Answers
The term measurement has to do with the process of obtaining the value of a quantity. There are several units that could be used in measurement but the SI unit is the internationally accepted unit of measurement.
Let us now match the words and the vocabulary;
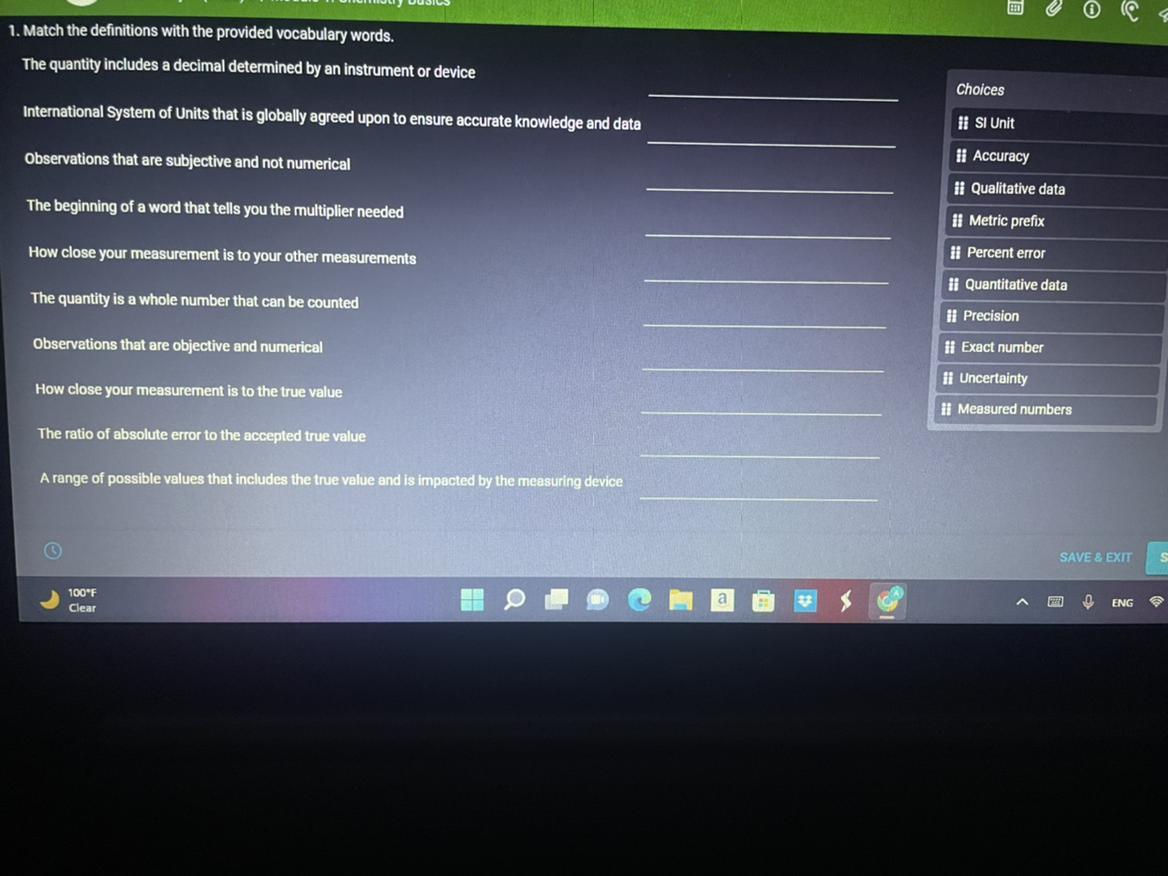
The quantity includes a decimal determined y the device or instrument - UncertaintyInternational units that are globally accepted - SI unitObservations that are subjective and not numerical - Qualitative dataThe beginning of a word that tells you the multiplier needed - metric prefixHow close your measurement is to your other measurements - precisionThe quantity is a whole number that can be counted - exact numberObservations that are objective and numerical - quantitative dataHow close your measurement is to the true value - AccuracyThe ratio of the absolute error to the accepted true value - percent errorA range of values that includes the true value and is affected by the measuring device - uncertaintyLearn more about error:https://brainly.com/question/19575648
#SPJ1
Related Questions
FeBr3+Ba(OH)2——-Fe(OH)3+BaBr2
Answers
Answer:
Balanced Chemical Equation
2FeBr3 + 3Ba(OH)2 → 2Fe(OH)3 + 3BaBr2
Explanation:
Reaction Information
Ferric Bromide + Barium Hydroxide = Iron(III) Hydroxide + Barium Bromide
Reaction Type
Double Displacement (Metathesis)
Reactants
Ferric Bromide - FeBr3
FeBr3
Molar Mass of Br3Fe Oxidation State of Br3Fe
Barium Hydroxide - Ba(OH)2
Caustic Baryta Barium Hydroxide Lime Ba(OH)2 Barium Dihydroxide Hydrate
Molar Mass of BaH2O2 Oxidation State of BaH2O2
Products
Iron(III) Hydroxide - Fe(OH)3
Ferric Hydroxide Ferric Oxide Yellow
Molar Mass of FeH3O3 Oxidation State of FeH3O3
Barium Bromide - BaBr2
Molar Mass of BaBr2 Oxidation State of BaBr2
FeBr3 + Ba(OH)2 = Fe(OH)3 + BaBr2
Instructions
To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above.
Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F.Ionic charges are not yet supported and will be ignored.Replace immutable groups in compounds to avoid ambiguity. For example, C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will.Compound states [like (s) (aq) or (g)] are not required.You can use parenthesis () or brackets []i hope it's help you
