulb does not create as much as much light energy as the fluorescent light bulb.
B. When the fluorescent light bulb is lit, more light energy is visible than thermal energy.
C. The thermal energy is stored in the incandescent light bulb before it is transformed into light energy.
D. More thermal energy is visible when the fluorescent light bulb is lit.

Answers
A. The incandescent light bulb does not create as much as much light energy as the fluorescent light bulb.
Why do you think fluorescent bulbs are more energy-efficient than incandescent ones?Incandescent light bulbs have the drawback of wasting a lot of electricity due to heat. All the energy used to create heat is a waste because heat does not produce light, and the light bulb's intended function is to produce light. Thus, incandescent lamps are quite ineffective.
Why does a fluorescent tube produce light that is brighter and uses less energy than an incandescent lamp?Mercury vapor is excited by an electric current in the gas to produce short-wave ultraviolet light, which illuminates a phosphor coating inside the lamp. Compared to an incandescent lamp, a fluorescent lamp is far more efficient at converting electrical energy into usable light.
To know more about the fluorescent light visit:
https://brainly.com/question/8979272
#SPJ1
What is the mass in grams of 12.9 moles of water, H2O?
Answers
Answer:
232 g.
Explanation:
12.9 mol x 18.106g = 232 g H₂O
a polygon with four equal sides ands angle
Answers
A rhombus is a parallelogram with all four sides congruent to each other. diamond-like shape. A square is a parallelogram with four congruent sides and four right angles. In other words, a square is a rectangle and a rhombus.
7. Apply Scientific Reasoning
Hooke
and Van Leeuwenhoek made their discoveries
around the same time. More than 150 years
later, Schleiden, Schwann, and Virchow all
made breakthroughs within a few years of each
other. What are some possible reasons for the
sudden development of the cell theory after
such a long break?
Answers
The 150-year gap in the scientific discovery could be because many individuals believed the theory of spontaneous generation.
What is the theory of spontaneous generation?The theory of Spontaneous generation is a superseded scientific theory that held that living creatures could arise from nonliving matter and that such processes were commonplace and regular.
The theory of Spontaneous generation is incorrect and also obsolete. This theory was formulated by Aristotle.
The theory of Spontaneous generation aims to explain the seemingly sudden emergence of organisms such as rats, flies and maggots within rotting meat and other decomposable items by suggesting that organisms do not descend from other organisms or from a parent, and that only require that certain conditions in their environment be fulfilled in order for creation to occur.
Many scientist disapproved the theory of Spontaneous generation and made studies and experiments to that effect. Such scientist's includes persons like Francesco Redi, John Needham, Lazzaro Spallanzani and many more people.
Learn more about theory of Spontaneous generation at: https://brainly.com/question/15387713
#SPJ1
One way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is by titrating a sample of silver nitrate solution. Any chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. Suppose an EPA chemist tests a sample of groundwater known to be contaminated with nickel(II) chloride, which would react with silver nitrate solution like this:
Answers
Answer:
6.5 mg/L.
Explanation:
Step one: write out and Balance the chemical reaction in the Question above:
NiCl2 + 2AgNO3 =====> 2AgCl + Ni(NO3)2.
Step two: Calculate or determine the number of moles of AgCl.
So, we are given that the mass of AgCl = 3.6 mg = 3.6 × 10^-3 g. Therefore, the number of moles of AgCl can be calculated as below:
Number of moles AgCl = mass/molar mass = 3.6 × 10^-3 g / 143.32. = 2.5118 × 10^-5 moles.
Step three: Calculate or determine the number of moles of NiCl2.
Thus, the number of moles of NiCl2 = 2.5118 × 10^-5/ 2 = 1.2559 × 10^-5 moles.
Step four: detemine the mass of NiCl2.
Therefore, the mass of NiCl2 = number of moles × molar mass = 1.2559 × 10^-5 moles × 129.6 = 1.6 × 10^-3 g.
Step five: finally, determine the concentration of NiCl2.
1000/ 250 × 1.6 × 10^-3 g. = 6.5 mg/L.
Complete the mass balance expression for a saturated solution of Ag2CO3. Note at least one blank is a numeric coefficient. (Note: Ignore any subsequent reactions of Ag^+)
[ ] = _ ( [ ] + [ ] + [ ] )
Hint: Consider how Ag2CO3 dissolves in water and what reactions, if any, each ionic product would further be involved with water.
