Metal plating is done by passing a current through a metal solution. For example, an item can become gold plated by attaching the item to a power source and submerging it into an Au³⁺ solution. The item itself serves as the cathode, at which the Au³⁺ ions are reduced to Au(s). A piece of solid gold is used as the anode and is also connected to the power source, thus completing the circuit. What mass of gold is produced when 15.1 A of current are passed through a gold solution for 31.0 min?
Answers
Answer:
172 g
Explanation:
Let's consider the reduction of Au³⁺ to Au.
Au³⁺(aq) + 3 e⁻ → Au(s)
In order to find the mass of gold produced, we will use the following relations.
1 min = 60 s1 A = 1 C/sThe charge of 1 mole of electrons is 96,468 C (Faraday's constant).1 mole of Au is deposited when 3 moles of electrons circulate.The molar mass of Au is 196.97 g/mol.The mass of gold produced when 15.1 A of current are passed through a gold solution for 31.0 min is:
\(31.0min \times \frac{60s}{1min} \times \frac{15.1C}{s} \times \frac{1mole^{-} }{96,468C} \times \frac{3molAu}{1mole^{-} } \times \frac{196.97gAu}{1molAu} = 172 gAu\)
Related Questions
While isobaric heat can be measured by using the coffee cup calorimeter, what kind of device would be needed to measure the reaction heat under isochoric condition? Please search literature to answer the question.
To measure the reaction heat more accurately at isobaric condition, what modification(s) would you suggest making on the coffee cup calorimeter? Please justify the suggested change(s).
Answers
To measure reaction heat under isochoric conditions, a bomb calorimeter is needed.
This device is designed to maintain a constant volume (isochoric) during the reaction, allowing for accurate measurement of reaction heat. To improve the accuracy of the coffee cup calorimeter for measuring reaction heat under isobaric conditions, a modification that could be made is to use a stirring device to ensure uniform mixing of the reactants and to minimize heat loss to the surroundings.
Additionally, a lid with a small hole could be placed over the top of the calorimeter to prevent heat loss while still allowing for pressure equalization. These modifications would help to minimize errors in heat measurement and improve the accuracy of the results obtained.
To know more about the Calorimeter, here
https://brainly.com/question/24150308
#SPJ1
How did he show that these particles had a charge on them?
Answers
J.J. Thomson discovered electrons and their negative charge through the cathode ray experiment, leading to the development of the plum pudding model of the atom.
J.J. Thomson, a British physicist, was the first to discover electrons in 1897.
He conducted the cathode ray experiment to identify the negatively charged particles.
The cathode ray tube is a vacuum-sealed glass tube with two electrodes at each end: a cathode and an anode.
When a high voltage electrical current is applied to the electrodes, the tube glows, indicating that the cathode rays are being emitted from the cathode and traveling through the tube towards the anode.
The cathode rays were found to have a negative charge, according to Thomson.
These rays were identified as particles by the presence of a magnet, which caused the particles to bend in the direction opposite to the magnet's polarity.
This discovery indicated that the particles had a charge on them because they were deflected by the magnetic field, which is only possible if the particles have an electric charge.
Thomson further concluded that these particles were about 1,000 times smaller than hydrogen atoms because of the degree of deflection they experienced in the magnetic field.
Furthermore, Thomson created the plum pudding model of an atom, in which electrons are dispersed throughout a positively charged matrix, based on his findings.
For more such questions on electrons
https://brainly.com/question/26084288
#SPJ8
calculate the number of moles for the quanity 8.06 x 1021 atoms of Pt
Answers
The number of moles for the quanity 8.06 x\(10_{21\) atoms of Pt is approximately 2.61 grams.
To calculate the number of moles for a given quantity of atoms, we can use Avogadro's number and the molar mass of the element. Avogadro's number is 6.022 x 10²³ atoms/mol.
In this case, you have 8.06 x 10²¹ atoms of Pt. To find the number of moles, divide this quantity by Avogadro's number:
8.06 x 10²¹ atoms Pt / 6.022 x 10²³ atoms/mol = 0.0134 mol Pt
So, there are approximately 0.0134 moles of Pt in 8.06 x 10²¹ atoms of Pt.