How to prepare dry crystals of the soluble salt cobalt(II) chloride-6-water from the insoluble base cobalt(II) carbonate.
Answers
The acid hydrogen chloride(HCl) could be used to prepare dry crystals of salt cobalt(II) chloride-6-water from the insoluble base cobalt(II) carbonate.
How are cobalt(II) chloride-6-water salts prepared?A beaker with acid (HCl) added should be heated over a bunsen burner flame. Stir the heated, acid with the insoluble base cobalt(II) carbonate until the base is overly present. To get rid of the extra base, filter the mixture into an evaporating basin. Heat the solution to cause water to evaporate and to reach saturation. Dip a cool glass rod into the solution to examine whether crystals grow on the end to determine if the solution is saturated. Allow the filtrate to dry and crystallize in a warm area.Equation of the reaction:
cobalt(II) carbonate + Hydrogen chloride => cobalt(II) chloride + Hydrogen-carbonate
Learn more about salt here:
brainly.com/question/5306491
#SPJ1
how many moles of ammonia could be obtained from a maximum of 5.0 moles of nitrogen
Answers
Answer:
10 moles of ammonia could be obtained from a maximum of 5.0 moles of nitrogen.
Explanation:
The balanced reaction is:
N₂ + 3 H₂ → 2 NH₃
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
N₂: 1 moleH₂: 3 molesNH₃: 2 molesThen you can apply the following rule of three: if by reaction stoichiometry 1 mole of nitrogen produces 2 moles of ammonia, 5 moles of nitrogen produces how many moles of ammonia?
\(moles of ammonia=\frac{5 moles of nitrogen*2 moles of ammonia}{1 mole of nitrogen}\)
moles of ammonia= 10
10 moles of ammonia could be obtained from a maximum of 5.0 moles of nitrogen.
A scientist fills two test tubes with different aqueous solutions and adds different compounds to each solution. After the reactions are complete, the scientist measures the temperature of each solution. The table shows the results of the experiment.
Test Tube1 Test Tube2
Initial Temperature 20°C | 20°C
Final Temperature
25°C | 19°C
Which statement best explains the results?