Answers
The mass balance expression for a saturated solution of Ag₂CO₃ can be written as:
\(\mathrm{[Ag_2CO_3] = Ksp/([Ag+]^2 [CO_3^2-])}\)
What is solubility product constant?The degree to which an ionic compound dissolves in water depends on the solubility product constant (Ksp), which is a thermodynamic equilibrium constant. It is referred to as the sum of the ion concentrations in a saturated solution of the compound, each concentration being raised to the power of the ion's stoichiometric coefficient in the chemical equation for the compound's dissolution.
Where [Ag₂CO₃] is the molar solubility of Ag₂CO₃ in the solution, Ksp is the solubility product constant of Ag₂CO₃, and [Ag⁺] and [CO₃²⁻] are the concentrations of silver ions and carbonate ions in the solution, respectively.
Ag₂CO₃ dissolves in water to form Ag⁺ and CO₃²⁻ ions according to the following equation:
Ag₂CO₃(s) ⇌ 2Ag+(aq) + CO₃²⁻(aq)
However, the Ag+ and CO₃²⁻ ions may further react with water to form other species. For example, Ag+ ions can form the complex ion Ag(H₂O)₂⁺:
Ag+(aq) + 2H₂O(l) ⇌ Ag(H₂O)2+(aq) + H₃O+(aq)
Therefore, the complete mass balance expression for a saturated solution of Ag₂CO₃ is:
[Ag₂CO₃] = Ksp/([Ag+]² [CO₃²⁻])
where [Ag+] = [Ag(H₂O)2+] + [AgOH(aq)]
and [CO₃²⁻] = [HCO₃⁻(aq)] + [CO₃²⁻(aq)] + [OH-(aq)]
To know more about solubility product constant, visit:
brainly.com/question/1419865
#SPJ1
What is the most accurate glassware for measuring a specific volume?
Answers
Volumetric glassware
There are five kinds of glassware to measure specific volume. Volumetric pipets, burets, volumetric flasks, beakers, and graduated cylinders.
The most accurate of the mentioned above are burets, volumetric pipets, and volumetric flasks.

Problem: 8.41 g magnesium oxide reacts with 5.40 L of carbon dioxide to form 14.45 g of a compound that is 28.83% magnesium, 14.24% carbon, and 56.93% oxygen. What is the percent yield?
Answers
The yield is 108.2% in percentage. As this result is higher than 100%, it is possible that the experimental process or the measurements had errors.
What occurs when carbon dioxide and magnesium oxide interact?In mild conditions, magnesium oxide works well as a catalyst for the cycloaddition of carbon dioxide to epoxides; when carbon dioxide reacts with (R)-styrene oxide in the presence of magnesium oxide, (R)-phenyl carbonate is produced in 97% of the time with stereochemistry retained.
We must first estimate the theoretical yield of the compound generated in order to calculate the percent yield.
molar mass of MgO = 24.31 g/mol (for Mg) + 15.99 g/mol (for O) = 40.30 g/mol
moles of MgO = 8.41 g / 40.30 g/mol = 0.2087 mol MgO
moles of compound formed = 0.2087 mol MgO = 0.2087 mol compound
mass of compound = (28.83% Mg / 100%) x 14.45 g = 4.16 g Mg
(14.24% C / 100%) x 14.45 g = 2.06 g C
(56.93% O / 100%) x 14.45 g = 8.23 g O
= 14.45 g of the compound formed
percent yield = (actual yield / theoretical yield) x 100%
actual yield = 14.45 g
theoretical yield = 0.2087 mol x (24.31 g/mol + 15.99 g/mol + 3(16.00 g/mol)) = 13.36 g
percent yield = (14.45 g / 13.36 g) x 100% = 108.2%.
