match the statement to the correct answer. (some reagents can be used more than once.) the flowchart along with the report sheets in experiment 5 should be very helpful. - in test b1 this is the first reagent added to the sample. - in test a2 you add this reagent to the test where a precipitate was formed. f. in test a1 you add this reagent to tests where a precipitate forms. e. concentrated ammonia (nh3) will combine with water to form these ions in solution. l. mixing agno3 and nacl will produce what white precipitate. o. the name for a negatively charged ion.
Answers
The statement "some reagents can be used more than once" is a general statement that can be applied to many experiments, including Experiment 5.
In this experiment, it is important to follow the flowchart and report sheets closely to ensure that the correct reagents are added at the correct time.
In Test B1, the first reagent added to the sample is not specified, so it is important to refer to the flowchart and report sheets for guidance.
In Test A2, you add a reagent to the test where a precipitate was formed. Again, it is important to follow the flowchart and report sheets closely to identify which reagent is required in this test.
In Test A1, you add a reagent to tests where a precipitate forms. Once again, it is important to consult the flowchart and report sheets to determine which reagent is required.
Concentrated ammonia (NH3) will combine with water to form ammonium ions (NH4+) and hydroxide ions (OH-) in solution.
Mixing AgNO3 and NaCl will produce a white precipitate of silver chloride (AgCl).
The name for a negatively charged ion is an anion.
Overall, it is important to carefully follow the instructions provided in the flowchart and report sheets to ensure accurate and reliable results in Experiment 5. Additionally, understanding the properties and behaviors of the reagents used in the experiment can help to ensure successful outcomes.
For more such questions on reagents
https://brainly.com/question/29713522
#SPJ11
Related Questions
it is difficult to cut the steam of water
Answers
It is difficult to cut the steam of water because of the unique properties of water and steam.Water and steam are two different states of matter, but they have a common property - they are both molecules of H2O. Steam is formed when water is heated, and the molecules of H2O begin to move faster and further apart from one another.
This results in steam, which is a gas and not a liquid like water. It is more challenging to cut the steam of water than the liquid water due to its unique properties.Therefore, the difficulty of cutting steam of water is due to the following properties of steam:Low density: Steam has low density because of the increased space between the water molecules due to heating. This means that steam takes up more space and is lighter than water, making it difficult to cut or separate from the atmosphere.Gaseous state: Steam is a gaseous state, which means it does not have a definite shape or volume like liquid water. Therefore, cutting steam would be difficult as it does not have a defined structure. Moreover, steam would disperse instantly if it is cut due to its gaseous form and become difficult to capture.Very hot: Steam is at a temperature that is harmful to human skin, and can cause severe burns. This means that cutting steam is also a safety concern, which makes the process even more challenging.For such more question on molecules
https://brainly.com/question/475709
#SPJ8
If the pressure of 50.0mL of oxygen gas at 100 ºC increases from 735mmHg to 925mmHg, what is the final volume
Answers
Given :
The pressure of 50.0 mL of oxygen gas at 100 ºC increases from 735 mm Hg to 925 mm Hg.
Temperature remains constant.
To Find :
The final volume.
Solution :
735 mm Hg = 0.967 atm
935 mm Hg = 1.230 atm
We know, at constant temperature :
\(P_iV_i=P_fV_f\\\\V_f=\dfrac{P_iV_i}{P_f}\\\\V_f=\dfrac{0.967\times 50}{1.230}\\\\V_f=39.31 \ mL\)
Therefore, final volume is 39.31 ml.
Hence, this is the required solution.
How many moles of BaS would be used to make 1200 mL of a 10.OM solution?
Answers
The concentration of CI ion in a sample of H,0 is 15.0 ppm. What mass of CI ion is present in 240.0 mL of H,0, which has a density of 1.00 g/mL?
Answers
Answer:
Mass of solute = 0.0036 g
Explanation:
Given data:
Concentration of Cl⁻ = 15.0 ppm
Volume of water = 240 mL
Mass of Cl⁻ present = ?
Solution:
1 mL = 1 g
240 mL = 240 g
Formula:
ppm = mass of solute / mass of sample ×1,000,000
by putting values,
15.0 ppm = (mass of solute / 240 g) ×1,000,000
Mass of solute = 15.0 ppm × 240 g / 1,000,000
Mass of solute = 0.0036 g
What fraction of a 100 g sample of K - 42 will remain after 24.8 hours?