The molar mass of Pt (platinum) is 195.08 g/mol. To convert the number of moles to grams, multiply the number of moles by the molar mass:
0.0134 mol Pt x 195.08 g/mol = 2.61 g Pt
Therefore, there are approximately 2.61 grams of Pt in 8.06 x10²¹ atoms of Pt.
In summary, the number of moles for the quantity 8.06 x 10²¹ atoms of Pt is approximately 0.0134 moles. This is equivalent to approximately 2.61 grams of Pt. Remember to use Avogadro's number and the molar mass to perform these calculations accurately.
Know more about moles here:
https://brainly.com/question/29367909
#SPJ8
A solution was prepared by dissolving 125.0 g of KCl in 315 g of water.
Calculate the molality of KCl . (The formula weight of KCl is 74.6 g/mol . The formula weight of water is 18.0 g/mol .)
Express the concentration of KCl in molality to two decimal places.
Answers
The molality of the KCl solution, given the data is 5.33 M
What is molality?Molality is defined as the mole of solute per unit kilogram of water. Mathematically, it can be expressed as:
Molality = mole / mass of water (in Kg)
How to determine the mole of KClFrom the question given above, the following data were obatined
Mass of KCl = 125 gMolar mass of KCl = 74.6 g/mol Mole of KCl =?Mole = mass / molar mass
Mole of KCl = 125 / 74.6
Mole of KCl = 1.68 mole
How to determine the molality of KCl solutionMole of KCl = 1.68 mole Mass of water = 315 g = 315 / 1000 = 0.315 KgMolality =?The molality of the solution can be obtained as follow:
Molarity = mole / mass of water (in Kg)
Molality = 1.68 / 0.315
Molality = 5.33 M
Learn more about Molality:
https://brainly.com/question/4251997
#SPJ1
What the volume of 59.2 grams of number 94. The density of plutonium is 19.8 g/cm^ 3
A) 377 cm^3
B) 0.0537 cm^3
C) 2.99 cm^3
D) 3.33 cm^3
Answers
Answer:
C) 2.99 cm^3
Explanation:
ρ=m/V
V=m/ρ=59.2/19.8=2.99 cm^3
\({ \red {\sf{Define \: Transportational}}}\)
2+x=19
Find x
Answers
Easy!!
2+17=19
#hope that helps
Answer:
To find the answer you may
2+17=19
Explanation:
so that is the answer I'm sorry,to not give all the solutions
how many molecules of potassium chloride will react if 21.89 grams KCl are added to the solution
Answers
There are approximately 1.765 x 10²³ molecules of KCl in 21.89 grams of KCl.
What is meant by potassium chloride ?Potassium chloride (KCl) is a compound made up of potassium and chloride ions. It is a colorless, odorless salt that is commonly used in a variety of applications.
Molar mass of KCl is 74.55 g/mol; number of moles = Mass/ Molar mass
So, the number of moles = 21.89 g ÷ 74.55 g/mol = 0.2936 mol
and the number of molecules = Number of moles * Avogadro's number
Number of molecules = 0.2936 mol x 6.02 x 10²³ molecules/mol
Number of molecules = 1.765 x 10²³ molecules
Therefore, there are approximately 1.765 x 10²³ molecules of KCl in 21.89 grams of KCl.
To know more about potassium chloride, refer
https://brainly.com/question/25380525
#SPJ1
Calculate the numerical value of Kc for
the following reaction if the equilibrium
mixture contains 0.0450 M PCI 3, 0.116
M Cl₂, and 25.8 M PCI 5.
PC 3 (g) + Cl₂ (g) ⇒ PCI5 (g)
(the 5 in PCl is a subscript)

Answers
The numerical value of Kc for the given reaction, with the equilibrium mixture containing 0.0450 M PCl3, 0.116 M Cl2, and 25.8 M PCl5, is approximately 4942.03.