To know more about magnesium visit:-
https://brainly.com/question/1533548
#SPJ1
738.90 m has ____ significant figures
Answers
Answer: 4
Explanation: because the zero doesn't count
which shows the correct order of stelps during the formation of an ionic bond
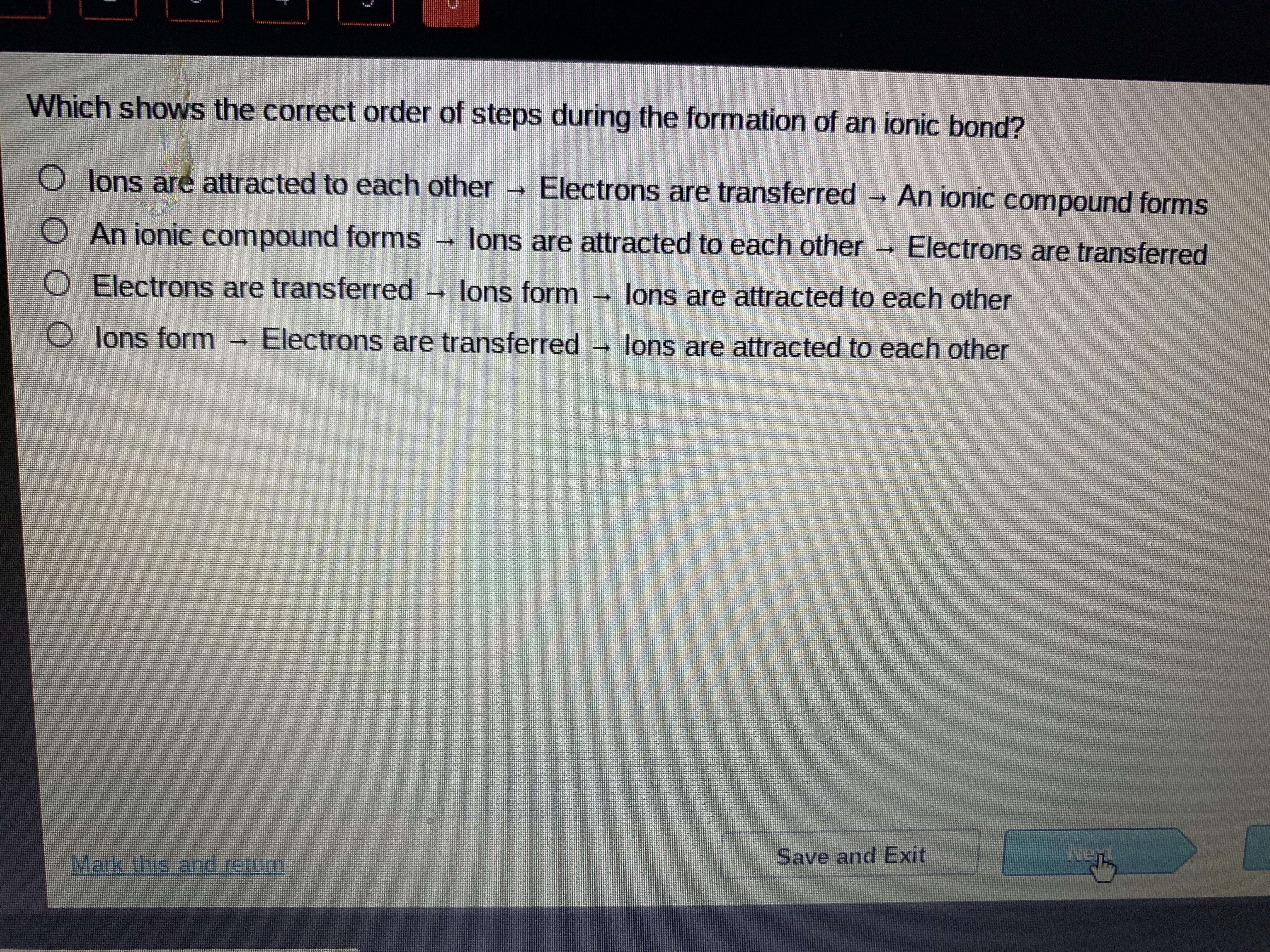
Answers
Answer:
The third one
Explanation:
HELP I HAVE A PICTURE

Answers
A maritime tropical meeting a continental tropical, will produce this kind of weather.
What happens when maritime tropical meeting continental tropical?When maritime tropical air masses meet continental tropical air masses, it can lead to the formation of weather patterns that can have significant impacts on the local and regional weather conditions.
Maritime tropical air masses are warm and humid air masses that form over the tropical and subtropical oceans, while continental tropical air masses are hot and dry air masses that form over land areas in the subtropics and tropics. When these two air masses meet, the warm, moist maritime tropical air rises over the denser, drier continental tropical air.