Answers
Answer:
1/4
Explanation:
From the question given above, the following data were:
Original amount (N₀) = 100 g
Time (t) = 24.8 h
Fraction remaining =?
NOTE: The half-life of K–42 is 12.4 h
Next, we shall determine the number of half-lives that has elapse. This can be obtained as follow:
Time (t) = 24.8 h
Half-life (t½) = 12.4 h
Number of half-lives (n) =?
n = t / t½
n = 24.8 / 12.4
n = 2
Finally, we shall determine the fraction remaining. This can be obtained as follow:
Number of half-lives (n) = 2
Fraction remaining =?
Fraction remaining = 1/2ⁿ
Fraction remaining = 1/2²
Fraction remaining = 1/4
Hydrogen gas at a pressure of 740. mmHg has a volume of 2.00 L at a temperature of 25.0°C. What is the temperature of this gas at 3.50 L and
standard pressure?
Answers
Answer:
The final temperature of hydrogen gas is 537.63 K.
Explanation:
Given data:
Initial volume = 2.00 L
Initial pressure = 740 mmHg (740/760 = 0.97 atm)
Initial temperature = 25 °C (25 +273 = 298 K)
Final temperature =?
Final volume = 3.50 L
Final pressure = standard = 1 atm
Formula:
According to general gas equation:
P₁V₁/T₁ = P₂V₂/T₂
P₁ = Initial pressure
V₁ = Initial volume
T₁ = Initial temperature
P₂ = Final pressure
V₂ = Final volume
T₂ = Final temperature
Solution:
P₁V₁/T₁ = P₂V₂/T₂
T₂ = P₂V₂T₁ / P₁V₁
T₂ = 1 atm × 3.5 L × 298 K / 0.97 atm × 2.00 L
T₂ = 1043 atm .L. K / 1.94 atm. L
T₂ = 537.63 K
The substances sodium (Na), oxygen (O₂), and sodium oxide (Na2O) participate in this chemical reaction:
4Na + O2 → 2Na₂O.
Which statement best describes the reaction?
Answers
Sodium oxide is created by the chemical reaction statement best describes the reaction .
What is chemical reaction ?
Chemical reactions happen absolutely everywhere. While we sometimes associate chemical reactions with the sterile environment of test tubes and laboratories, nothing is farther from the truth. In fact, a vast number of transformations are creating a dizzying and almost incomprehensible series of new matter and energy changes in our world every second of every day.
In nature, chemical reactions are much more uncontrollable and sometimes much more complex than in the laboratory. Chemical reactions usually occur as needed. The fire raging in the forest, the slow rusting process of iron over the years in the presence of oxygen and water, or the delicate ripening of the fruit on the tree, the process of transforming various chemicals... ( The transformation of one group of substances (products) into another group of substances (products) is a so-called chemical reaction.
To learn more about Chemical reaction , click the link below ;
https://brainly.com/question/11231920
#SPJ9
Molecules can be composed of
__. Select all that apply. ...
two of more of the same atoms
two or more smaller molecules
two or more different atoms
one or more particle
Answers
Answer:
Molecules can be composed of two or more of the same atom
Explanation:
What's you're favorite year?
Answers
Answer:
2020 cause of the lockdown
Explanation:
Write the equilibrium constant expression for this reaction:
NH+4(aq) → NH3(aq)+H+(aq)
Answers
The equilibrium constant expression for the given reaction is:-
Equilibrium constant = [NH₃] [H⁺] / [NH₄⁺]
An equilibrium constant is the ratio of the concentration of products to the concentration of the reactant raised to the power of its coefficients.
The rate constants are constant at some particular temperature. It is equal to the rate constant for the forward reaction divided by the rate constant for the backward reaction.
aA + bB ⇄ cC + dD
The equilibrium constant for the reaction is given as
NH₄⁺ (aq) → NH₃ (aq) + H⁺ (aq)
The expression is given as
Equilibrium constant = [NH₃] [H⁺] / [NH₄⁺]
To learn more about the equilibrium constant, visit: https://brainly.com/question/4742332
#SPJ9
What mass of iron (III) sulfide solid product can be made from reaction of 9.34g of aqueous iron (III) chloride with excess sodium solution?