A. The products in test tube 1 have a lower potential energy than the products in test tube 2.
B. The reaction in test tube 1 has a higher activation energy than the reaction in test tube 2.
C. Test tube 1 has a catalyst, and test tube 2 does not.
D. The reaction in test tube 1 is exothermic, and the reaction in test tube 2 is not.
Answers
Answer:
D. The reaction in test tube 1 is exothermic, and the reaction in test tube 2 is not.
Explanation:
just took it
The option that best explains the result is option D. That is, the reaction in test tube 1 is exothermic, and the reaction in test tube 2 is not.
Change in chemical reaction:In a chemical reaction, heat is either absorbed or released.
In a reaction where there is absorption of heat from the surrounding is called an endothermic reaction.
While in a reaction where heat is released into the surrounding is called exothermic reaction.
From the experiment, there is increase in temperature, from 20 to 25°C, which shows the release of temperature to the surrounding.
Therefore, the reaction in test tube 1 is exothermic, and the reaction in test tube 2 is not.
Learn more about exothermic reaction here:
https://brainly.com/question/2924714
Which equation is used to help form the combined gas law? mc009-1. Jpg mc009-2. Jpg mc009-3. Jpg mc009-4. Jpg.
Answers
The combined gas law equation has been \(\dfrac{P_1V_1}{T_1}=\dfrac{P_2V_2}{T_2}\).
The combined gas law has been assigned to the ideal gas. It has been stating that ideal gas are having negligible inter-molecular attraction and collision resulting in the absence of pressure and volume from the particles.
In an ideal gas the equation has been given as:
\(PV=nRT\)
Where, P has been the pressure of the gas
V has been the volume of the gas
n has been the moles of the gas
R has been a constant
T has been the temperature of the gas
The combined gas law has been given as the change in the pressure, and volume for a gas. It has been given as:
\(\dfrac{P_1V_1}{T_1}=\dfrac{P_2V_2}{T_2}\)
For more information about combined gas law, refer to the link:
https://brainly.com/question/13154969
Answer:b
Explanation:
Which statement explains the relationship between the amount of energy it takes to break a bond and the amount of energy released when the same bond is formed? (1 point)
A.The amount of energy it takes to break a bond is always greater than the amount of energy released when the bond is formed.
B.The relationship depends on the particular bond.
C.The amounts of energy are always equal.
D.The amount of energy it takes to break a bond is always less than the amount of energy released when the bond is formed.
Answers
The statement that best explains the relationship between the amount of energy it takes to break a bond and the amount of energy released when the same bond is formed is that The amount of energy it takes to break a bond is always less than the amount of energy released when the bond is formed.
For better understanding let's explain what the statement means
The standard that guides the breaking of bond is that bonds breaking between atoms needs adding energy that is stronger the bond is need and when it is, then more energy is also needed to to break the bond.From the above we can therefore say that the answer The statement that best explains the relationship between the amount of energy it takes to break a bond and The amount of energy it takes to break a bond is always less than the amount of energy released when the bond is formed, is correct
Learn more from
https://brainly.com/question/24717620
Answer:
the amount of energy it takes to break a bond is always less than the amount of energy released when the bond is formed.
Explanation:
the water in a beaker has a volume of 50 millimeters, is this an extensive property?
Answers
No, the volume of water in a beaker is not an extensive property.
Extensive properties are those that depend on the amount or size of the substance being measured. In other words, they are properties that change with the quantity of the substance. Examples of extensive properties include mass, volume, and total energy.
In the given scenario, the volume of water in the beaker is 50 milliliters. This volume remains the same regardless of the quantity of water present. Whether it's 50 milliliters or 500 milliliters, the volume measurement does not change. Therefore, the volume of water in the beaker is an example of an intensive property.
Intensive properties are independent of the amount or size of the substance. They are characteristics that remain constant regardless of the quantity of the substance. Examples of intensive properties include temperature, density, and color.
It's important to note that the distinction between extensive and intensive properties depends on the specific property being considered. While volume is typically an extensive property for a bulk substance, in the case of a fixed volume of water in a beaker, it becomes an intensive property.