As the air rises, it cools and the water vapor in it condenses, leading to the formation of clouds and possibly precipitation. The amount and type of precipitation that forms depend on a variety of factors, such as the temperature and humidity of the two air masses, the strength and direction of the wind, and the topography of the land.
To know more about subtropics, visit:
https://brainly.com/question/30586789
#SPJ1
100 points answer in depth Describe the current model of the atom and the characteristics of each of the three subatomic particles.
Answers
Answer:
The current model of the atom and the characteristics of each of the 3 subatomic particles are the electron cloud which contains 1 subatomic particle the electron which has negative charge and weights 1/2000 AMU the electron cloud surrounds the nucleus, the nucleus contains two subatomic particles the proton which has positive charge and weights one AMU and the neutron which has neutral/no charge and weights one AMU.
If the concentration of OH- is 2.3 x 10-5 M, what is the pH?
Answers
The pH of a solution with a hydroxide ion concentration of 2.3 x 10^-5 M is approximately 9.362.
pOH = -log[OH-]
Since pH + pOH = 14 (at 25°C), we can rearrange the equation to solve for pH:
pH = 14 - pOH
Given that the concentration of OH- is 2.3 x 10^-5 M, we can calculate the pOH as follows:
pOH = -log(2.3 x 10^-5)
Using logarithm properties, we can simplify the expression:
pOH = -log(2.3) - log(10^-5)
Since log(10^-5) = -5, the equation becomes:
pOH = -log(2.3) - (-5)
Next, we need to calculate the value of -log(2.3):
log(2.3) ≈ -0.362
Substituting this value into the pOH equation:
pOH ≈ -0.362 - (-5)
pOH ≈ -0.362 + 5
pOH ≈ 4.638
Now, we can calculate the pH using the pH + pOH = 14 equation:
pH = 14 - 4.638
pH ≈ 9.362
Therefore, the pH of a solution with a hydroxide ion concentration of 2.3 x 10^-5 M is approximately 9.362. It's possible that the concentration was meant to be 2.3 x 10-9 M, which would give a pH of 9.64. Alternatively, if the concentration of H+ ions was given instead, we could use the formula pH = -log[H+] to find the pH of the solution.
For more usch questions on hydroxide
https://brainly.com/question/21393201
#SPJ11
Following a chemical reaction that produced 5.06 grams of magnesium chloride, the lab report was prepared to
document the results. The expected result was estimated to be 8.85 grams. What are the percent yield and percent
error that are to be included in the lab report?
Percent Yield
Percent Error
Answers
Answer:
1. Percentage yield = 57.2%
2. Percentage error = 74.9%
Explanation:
From the question given above, the following data were obtained:
Actual yield = 5.06 g
Experimental yield = 8.85 g
Percentage yield =?
Percentage error =?
1. Determination of the percentage yield.
Actual yield = 5.06 g
Experimental yield = 8.85 g
Percentage yield =?
Percentage yield = Actual yield /Experimental yield × 100
Percentage yield = 5.06 / 8.85 × 100
Percentage yield = 57.2%
2. Determination of the Percentage error.
Actual yield = 5.06 g
Experimental yield = 8.85 g
Percentage error =?
Percentage error = |Experimental – Actual| / Actual yield × 100
Percentage error = |8.85 – 5.06| / 5.06 × 100
Percentage error = 3.79 / 5.06 × 100
Percentage error = 74.9%
Molecular hydrogen and oxygen combine to form water. If you have 10.00g of oxygen and 10.0g of hydrogen react, once the reaction is complete, which reactant would you have left over and how much of it would you have left over?
Answers
The amount of hydrogen left over is 9.683 g.
Which reactant would you have left over?The balanced chemical equation for the reaction between molecular hydrogen (H₂) and oxygen (O₂) to form water (H₂O) is:
2H₂ + O₂ -> 2H₂O
The equation shows that two moles of hydrogen react with one mole of oxygen to produce two moles of water.
To determine which reactant is left over, we need to calculate how much of each reactant is required for the reaction and compare it with the actual amount provided.
The reactant that is not completely consumed will be left over.