Answers
3.99 g of Fe₂S₃ solid product can be made from the given amount of FeCl₃.
A balanced equation for the reaction is:
FeCl₃ (aq) + 3Na₂S (aq) → Fe₂S₃ (s) + 6NaCl (aq)
In the stoichiometry of the reaction, 1 mole of FeCl₃ reacts with 1/3 moles of Fe₂S₃, or that 3 moles of FeCl₃ react with 1 mole of Fe₂S₃.
Moles of FeCl₃ = mass / molar mass
Moles of FeCl₃ = 9.34 g / 162.2 g/mol
Moles of FeCl₃ = 0.0575 mol
Since the reaction uses 3 moles of FeCl₃ to produce 1 mole of Fe₂S₃,
Moles of Fe₂S₃ = (0.0575 mol FeCl₃) / 3
= 0.0192 mol Fe₂S₃
Mass of Fe₂S₃ = moles × molar mass
Mass of Fe₂S₃ = 0.0192 mol × 207.9 g/mol
Mass of Fe₂S₃ = 3.99 g
To learn more about FeCl₃, follow the link:
https://brainly.com/question/28299457
#SPJ1
helpp plz I’ll mark brainiest
choose all statements that describe acids

Answers
Which statement about bulk properties of matter and the electrostatic forces between molecules is correct?
a Bulk properties can be used to compare the strength of electrostatic forces between molecules in different materials.
b The bulk properties of a material can be inferred by measuring the strength of the electrostatic forces between molecules.
c Bulk properties can be changed to strengthen or weaken the electrostatic forces between molecules in a material.
d The bulk properties of a material determine the strength of the electrostatic forces between molecules.
Answers
Bulk properties show the strength of electrostatic forces in molecules.
The bulk properties of matter are the properties of matter such as the boiling point, melting point, vapor pressure etc. These properties of matter depends on electrostatic forces between molecules.
The true statement regarding the bulk properties of matter and the electrostatic forces between molecules is "bulk properties can be used to compare the strength of electrostatic forces between molecules in different materials."
Learn more about bulk properties: https://brainly.com/question/25967414
The statement true about the bulk properties has been that the bulk properties of a material can be inferred by measuring the strength of the electrostatic forces between molecules. Thus, option B is correct.
Bulk properties of matter have been described as properties that do not change with the same composition. The bulk property of matter has been the volume, density, temperature, thermal energy, etc.
The electrostatic force has been the interaction between the molecules. With the inference of the electrostatic force present between the molecules, there has been the interpretation of the bulk properties. Thus, option B is correct.
For more information about the bulk properties, refer to the link:
https://brainly.com/question/25706640
please fast!!!!!!!!!!!!!!!!!!!!!!
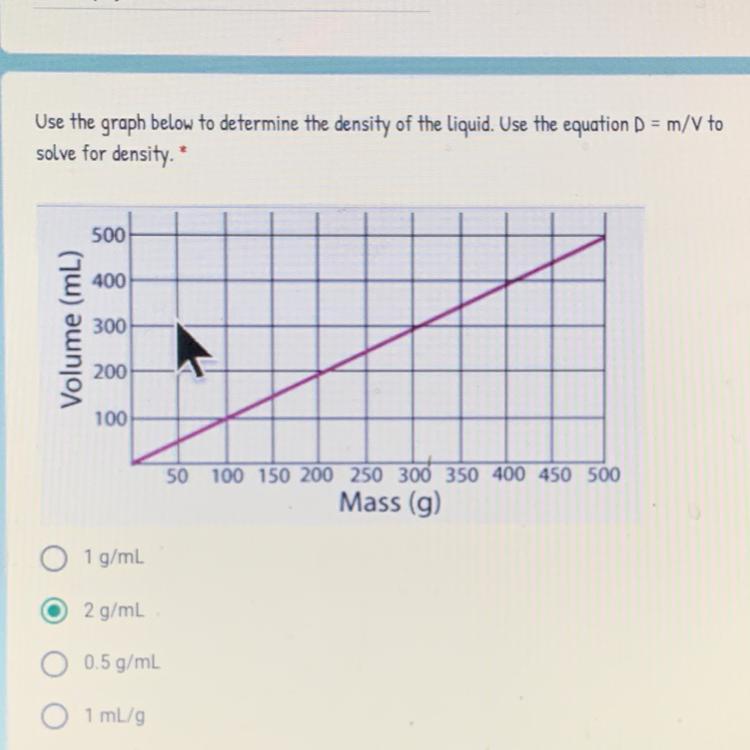
Answers
Answer:
1 g/mL.