In summary, the volume of water in a beaker is not an extensive property but rather an intensive property because it does not change with the quantity of the substance.
For more such questions on extensive property visit:
https://brainly.com/question/13055036
#SPJ8
What volume of oxygen can be produced from the decomposition of 25 g of aluminum chloride at 28°C in 750 mmHg
Answers
Answer:
Therefore, approximately 8.5 mL of chlorine gas can be produced from the decomposition of 25 g of aluminum chloride at 28°C and 750 mmHg.
Explanation:
The decomposition of aluminum chloride will not produce oxygen. Instead, it produces aluminum oxide and chlorine gas. The balanced chemical equation for this reaction is:
2AlCl3(s) → Al2O3(s) + 3Cl2(g)
To determine the volume of chlorine gas produced from the decomposition of 25 g of aluminum chloride, we need to use the ideal gas law, which is PV = nRT, where P is the pressure of the gas in atmospheres, V is the volume of the gas in liters, n is the number of moles of gas, R is the ideal gas constant (0.0821 L·atm/mol·K), and T is the temperature of the gas in Kelvin.
First, we need to determine the number of moles of aluminum chloride. The molar mass of AlCl3 is 133.34 g/mol, so:
25 g AlCl3 × (1 mol AlCl3/133.34 g AlCl3) = 0.187 moles AlCl3
According to the balanced chemical equation, 2 moles of AlCl3 will produce 3 moles of Cl2 gas. Therefore, 0.187 moles of AlCl3 will produce:
0.187 moles AlCl3 × (3 moles Cl2/2 moles AlCl3) = 0.2805 moles Cl2
Now we can use the ideal gas law to calculate the volume of Cl2 gas produced. We need to convert the temperature from Celsius to Kelvin by adding 273.15.
PV = nRT
V = nRT/P
V = (0.2805 mol) × (0.0821 L·atm/mol·K) × (301.15 K) / (750 mmHg × 1 atm/760 mmHg)
V = 0.0085 L or 8.5 mL (rounded to two significant figures)
Therefore, approximately 8.5 mL of chlorine gas can be produced from the decomposition of 25 g of aluminum chloride at 28°C and 750 mmHg.
What is the correct formula for the product of the combination reaction between calcium metal and oxygen gas?.
Answers
Oxygen and calcium react to generate calcium oxide. The chemical made of calcium and oxygen has the formula CaO. This reaction's chemical formula is 2Ca + O2 2CaO.
What are the eight different kinds of chemical reactions?the eight types of chemical reactions that are most frequently encountered are: decomposition reaction, combination reaction, combustion reaction, single displacement reaction, double displacement reaction, precipitation reaction, and redox reaction.
What four traits do chemical reactions have?The evolution of a gas, precipitate formation, color change, temperature change, and state change are significant aspects.
Learn more about redox reaction here:
https://brainly.com/question/13293425
#SPJ4
Explain how chemists could use trends in atomic radius to predict the size of a new element that has not yet been discovered
Answers
Atomic radius decreases along a period and increases along a group so if a new element is to be added in periodic table it must have larger atomic radius in group and smaller atomic radius in period than their respective preceding elements.
What is periodic table?Periodic table is a tabular arrangement of elements in the form of a table. In the periodic table, elements are arranged according to the modern periodic law which states that the properties of elements are a periodic function of their atomic numbers.
It is called as periodic because properties repeat after regular intervals of atomic numbers . It is a tabular arrangement consisting of seven horizontal rows called periods and eighteen vertical columns called groups.
Elements present in the same group have same number of valence electrons and hence have similar properties while elements present in the same period show gradual variation in properties due to addition of one electron for each successive element in a period.
Learn more about periodic table,here:
https://brainly.com/question/11155928
#SPJ2
An excerpt from a fantasy periodic table, including molar masses, is shown below. Using this information, what is the molecular formula for a substance with a molar mass of 1644.28 g/mol and an empirical formula of Bg2DGr3?