To calculate the numerical value of Kc for the given reaction, we need to set up an expression for the equilibrium constant using the concentrations of the species involved.
The balanced equation for the reaction is:
PCl3 (g) + Cl2 (g) ⇌ PCl5 (g)
The equilibrium constant expression, Kc, is given by:
Kc = [PCI5] / ([PCl3] * [Cl2])
Given the equilibrium concentrations:
[PCl3] = 0.0450 M
[Cl2] = 0.116 M
[PCI5] = 25.8 M
Substituting these values into the equilibrium constant expression, we have:
Kc = (25.8 M) / ((0.0450 M) * (0.116 M))
Calculating Kc:
Kc = 25.8 M / (0.00522 M^2)
Kc = 4942.03
Therefore, the numerical value of Kc for the given reaction, with the equilibrium mixture containing 0.0450 M PCl3, 0.116 M Cl2, and 25.8 M PCl5, is approximately 4942.03.
For more such questions on equilibrium visit:
https://brainly.com/question/18849238
#SPJ11
if 0.75 g of gas at 40 atm of pressure dissolves in 1.25 l of water at 25°c, how much will dissolve in 2.0 l of water at 3.0 atm of pressure and the same temperature
Answers
In this case, we can use Henry's law, which states that the mole fraction of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. Mathematically, we can write:
x = k * P
where x is the mole fraction of the gas in the liquid, P is the partial pressure of the gas above the liquid, and k is the proportionality constant, which depends on the gas and the liquid.
To solve this problem, we need to use the given information to find the value of k, and then use Henry's law to find the mole fraction of gas in the second solution.
First, we can use the given information to find the value of k:
x = k * P
0.75 g of gas at 40 atm of pressure dissolves in 1.25 l of water, which has a density of about 1 g/cm^3 at 25°C. The molar mass of the gas is not given, but we can assume that it is a simple gas such as nitrogen, oxygen, or carbon dioxide, which have molar masses of about 28 g/mol, 32 g/mol, and 44 g/mol, respectively. Let's assume that the gas is nitrogen, which has a molar mass of 28 g/mol. Then, the number of moles of gas dissolved in the water is:
n = m / M = 0.75 g / 28 g/mol ≈ 0.0268 mol
The total number of moles in the solution is equal to the number of moles of gas plus the number of moles of water:
n_total = n_gas + n_water
n_total = n_gas + V_water / M_water
where V_water is the volume of water and M_water is the molar mass of water (18 g/mol).
Since the density of water is about 1 g/cm^3, we can convert the volume of water from liters to cubic centimeters:
1.25 l = 1250 cm^3
Then, we can calculate the total number of moles in the solution:
n_total = 0.0268 mol + 1250 cm^3 / 1000 cm^3/mol
n_total ≈ 0.048 mol
The mole fraction of the gas in the solution is equal to the number of moles of gas divided by the total number of moles:
x = n_gas / n_total
x = 0.0268 mol / 0.048 mol
x ≈ 0.558
Now we can use Henry's law to find the mole fraction of gas in the second solution:
x = k * P
We want to find x when P = 3.0 atm and V_water = 2.0 L. Since the temperature is the same as in the first solution, we can assume that k has the same value as before.
k = x / P
k = 0.558 / 40 atm
k ≈ 0.014
x = k * P
x = 0.014 * 3.0 atm
x = 0.042
Therefore, the mole fraction of gas in the second solution is about 0.042. To find the number of moles of gas in the second solution, we can use the same formula as before:
n_gas = x * n_total
n_gas = 0.042 * 0.048 mol
n_gas ≈ 0.002 mol
Finally, we can convert the number of moles of gas to mass using the molar mass of nitrogen:
m_gas = n_gas * M_gas
m_gas = 0.002 mol * 28 g/mol
m_gas = 0.056 g
Therefore, approximately 0.056 g of gas will dissolve in 2.0 L of water at 3.0 atm of pressure and the same temperature.