First, we need to convert the masses of hydrogen and oxygen to moles using their respective molar masses:
Molar mass of hydrogen (H2) = 2.016 g/mol
Molar mass of oxygen (O2) = 31.9988 g/mol
Number of moles of hydrogen = 10.0 g / 2.016 g/mol = 4.96 mol
Number of moles of oxygen = 10.00 g / 31.9988 g/mol = 0.3125 mol
From the balanced equation, we can see that 2 moles of hydrogen react with 1 mole of oxygen.
Therefore, the number of moles of oxygen required to react with 4.96 moles of hydrogen is:
0.5 x 4.96 = 2.48 mol
Since we only have 0.3125 mol of oxygen, it is the limiting reactant, and hydrogen is in excess.
To find out how much hydrogen is left over, we need to calculate how much hydrogen was used in the reaction:
Number of moles of water produced = Number of moles of oxygen used = 0.3125 mol
Number of moles of hydrogen used = 0.5 x 0.3125 = 0.15625 mol
The total number of moles of hydrogen we started with was 4.96 mol, and 0.15625 mol of hydrogen was used in the reaction.
Therefore, the amount of hydrogen left over is:
4.96 mol - 0.15625 mol = 4.80375 mol
Finally, we can convert this back to a mass:
Mass of hydrogen left over = 4.80375 mol x 2.016 g/mol = 9.683 g
Learn more about reactant here: https://brainly.com/question/26283409
#SPJ1
Answer:
The amount of hydrogen left over is 9.683 g.
Explanation:
what is the change in mass of A in
60 minutes?
Mass of A (g)
12.4
10.4
9.1
7.7
6.2
Time
O
15
30
45
60
Answers
Answer:
To determine the change in mass of A over the given time period, we need to find the difference between the initial mass of A and the final mass of A.
From the given table, we can see that the initial mass of A at t = 0 (start time) is 12.4 g and the final mass of A at t = 60 minutes (end time) is 6.2 g.
Therefore, the change in mass of A over 60 minutes is:
Final mass of A - Initial mass of A
= 6.2 g - 12.4 g
= -6.2 g
The negative sign indicates that the mass of A decreased over time, which means that A underwent some kind of reaction or process that caused it to lose mass.
The change in mass of A over 60 minutes is -6.2 grams.
To determine the change in mass of A over 60 minutes, we need to compare the initial mass to the final mass.
From the given information, we can see that the mass of A decreases over time.
Let's calculate the change in mass.
Initial Mass of A: 12.4 g
Final Mass of A: 6.2 g
Change in Mass of A = Final Mass of A - Initial Mass of A
= 6.2 g - 12.4 g
= -6.2 g
The change in mass of A over 60 minutes is -6.2 grams.
Note that the negative sign indicates a decrease in mass.
For such more questions on mass
https://brainly.com/question/1838164
#SPJ8
Calculate the pH for the following buffer solutions with steps.
1. 7.4862 grams of acetic acid and 10.4695 grams of sodium acetate are dissolved in enough deionized water to make 250.0 mL of solution.
2. 11.8115 grams of trimethylamine and 18.7088 grams of trimethylammonium chloride are dissolved in enough deionized water to make 500.0 mL of solution.
Thank You:)
Answers
Answer:
Answer is shown on the paper :D
Explanation:
Remember to follow sig-figs.


The resistance of a solution of copper sulfate is measured at 0.51 ohms when two electrodes are 4.00 cm apart. If resistance is directly proportional to the distance between electrodes, what is the distance between the electrodes if the resistance is 8 ohms?
Answers
When the resistance changes , the distance between the electrodes will get changed to 62.74 cm
The resistance and length formula clearly states that, for a given material, the resistance is precisely related to its length. The resistance value of a material increases with an increase in length. The material's resistance value will drop as its length does as well.
Here it is given that ,
Resistance of solution of CuSO₄ (R₁) = 0.51 ohms,
Distance between electrodes (d₁)= 4.00cm
When resistance changes,
R₂= 8 ohms
We have to calculate d₂,
d₂=?
It is given that ,
Resistance is directly proportional to distance between electrodes,
R∝ d
R₁/R₂= d₁/d₂
0.51/8=4/d₂
d₂= 8×4/0.51
=62.74 cm
So, the distance between the electrodes will be 62.74 cm , it is directly proportional to the resistance, it increases as the resistance increases.