Bg8D4Gr12
Bg10D5Gr15
Bg4D2Gr6
Bg6D3Gr9
Answers
The molecular formula for the substance with a molar mass of 1644.28 g/mol and an empirical formula of Bg₂DGr₃ is Bg₁₀D₅Gr₁₅.
To determine the molecular formula, we need to calculate the molecular weight of the empirical formula, which can be done by adding up the molar masses of the atoms in the empirical formula.
The molar mass of Bg₂DGr₃ is:
(2 x 10.81 g/mol) + (3 x 2.01 g/mol) + (3 x 72.63 g/mol) = 328.50 g/mol
Next, we can divide the given molar mass by the empirical formula mass to get the ratio between the empirical formula and the molecular formula:
1644.28 g/mol ÷ 328.50 g/mol = 5
This tells us that the molecular formula contains 5 times as many atoms as the empirical formula. To find the molecular formula, we simply multiply the subscripts in the empirical formula by 5:
Bg₁₀D₅Gr₁₅
Learn more about Molecular here:
https://brainly.com/question/14614762
#SPJ1
Un elemento posee dos isótopos cuyos números de masa suman 68 y la semisuma de sus neutrones es 19. Determine el grupo de este elemento en la Tabla Periódica Actual.
Answers
Answer:
The element belongs to group 15 of the present periodic table
Explanation:
Isotopes have the same proton number, what differs is that they have different number of neutrons.
Now since the half-sum of their neutrons is 19, the total number of neutrons would be 19 * 2 = 38 neutrons.
Also, their masses add up to be 68, since the total mass equals sum of neutrons and sum of protons, this means that the total number of protons is 68-38 = 30
Since this is equal in both isotopes, this means that the proton number of the element is 30/2 = 15
The atomic number is the number of protons in the nucleus of an atom. Since the atomic number is 15, this element is phosphorus and it belongs to group 15 of the present periodic table
In the notations C-12 and C-14, what do the numbers after the element
symbol and hyphen represent?
Answers
Convert the speed of light, 3.0 x 108 m/s to km/day.
Answers
The speed of light in terms of km/day is \(2.59\)×\(10^{10}\) km/day.
What is speed of light?The speed of light is \(3.0\)×\(10^{8}\) \(m/s\).
1 \(m/s\) = 86.4 km/day
by using this \(3.0\)×\(10^{8}\) \(m/s\) can be converted to \(2.59\)×\(10^{10}\) km/day.
The speed of light in a vacuum, commonly abbreviated as c, is one universal physical constant that is important in many fields of physics. The actual speed of light is 299,792,458 meters per second, or c.
In physics class, Einstein had already learnt what a light beam was: a collection of oscillating electric and magnetic fields moving at the observed speed of light, or 186,000 miles per second.
Thus, somewhat strangely, de Rham claims that the only thing that can travel faster than the speed of light is light itself, but only when it is not in the vacuum of space. It is interesting to note that light will never travel faster than 186,282 miles per second regardless of the medium.
To learn more about speed of light from the given link:
brainly.com/question/394103
#SPJ1
During a chemical reaction, a fixed amount of Iron combines completely with a fixed amount of oxygen to form Iron oxide. Which statement best describes the mass of the iron odide that is
produced?
a)The mass is equal to the mass of the Iron minus the mass of the oxygen before reacting.
B)The mass is equal to the mass of the Iron plus the mass of the oxygen.
C)The mass is greater than the combined mass of both the Iron and the oxygen
D)The mass is less than the mass of the Iron but greater than the mass of the oxygen before reacting.
Answers
Answer: the answer is C
Explanation:
differentiate between Physical and chemical changes
Answers
Answer:
In a physical change the appearance or form of the matter changes but the kind of matter in the substance does not. However in a chemical change, the kind of matter changes and at least one new substance with new properties is formed.