When the equation
_Pb2+ + Au²+ →__pb4+ + _Au
is correctly balanced using the smallest whole-number coefficients, the coefficient of Pb2+ will be
1.1
2.2
3.3
4.4
Submit Answer
Hide Toolbar
Answers
The answer is option 2 , the smallest whole-number coefficients, the coefficient of Pb²⁺ will be 2.
What is a Balanced Chemical Equation ?
Law of conservation of mass states that mass can neither be created nor be destroyed but it can only be transformed from one form to another form.
This also means that total mass on the reactant side must be equal to the total mass on the product side.
There are two types of numbers that appear in chemical equations.
There are subscripts, which are part of the chemical formulas of the reactants and products;
and there are coefficients that are placed in front of the formulas to indicate how many molecules of that substance is used or produced.
Balance The Equation:
Pb²⁺ + Au²⁺ = Pb⁴⁺ + Au
Label Each Compound With a Variable
Label each compound with a variable to represent the unknown coefficients.
aPb²⁺ + bAu²⁺ = c Pb⁴⁺ + d Au
Create a System of Equations
Create an equation for each element where each term represents the number of atoms of the element in each reactant or product.
Pb: 2a + 0b = 4c + 0d
Au: 0a + 2b = 0c + 1d
e: 0a + -1b = -1c + 0d
2a - 4c = 0
2b - 1d = 0
-1b + 1c = 0
Solve For All Variables
Use substitution,
Substitute Coefficients and Verify Result
2Pb²⁺ + Au²⁺ = Pb⁴⁺ + 2 Au
So the answer is 2 , option 2
To know more about Balanced Chemical Equation
https://brainly.com/question/15052184
#SPJ1
Which is a symbol that represents SI units for temperature?
0 °C
g
OL
OF
Answers
Answer:
0 °C
Explanation:
Answer:
0 C
Explanation:
Use the balanced equation to answer each question.
2 C2H6(g) + 5 O2(g) delta 4 CO(g) + 6 H2O(g)
How many moles of C2H6 are needed to form 2.0 moles of CO?
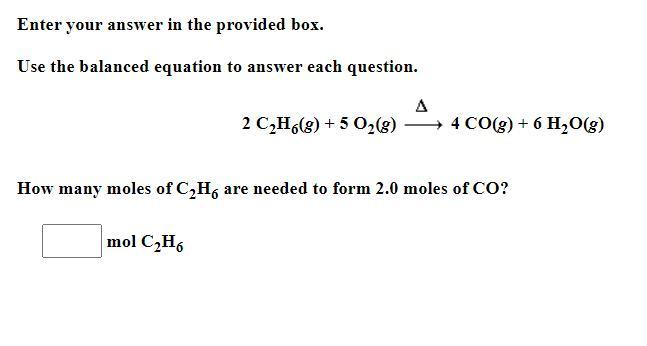
Answers
Answer:
2 moles of C2H6 are required to form 4 moles of CO
therefore for 2 moles of CO, 1 mole of C2H6 is required
13C NMR is a technique in which the total number of signals represents the number of unique carbon atoms in a molecule. Propose a structure that is consistent with the following data.
a. The IR includes peaks at 1603 and 1495 cm^-1
b. The 13c NMR has a total of 7 signals
c. The compound has one acidic proton.
Answers
Answer:
D. Poop Butt.
Explanation:
Based on the given data, we can propose a possible structure that fits the criteria: a. carbonyl group (C=O) and an aromatic ring b. there are seven unique carbon environments. c. Presence of a functional group like a carboxylic acid or phenol .
a. The IR peaks at 1603 \(cm^{-1}\) and 1495\(cm^{-1}\)suggest the presence of both a carbonyl group (C=O) and an aromatic ring.
b. The 13C NMR having a total of 7 signals indicates that there are seven unique carbon environments in the molecule.
c. Considering the presence of an acidic proton, it suggests the presence of a functional group like a carboxylic acid (COOH) or phenol (\(C_6H_5OH\)).
Putting all this information together, a possible structure that fits the data could be benzoic acid (\(C_6H_5COOH\)). It contains a benzene ring (giving 6 unique carbon environments), a carbonyl group (giving 1 unique carbon environment), and an acidic proton in the carboxylic acid group. This structure satisfies all the given data.