Explanation:
From the question given above, the following data were obtained:
Volume = 100 mL
Mass = 100 g
Density =?
Density of a substance is simply defined as the mass of the substance per unit volume of the substance. The density can be expressed mathematically as:
Density (D) = mass (m) / volume (V)
D = m / V
With the above formula, we can obtain the density of the liquid as follow:
Volume (V) = 100 mL
Mass (m) = 100 g
Density (D) =?
D = m / V
D = 100 / 100
D = 1 g/mL
Thus, the density of the liquid is 1 g/mL
Given: H2 + O 2 → H2O1
the reaction occurs at ST.P a) Balance the chemical equation. (1 pts) b) Calculate the number of moles of the reactants needed to obtain 45 liner of H2O (2 pt) 4) Deduce the volume of the reactants (2 pts)
Answers
a) The balanced chemical equation for the reaction is: 2H₂ + O₂ → 2H₂O
b) the number of moles of O₂ required is approximately 1.004 moles.
c) approximately 45 liters of H₂ and 22.5 liters of O₂ are needed to obtain 45 liters of H₂O.
a) Balancing the chemical equation:
The balanced chemical equation for the reaction is: 2H₂ + O₂ → 2H₂O
b) Calculating the number of moles of the reactants needed to obtain 45 liters of H₂O:
From the balanced equation, we can see that for every 2 moles of H₂O produced, we need 2 moles of H₂ and 1 mole of O₂. Since the stoichiometry is based on moles, we need to convert the given volume of H2O into moles.
To convert volume to moles, we need to use the ideal gas law, PV = nRT. At standard temperature and pressure (STP), the molar volume of an ideal gas is 22.4 liters.
Given that we have 45 liters of H2O, we can calculate the number of moles as follows:
moles of H₂O = (volume of H₂O) / (molar volume at STP)
= 45 liters / 22.4 liters/mol
≈ 2.008 moles of H₂O
Since the stoichiometry of the reaction is 2 moles of H₂O for every 2 moles of H₂, we need an equal number of moles of H₂. Therefore, the number of moles of H₂ required is also approximately 2.008 moles.
For O₂, since the stoichiometry is 1 mole of O₂ for every 2 moles of H₂O, we need half the number of moles of H₂O. Thus, the number of moles of O₂required is approximately 1.004 moles.
c) the volume of the reactants:
Since the stoichiometry of the balanced equation is 2 moles of H₂for every 1 mole of O₂ and 2 moles of H₂O, we can deduce the volume of the reactants based on their molar volumes at STP.
For 2.008 moles of H₂, the volume can be calculated as follows:
volume of H₂= (moles of H₂) * (molar volume at STP)
= 2.008 moles * 22.4 liters/mol
≈ 45 liters of H₂
For 1.004 moles of O₂, the volume can be calculated similarly:
volume of O₂= (moles of O₂) * (molar volume at STP)
= 1.004 moles * 22.4 liters/mol
≈ 22.5 liters of O₂
Therefore, approximately 45 liters of H₂and 22.5 liters of O₂ are needed to obtain 45 liters of H₂O
for more questions on chemical
https://brainly.com/question/29886197
#SPJ8
An ion can be created when a .................... is lost or gained by an element.
Answers
Answer:
electron and ignore this woejdnks
I need help, due in 30 mins

Answers
Answer:
1.Reaction rate is how fast a chemical reaction proceeds.
2.Nature of the reaction, Pressure factor
, Solvent, Catalyst and inhibitors.
3.Physical change is a temporary change. A chemical change is a permanent change. A Physical change affects only physical properties i.e. shape, size, etc.