I HOPE THIS WILL HELP YOU IF NOT THEN SORRY HAVE A GREAT DAY:)what is the total number of neutrons in an atom of k-42
Answers
Answer:
23
This particular isotope of potassium-42 contains 42 nucleons (i.e., protons and neutrons, combined;) Like other isotopes of potassium 19 out of these nucleons are protons; the rest 42−19=23 are therefore neutrons.
Answer:
23 neutrons
Explanation:
3 points
14) Calculate and round the density to the correct number of significant
digits. *
mass
Density =
volume
What is the density of an object with
a mass of 320 grams and a volume
of 47.5 ml?
6.0 g/ml
6.74 g/ml
6.7 g/ml
O 7 g/ml

Answers
Answer:
density=6.74g/ml
:320g÷47.5ml
d=6.74g/ml
thank you
I hope this is helpful
Scientists have been able to cause some bacteria to produce insulin that humans then use for medicine. This is a result of which technology?
is it:
cloning
bioprinting
genetic modification
selective breeding
Answers
Please Help ASAP Students set up a controlled experimernt. They put five
crickets in each of three identical containers. They set one
container to 15C, one to 20°C, and one to 25°C. Then they
count the number of cricket chirps in each container during
1 hour. What are the two constants in this experiment? You have to pick to answers.
A: The number of crickets in each container.
B: The temperature of each container.
C: The number of cricket chirps.
D: The kind of containers used.
Answers
Answer: A
Explanation:in learned this last year:)
A: The number of crickets in each container. and
C: The number of cricket chirps.
are possibly the right answers
covalent bonding occurs in both molecular and covalentnetwork solids. which of the following statements best explains why these two kinds of solids differ so greatly in their hardness and melting points? (a) the molecules in molecular solids have stronger covalent bonding than covalent-network solids do. (b) the molecules in molecular solids are held together by weak intermolecular interactions. (c) the atoms in covalent-network solids are more polarizable than those in molecular solids. (d) molecular solids are denser than covalent-network solids.
Answers
Both molecular and covalent network solids exhibit covalent bonding. Weak intermolecular interactions keep the molecules in molecular solids together.
Explain the covalent bond.When two atoms share an electron pair, an interatomic interaction known as a covalent bond is formed. The binding is brought about by the electrical attraction of their nuclei to the same electrons. A covalent bond is formed when the combined energy of the bonded atoms is lower than the energy of the unbonded atoms.
How do hydrogen atoms form covalent connections with one another?The identical electron pair of the covalent connection pulls the two hydrogen atoms in. The bond is shown as either a solid line or two "dots." Every hydrogen atom acquires an electron arrangement resembling that of helium. The bond length is the separation between atomic nuclei at equilibrium.
Learn more about atomic nuclei here:-
https://brainly.com/question/7805095
#SPJ4
What causes the sea floor to move apart at a sea floor spreading center A density B continental drift C paleomagnetism D convection currents
Answers
Answer: D convection currents
Explanation:
The seafloor spreading is a phenomena that occurs due to liberation of heat from the convection currents generated in the mantle. It makes the earth crust more plastic and less dense. This happens at divergent plate boundaries. As the plates move apart, the less denser material rises. It leads to the formation of mountain and crust cracks.
based on your experience in the simulation wat do the numbers 3 and 4 refer to in 3NH4
Answers
Answer:
●3 refer to No. of molecule
There are 3 molecule of NH4
●4 refer to atom of hydrogen
since there are 3 molecules of NH4.Each molecule containing 4 H.Therefore there are 12 H atom in the compound
standard units of measure for density
Answers
Answer:
g/cm³ for solids,
g/ml for liquids
g/L for gases.
Explanation:
Though SI unit of density is kg/m³, for convenience we use g/cm³ for solids, g/ml for liquids and g/L for gases. Mathematically, density is defined as mass divided by volume: ρ=m/V
2 Points
Which intermolecular force would affect melting point the most?
O A. Dipole-dipole attractions
O B. Hydrogen bonding
O C. Nonpolar interactions
O D. Van der Waals forces
Answers
Answer:
Hydrogen bonding
Explanation:
Hydrogen bonding is the intermolecular force that would affect melting point the most. Therefore, the correct option is option B.