To know more about carboxylic acid, here
brainly.com/question/33933714
#SPJ2
Which type of energy does alpha decay generate?
O kinetic
O potential
O sound
O electromagnetic
Answers
Answer: kinetic or A :)
Explanation: kinetic energy Alpha particles have typical kinetic energy of 5 MeV (or ≈ 0.13% of their total energy 110 TJ/kg) and have a speed of about 15 000 000 m/s or 5% of the speed of light.
hope this helped !! - hailey
) A 3.50 gram sample of a hydrate of copper sulfate yields 2.10 g of anhydrous copper (II) sulfate.a. Determine the mass percent of water in the hydrate.
Answers
The mass percent is described by the following equation:
\(Mass\%(H_2O)=\frac{MassH_2O}{MassCuSO_4(H_2O)_x}\times100\%\)\(Mass\%(H_2O)=\frac{MassCuSO_4(H_2O)_x-MassCuSO_4}{MassCuSO_4(H_2O)_x}\times100\%\)CuSO4 corresponds to anhydrous copper (II) sulfate
CuSO4(H2O)x will be hydrate of copper sulfate
H2O corresponds to the water. The mass of water as we saw in the equation is the total mass of hydrate of copper sulfate minus the mass of anhydrous copper (II) sulfate
So, we can replace the known masses:
\(Mass\%(H_2O)=\frac{3.50g-2.10g}{3.50g}\times100\%\)\(Mass\%(H_2O)=40.0\%\)Answer: The mass percent of water in the hydrate is 40.0%
Which redox reaction would most likely occur if silver and copper metal were
added to a solution that contained silver and copper ions?
Answers
The redox reaction most likely occur if silver and copper metal were added to a solution that contained silver and copper ions is ;
Cu²⁺ + 2Ag → Cu + 2Ag⁺
What is Redox Reaction ?
A chemical reaction that takes place between an oxidizing substance and a reducing substance.
The oxidizing substance loses electrons in the reaction, and the reducing substance gains electrons.
On the reduction potential chart, we have that silver ion (Ag) is a stronger oxidizing agent than, Copper (Cu), which is a reducing agent the compared to silver
The redox reaction most likely occur if silver and copper metal were added to a solution that contained silver and copper ions is ;
Cu²⁺ + 2Ag → Cu + 2Ag⁺
Learn more about Redox Reaction here ;
https://brainly.com/question/13293425
#SPJ1
Comparing Earth's Inner Layers
Use what you've learned to match Earth's inner layers to their correct descriptions,
outer core
is under the asthenosphere
lower mantle
is made of liquefied metal
inner core
has a tarry consistency
asthenosphere
is under great pressure and is solid
Intro
Done

Answers
Answer:
Outer core is made of liquidified metal
Lower mantle is under the asthenosphere
Inner core is under great pressure and is solid
Asthenosphere has a tarry consistency
Answer:
Outer core is made of liquidified metal
Lower mantle is under the asthenosphere
Inner core is under great pressure and is solid
Asthenosphere has a tarry consistency
ANSWER QUICK!!!! GIVING BRAINIEST!!!

Answers
Answer:
B.
Explanation:
Can you give me brainliest? i need to rank up
Answer:
2nd One is correct
Does MgSO4 react with ZnSO4?
Answers
There is no reaction between zinc II sulfate and magnesium sulfate.
Is there a reaction?We know that a reaction is said to occur when two species are combined and there are new products that appear in the system. This implies that if there are no new substances that appear in the system we can not say that a chemical reaction has taken place.
In this case, we can see that we can not be able to observe any change when wee mix a solution of magnesium sulfate and zinc II sulfate since the both of them have the same anion.
Learn more about reaction:https://brainly.com/question/28984750
#SPJ1
How much water has to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M?
Answers
Approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
To find the amount of water that needs to be evaporatedThe relationship between the initial and final concentrations and volumes must be taken into account.