4. One example of the effect of temperature is the use of lightsticks or glowsticks.
5.When the particle size of a fixed mass of a solid reactant becomes smaller, the total exposed surface area becomes larger, the rate of reaction increases. an example could be ice and water when the atoms are stuck together a solid but all over the place as a liquid.
6. Sometimes a reaction depends on catalysts to do their job. In that case, changing the concentration of the catalyst can speed up or slow down the reaction. For example, enzymes speed up biological reactions, and their concentration affects the rate of reaction.
7.A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. For example, a catalyst could cause a reaction between reactants to happen at a faster rate or at a lower temperature than would be possible without the catalyst.
Explanation:
I hope this helps have a great day! :)
I can distunguish between elements, compounds and mixtures.
3.1 I can describe what elements anre and give examples of them.
3.2 I can describe what mixtures are and give examples of them.
3.3 I can describe what compounds are and give examples of them.
0 0
This picture represents..
O a compound
O an element
O a mixture of elements
O a mixture of compounds

Answers
Answer:
an element
Explanation:
that is the answer yep
it represent an element
Following a chemical reaction that produced 5.06 grams of magnesium chloride, the lab report was prepared to
document the results. The expected result was estimated to be 8.85 grams. What are the percent yield and percent
error that are to be included in the lab report?
Percent Yield
Percent Error
Answers
Answer:
1. Percentage yield = 57.2%
2. Percentage error = 74.9%
Explanation:
From the question given above, the following data were obtained:
Actual yield = 5.06 g
Experimental yield = 8.85 g
Percentage yield =?
Percentage error =?
1. Determination of the percentage yield.
Actual yield = 5.06 g
Experimental yield = 8.85 g
Percentage yield =?
Percentage yield = Actual yield /Experimental yield × 100
Percentage yield = 5.06 / 8.85 × 100
Percentage yield = 57.2%
2. Determination of the Percentage error.
Actual yield = 5.06 g
Experimental yield = 8.85 g
Percentage error =?
Percentage error = |Experimental – Actual| / Actual yield × 100
Percentage error = |8.85 – 5.06| / 5.06 × 100
Percentage error = 3.79 / 5.06 × 100
Percentage error = 74.9%
Pls solve these 2 questions I will mark you as the brainliest
Which of the following is a way to conserve resources?
leave lights on
go one more trips
plan longer trips
take shorter showers
Answers
I need friends and I’m in middle school :)
Answers
Answer:
Ok?
Explanation:
molarity of 74.6 g C₂H6O in 2.36 L of solution
Answers
Answer:
0.686 M
Explanation:
To find the molarity of C₂H₆O, you need to (1) convert grams to moles (using the molar mass of C₂H₆O) and then (2) calculate the molarity (using the molarity ratio). The final answer should have 3 sig figs like the given values.
(Step 1)
Atomic Mass (C): 12.011 g/mol
Atomic Mass (H): 1.008 g/mol
Atomic Mass (O): 15.999 g/mol
Molar Mass (C₂H₆O): 2(12.011 g/mol) + 6(1.008 g/mol) + 15.999 g/mol
Molar Mass (C₂H₆O): 46.069 g/mol
74.6 g C₂H₆O 1 mole
------------------------- x -------------------- = 1.62 moles C₂H₆O
46.069 g
(Step 2)
Molarity = moles / volume (L)
Molarity = 1.62 moles C₂H₆O / 2.36 L
Molarity = 0.686 M
What creates an electric current in
a battery?

Answers
Answer: The answers are kinda blury but use this info to help u out or read over the passage
Explanation:A battery is a device that stores chemical energy and converts it to electrical energy. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. The flow of electrons provides an electric current that can be used to do work.
Answer: metal bending
Explanation: is the metal
1 A pump with an 80% efficiency drives water up between two reservoirs through a piping system of total length L = 15 and circular cross -section diameter d = 7 cm. The reservoirs are open to the atmosphere at an ambient temperature of 20 C. The difference in elevation between the free surface can be accounted for by the local loss coefficients Kentrance Kexit 1.0, Kelbow 0.4. If the volumetric flow rate in the system Q = 10 Liter/s, and the surface roughness of the pipe is = 0.15 mm, calculate:
(a) The average water flow velocity in the pipe.