What is intermolecular force?The electro - magnetic forces of attraction but rather repulsion that act between atoms or other types of nearby particles are examples of intermolecular forces, which mediate interaction between molecules. When compared to intramolecular forces, which include covalent bonds and other forces that hold molecules together, these forces are weaker.
The intermolecular force, which depends on the kinetic energy between atoms as well as the tiny electrically charged positive and negative charges on various sections of a molecule, is the total of all the forces connecting two nearby molecules. Hydrogen bonding is the intermolecular force that would affect melting point the most.
Therefore, the correct option is option B.
To know more about intermolecular force, here:
https://brainly.com/question/17111432
#SPJ7
Consider the following pair of reactions. Predict the type of substitution mechanism, predict which reaction of the pair will occur at the faster rate, and draw the correct organic product
Answers
The reaction with S_N₂mechanism is likely to be faster than the reaction with S_N₂ mechanism. This is because the carbocation intermediate formed in S_N₁ mechanism is more stable.
The pair of reactions given below is:
CH₃Cl + NaOH→CH₃OH + NaCl
CH₃I + NaOH→CH₃OH + NaI
The type of substitution mechanism:
The first reaction involves S_N₁ mechanism (unimolecular nucleophilic substitution). The second reaction involves S_N₂ mechanism (bimolecular nucleophilic substitution).
Prediction of the reaction that will occur at a faster rate:
The reaction with S_N₁ mechanism is likely to be faster. The rate of this reaction mainly depends on the stability of the carbocation intermediate formed after the initial step. In this case,CH₃Cl reacts to form a tertiary carbocation which is more stable than the primary carbocation formed in CH₃I.
Drawing the correct organic product:
CH₃Cl + NaOH→CH₃OH + NaCl
CH₃I + NaOH→CH_3OH + NaI
CH₃C reacts with NaOHin an S_N₁ mechanism to produceCH₃OH and NaCl.
CH₃ reacts withNaOH in an S_N₂mechanism to produce CH₃OH and NaCI.
To know more about unimolecular nucleophilic substitution visit:
brainly.com/question/32657850
#SPJ11
When a cold front is collied and a warm front is the same this occurs during a ? front
Answers
When a cold front and a warm front collide, it is called an occluded front. This weather phenomenon occurs when a cold front catches up to and overtakes a slower-moving warm front, pushing it upwards.
As the two fronts meet, the warm air is lifted, causing it to cool and condense, leading to the formation of clouds and precipitation. The type of precipitation that occurs during an occluded front can vary depending on the temperature and moisture content of the air masses involved. Generally, it is common to see rain or snowfall during an occluded front, and the intensity of the precipitation can be moderate to heavy. During an occluded front, the temperature can also change rapidly as the warm air mass is lifted and replaced by cooler air. If the air behind the occluded front is colder than the air ahead of it, the temperature will drop, leading to a sudden chill. However, if the air behind the occluded front is warmer, the temperature will rise, creating a sudden warmth.
In conclusion, an occluded front is a weather phenomenon that occurs when a cold front overtakes and lifts a slower-moving warm front, resulting in precipitation and sudden temperature changes. It is a unique weather event that requires careful monitoring by meteorologists to ensure the safety of people and property.
Learn more about occluded front here:
https://brainly.com/question/2546011
#SPJ11
Can someone please help me with the last column!! ASAP

Answers
The ratio of the volume and temperature of the gas in the given table is as follows:
0.72/276 = 0.002620.78/294 = 0.002650.84/313 = 0.002680.87/330 = 0.002630.93/355 = 0.002620.98/371 = 0.00264What is the relationship between the volume and temperature of a gas?Charles's law, also known as the law of volumes, describes the relationship between the volume and temperature of a gas at a constant pressure. According to this law, the volume of a gas is directly proportional to its absolute temperature (measured in Kelvin) when the pressure is constant.