Given: Initial concentration \((C^1) = 1 M Initial volume (V^1) = 250 mL\)
\((C^2) = 3 M final concentration\)
We can use the equation:
\(C^1 * V^1 = C^2 * V^2\)
Where:
\(V^2\)is the final volume of the solution
Rearranging the equation to solve for V2:
\(V^2 = (C^1 * V^1) / C^2\)
Substituting the given values:
\(V^2 = (1 M * 250 mL) / 3 M\)
\(V^2 = 250 mL / 3\)
\(V^2\) ≈ \(83.33 mL\)
To find the amount of water that needs to be evaporated, we subtract the final volume from the initial volume:
Amount of water to be evaporated = \(V^1 - V^2\)
Amount of water to be evaporated = 250 mL - 83.33 mL
Amount of water to be evaporated ≈ 166.67 mL
Therefore, approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
Learn more about Initial concentration here: brainly.com/question/30720317
#SPJ1
plz help as soon as you can
Oil of Vitriol is a substance that humankind has used for thousands of years! Today the substance is known by its scientific name sulfuric acid and has the molecular formula H2SO4. What is the molar mass of H2SO4?
Question 2 options:
49.067 g/mol
73.29 g/mol
98.08 g/mol
148.72 g/mol
Answers
Answer:
98.08 g/mol
Explanation:
Hope this helps.
At 25.0° C, a 10.00 L vessel is filled with 5.25 moles of Gas A and 7.75 moles of Gas B. What is the total pressure in atm?
Answers
Answer:
23.12 atm
Explanation:
First, add together the moles of the two samples:
5.25 moles + 4.20 moles = 9.45 moles
273 + 25 = 298 K for the temperature
volume is 10.0 L
Since we have moles now, we have to rearrange our ideal law equation to solve for pressure:
\(P = \frac{nRT}{V}\)
\(\frac{(9.45 moles) X (0.08206) X (298 K)}{10.0 L}\)
9.45 X .08206 X 298 all divided by 10.0 = 23.09202 atm (or 23.12)
297.85
Question 2
4 pts
If a sample of gas occupies 23.5 mL at 315 K and 14.8 atm of pressure, what volume will it occupy at 415 K and
12.3 atm?
Give your volume in ml, but do not include the units in the answer. Do not use scientific notation.
Question 3
4 pts
Answers
Answer:
37.25
Explanation:
\(P_1\) = Initial pressure = \(14.8\ \text{atm}\)
\(P_2\) = Final pressure = \(12.3\ \text{atm}\)
\(V_1\) = Initial volume = \(23.5\ \text{mL}\)
\(V_2\) = Final volume
\(T_1\) = Initial temperature = \(315\ \text{K}\)
\(T_2\) = Final temperature = \(415\ \text{K}\)
From ideal gas law we have
\(PV=nRT\)
\(\Rightarrow PV\propto \dfrac{1}{T}\)
So
\(\dfrac{P_1V_1}{T_1}=\dfrac{P_2V_2}{T_2}\\\Rightarrow V_2=\dfrac{P_1V_1T_2}{T_1P_2}\\\Rightarrow V_2=\dfrac{14.8\times 23.5\times 415}{315\times 12.3}\\\Rightarrow V_2=37.25\ \text{mL}\)
The final volume is \(37.25\ \text{mL}\)
What is the IUPAC name of the compound N2O?
Answers
Answer:
Nitrous Oxide
Explanation:
how would you confirm the presence of lead in an ore?
Answers
There are numerous ways to determine whether lead is present in an ore. Atomic absorption spectroscopy is a popular approach. With this method, an ore sample is dissolved in acid and then atomized in a flame or plasma.
The sample's atoms will absorb light at particular wavelengths that are peculiar to the element under investigation. The amount of light absorbed can be used to calculate how much lead is present in the sample. Inductively coupled plasma mass spectrometry and X-ray fluorescence spectroscopy are further techniques. It is crucial to remember that these procedures call for specialized tools and training, thus they ought to only be carried out in a lab by qualified experts.