Answers
how many moles in 1.505x10^23 Na atoms
Answers
Answer:
The answer is 0.25 molesExplanation:
To find the number of moles in a substance given it's number of entities we use the formula
\(n = \frac{N}{L} \\\)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question
N = 1.505 × 10²³ Na atoms
We have
\(n = \frac{1.505 \times {10}^{23} }{6.02 \times {10}^{23} } = \frac{1}{4} \\ \)
We have the final answer as
0.25 molesHope this helps you
Aluminum undergoes a single-displacement reaction with copper (II) sulfate to form aluminum sulfate and _______________.
Answers
Ans cooper
. Calculate ΔG for the following reaction at 25°C. Will the reaction occur (be spontaneous)? How do you know? NH3(g) + HCl(g) → NH4Cl(s) ΔH = -176.0 kJ ΔS = -284.8 J·K-1
Answers
Answer:
\(\triangle G = -911.296 \ kJ\)
Explanation:
ΔG = ΔH-TΔS
Where ΔH = -176 kJ = -176000 J , T = 25°C + 273 = 298 K , ΔS = -284.8 JK⁻¹
=> \(\triangle G =-176000 - (298)(-284.8)\)
=> \(\triangle G = -176000+84870.4\)
=> \(\triangle G = -91129.6 \ J\)
=> \(\triangle G = -911.296\ kJ\)
Since the value is negative, the reaction is spontaneous under standard conditions at 298 K and the reactants have more free energy than the products.
When we choose to use nonmanipulated IVs in our factorial experiments, we are conducting ________ research.
a. ex post facto
b. experimental
c. correlated-groups
d. parsimonious
Answers
When we choose to use nonmanipulated IVs in our factorial experiments, we are conducting experimental research.
When we choose to use nonmanipulated IVs (independent variables) in our factorial experiments, we are conducting experimental research. In experimental research, the researcher manipulates one or more variables (the independent variables) and measures the effect on another variable (the dependent variable). Factorial experiments are a type of experimental design that allow the researcher to study the effects of multiple independent variables simultaneously. Nonmanipulated IVs are variables that are not controlled or manipulated by the researcher, but rather are observed as they naturally occur. By using nonmanipulated IVs in our experiments, we are able to study the effects of these variables on the dependent variable while controlling for other factors that might influence the outcome.
To know more about parsimonious
https://brainly.com/question/13049462
#SPJ4
Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
What is the mole ratio of PbO2 to water
Answers
The mole ratio of PbO2 to water is 1:2.
The mole ratio of PbO2 to water, PbO2:H2O, is 1:2 when the reaction stoichiometry is taken into consideration.
The appropriate reaction is:
2 PbSO4 + 2 H2O ⇒ Pb + PbO2 + 2 H2SO4
The following numbers of moles of each component are involved in the reaction according to reaction stoichiometry, which describes the relationship between the amounts of reagents and products in a chemical reaction:
1 mole Pb
1 mole of PbO2
H2SO4: 2 mol
2 moles of PbSO4
Water: 2 moles
Then, you can see that according to the reaction's stoichiometry, 1 mole of PbO2 produces 2 moles of water (H2O).
Therefore,the mole ratio of PbO2 to water is 1:2.
Learn more about mole here;
https://brainly.com/question/26416088
#SPJ9
2. How might this population suddenly increase? How might this affect the ecosystem? (for tropical savannah.)
Answers
Explanation:
it can suddenly increase when more and more people have babies and the infant rate goes up. and how it might affect the ecosystem is the pollution that people give off.
Answer:
Explanation:
Due to less predators in the Savannah/habitat, the population could unexpectedly grow. This will have an impact on the environment because it will give rise to more prey as there will be fewer predators. If too many herbivores are present on one feeding site, competition for food will occur. If there are more plants in an area than normal, it can increase the population of animals that consume the plant. If the population of one animal rises, the population of animals that consume that animal could also rise.
Population growth isn't always good. A population may often grow too big to sustain the climate. Other modifications in limiting variables can cause a population to decline. The population will decrease if a population becomes diseased, and the population of animals that consume the diseased animals will decrease as well. Populations in nature typically balance themselves. Sometimes, they can't always restore a natural balance as man affects populations.