To learn more about resistance , please refer,
https://brainly.com/question/28135236
#SPJ1
A Sample of an Organic Compound Contain
0.624 Carbon, 0.065 hydrogen, 0·028 oxygen
(a) what is the Emperical formuler of the Compound.
(b) If the relative molecular mass of the Compound Is 1940 what is the moleculer
formular of the compound (C=12₁ H=1
N = 14,0= 16)
Answers
(a) The empirical formula of the compound is \(C_{29}H_ {36}O\)
(b) The molecular formula of the compound is approximately \(C_{8}H_{118}O_{3}\)
To determine the empirical formula of the organic compound, we need to find the simplest whole-number ratio of the elements present.
(a) The given percentages of carbon, hydrogen, and oxygen can be converted into moles by dividing them by their respective atomic masses:
Carbon: 0.624 g / 12.01 g/mol = 0.052 mol
Hydrogen: 0.065 g / 1.008 g/mol = 0.064 mol
Oxygen: 0.028 g / 16.00 g/mol = 0.0018 mol
Next, we divide each of the mole values by the smallest mole value (0.0018 mol in this case) to obtain the mole ratio:
Carbon: 0.052 mol / 0.0018 mol ≈ 29
Hydrogen: 0.064 mol / 0.0018 mol ≈ 36
Oxygen: 0.0018 mol / 0.0018 mol = 1
Therefore, the empirical formula of the compound is \(C_{29}H_ {36}O\)
(b) To find the molecular formula, we need the relative molecular mass of the compound, which is given as 1940 g/mol. The empirical formula mass can be calculated by summing the atomic masses in the empirical formula:
Empirical formula mass: (29 × 12.01 g/mol) + (36 × 1.008 g/mol) + (1 × 16.00 g/mol) = 588.94 g/mol
To find the multiplier, we divide the relative molecular mass by the empirical formula mass:
Multiplier: 1940 g/mol / 588.94 g/mol ≈ 3.29
Rounding to the nearest whole number, the molecular formula of the compound is approximately 3 times the empirical formula, resulting in \(C_{8}H_{118}O_{3}\).
In summary, the empirical formula of the compound is\(C_{29}H_ {36}O\), and the molecular formula is approximately \(C_{8}H_{118}O_{3}\).
Know more about empirical formula here:
https://brainly.com/question/1439914
#SPJ8
5 pois
Energy pyramids illustrate the transfer of 10% of the energy stored by
producers flowing from the bottom level to primary consumers. What
happens to the other 90% of the energy?
О
The remaining 90% is transformed for life activities such as movement, growth, or
released as heat.
The remaining 90% evaporates into the atmosphere.
The remaining 90% transforms into Hydrogen and Oxygen.
The remaining 90% is recombined to maker larger molecules.
Answers
What is the chemical equation of table salt
Answers
Answer: NaCl
Explanation:
Table salt is made up of 1 atom of sodium (Na) and 1 atom of chlorine (Cl)
in which location are there no changes in season
a north pole
B South Pole
C equator
D antártica
Answers
Answer: I'm going to say it's Antartica because the only seasons it have is summer and winter and it doesn't change.
Explanation:
Which of the following do all food chains in an ecosystem depend on?
A.
consumers
B.
competition
C.
decomposers
D.
producers
Answers
A mouthwash contains 22.5% alcohol(p=0.9652). If the bottle of mouthwash contains 355 mL. How many milliliters of alcohol are present(p=0.7893)?
Answers
The volume of the alcohol can be obtained as 79.9 mL.
What is the volume of the alcohol?We know that there are many different unit of concentration. The unit of concentration that we are going to focus on here is the volume per volume percent. This has to do with the volume of solute in a given volume of the solvent.
Thus we have;
Volume solution = 355 mL
Volume percent of the alcohol = 22.5%
Actual volume of the alcohol = x
We now have;
Volume percent = Volume of the alcohol (x)/volume of the solution * 100/1
22.5 = x/355 * 100/1
22.5 = 100x/355
x = 22.5 * 355/100
x = 79.9 mL
Learn more about volume percent :https://brainly.com/question/15461083
#SPJ1
Missing parts;
A mouthwash contains 22.5% (v/v) alcohol. If the bottle of mouthwash contains 355 mL, what is the volume, in milliliters, of alcohol