To know more about spectrometry, here
brainly.com/question/31075363
#SPJ1
How many moles of MgCl2 are present in 60.0 mL of 0.100 M MgCl2 solution
Answers
Taking into account the definition of molarity, the number of moles of MgCl₂ present in 60.0 mL of 0.100 M MgCl₂ solution is 0.006 moles.
Definition of molarityMolarity is a measure of the concentration of a solute in a solution and indicates the number of moles of solute that are dissolved in a given volume.
The molarity of a solution is calculated by:
molarity= number of moles of solute÷ volume
Molarity is expressed in units moles/L.
Number of moles of MgCl₂In this case, you have:
Molarity= 0.100 Mnumber of moles of MgCl₂= ?volume= 60 mL= 0.06 L (being 1000 mL= 2 L)Replacing in the definition of molarity:
0.100 M=number of moles of MgCl₂÷ 0.06 L
Solving:
0.100 M × 0.06 L= number of moles of MgCl₂
0.006 moles= number of moles of MgCl₂
Finally, the number of moles of MgCl₂ is 0.006 moles.
Learn more about molarity:
brainly.com/question/9324116
brainly.com/question/10608366
brainly.com/question/7429224
#SPJ1
A gas cylinder contains exactly 1 mole of oxygen
gas (O2). How many molecules of oxygen are in
the cylinder?
O 4.01 x 1022 molecules
O 6.02 x 1023 molecules
O 9.03 x 1024 molecules
2.89 x 1026 molecules

Answers
The number of molecules of oxygen in the cylinder is 6.02×10²³ molecules
Data obtained from the question Number of mole of oxygen = 1 moleNumber of molecule of oxygen =?From Avogadro's hypothesis, we understood that 1 mole of any substance contains 6.02×10²³ molecules. This implies that 1 mole of oxygen contains 6.02×10²³ molecules.
Since the cylinder contains 1 mole of oxygen, then the number of molecules of oxygen present in the cylinder is 6.02×10²³ molecules.
Learn more about Avogadro's number:
https://brainly.com/question/1445383
Answer:
the answer is B.
Explanation:
Find the percent composition of each element in CaSO4. Be sure to add the % sign
and the element.
*Ca is the 1st blank*
*S is the 2nd blank*
*O is the 3rd blank*
Answers
The percent composition of each element in CaSO4. S 1 23.21 O 4 46.32 Ca 1 29.00.
What does CaSO4 stand for?A calcium salt that occurs naturally is calcium sulphate, or CaSO4. It is most generally referred to as gypsum when it is present as the dihydrate CaSO42H2O. The sulphate is used as a soil conditioner as uncalcined gypsum.The recognised value for the mass percentage of water in the hydrated compound CaSO4•2H2O is 20.9%.The formula for percent composition is 100 multiplied by (mass of element/molecular mass).A compound's percentage composition is calculated by dividing the amount of each ingredient by the sum of all the individual elements in the compound and multiplying the result by 100.To learn more about percent composition refer to:
https://brainly.com/question/17021926
#SPJ1
PLEASE HELP with these problems ASAP!!! 30 points!!


Answers
Answer:
which?........................
Explain why the ability of PLP to catalyze an amino acid transformation is greatly reduced if the OH substituent of pyridoxal phosphate is replaced by OCH3. Explain why the ability of PLP to catalyze an amino acid transformation is greatly reduced if the substituent of pyridoxal phosphate is replaced by . One of the steps in all amino acid transformations is removal of a hydrogen atom from the OH substituent of pyridoxal phosphate. One of the steps in all amino acid transformations is removal of the OH substituent of pyridoxal phosphate. The hydrogen of the OH substituent forms a hydrogen bond with the nitrogen of the imine linkage. g
Answers
Answer:
Explanation:
It should be noted that, the principle behind the ability of PLP to catalyze an amino acid transformation is greatly reduced if the OH substituent of pyridoxal phosphate is replaced by OCH3 is that; the OH is able to form a H-bond with the N which puts partial (+) on the N. This makes it easier for the AA to add to the imine C
OCH3 cannot make this H-bond w N