In other words, as the temperature of a gas increases, its volume will also increase proportionally, and vice versa. Mathematically, Charles's law can be expressed as:
V/T = k
where V is the volume of the gas, T is its temperature in Kelvin, and k is a constant of proportionality.
Learn more about the volume and temperature of a gas at: https://brainly.com/question/17100204
#SPJ1
Match each term below with its definition or description.
1 The point in a titration when the added amount of standard reagent is equal to the amount of analyte being titrated.
2 The analyte is titrated with the standard reagent and the volume of standard solution required to complete the reaction is measured.
3 A reagent that is pure and stable, which can be used directly after weighing.
4 The analyte that is being analyzed in the titration.
5 Standard reagent is added in excess to ensure complete reaction with the analyte. The excess reagent is then titrated with a second standard reagent.
6 A solution, whose concentration is known, often made from a reagent of known purity.
7 The standard reagent of known concentration that is added from a buret to the analyte solution.
8 The analyte does not react directly with the titrant so it is converted to another form which will react with the titrant.
9 The point in a titration when a change in the analyte solution is observed, indicating equivalency.
10 It is added to the analyte solution and aids in the observation of the completion of the reaction.
a) End Point
b) Indicator
c) Direct Titration
d) Back Titration
e) Indirect Titration
f) Primary Standard
g) Standard Solution
h) Titrand
i) Equivalence Point
j) Titrant
Answers
Answer:
1. Equivalence point
2. Direct titration
3. Primary standard
4. Titrand
5. Back titration
6. Standard solution
7. Titrant
8. Indirect titration
9. End point
10. Indicator
Explanation:
1. The equivalence point is the tiration point at which the quantity or moles of the added titrant is sufficient or equal to the quantity or moles of the analyte for the neutralization of the solution of the analyte.
2. Direct titration is a method of quantitatively determining the contents of a substance
3. A primary standard is an easily weigh-able representative of the mount of moles contained in a substance
4. A titrand is the substance of unknown concentration which is to be determined
5. The titration method that uses a given amount of an excess reagent to determine the concentration of an analyte is known as back titration
6. A standard solution is a solution of accurately known concentration
7. A titrant is a solution that has a known concentration and which is titrated unto another solution to determine the concentration of the second solution
8. Indirect titration is the process of performing a titration in athe reverse order
9. The end point is the point at which the indicator indicates that the equivalent quantities of the reagents required for a complete reaction has been added
10 An indicator is a compound used to visually determine the pH of a solution.
The titration has been the neutralization reaction in which the titrand and the titrant react to form the salt and the water and help in the determination of the qualitative and quantitative properties.
What is an Endpoint?In a titration reaction, the endpoint has been the point at which the equivalent amount of reagent has been completely neutralized.The Indicator has been the chemical that changes to indicate the endpoint of the reaction.Direct titration involves the reaction for the quantitative determination of the substances.The back titration can be given as the reaction in which the excess reagent is used to titrate the second standard reagent in the reaction.Indirect titration can be given as the reaction of the analyte to convert to another form and then the analysis with the titrant.The primary standard has been the known concentration of the pure and stable weighing reagent.The standard solution has been the solution of the known concentration in the reaction.Titrand has been the unknown sample that has to be analyzed.The equivalence point is the concentration point at which the quantity of titrant added to the titrand has been equal.Titrant has been the known concentration of sample that has been added to equivalent the unknown sample.Learn more about titration here:
https://brainly.com/question/24704707
What is the first element to form according to Big Bang theory?
Answers
The Big Bang theory claims that hydrogen was the first element to form.
What is Hydrogen?Atomic number 1 and the letter H are used to identify the chemical element hydrogen. In the cosmos, it makes up around 75% of the elemental mass and is the lightest and most prevalent element. At room temperature and pressure, hydrogen is a colorless, tasteless, odorless gas that is non-toxic.
The Big Bang theory claims that hydrogen was the first element to form. The universe was made of a hot, dense plasma of protons, neutrons, and electrons in the initial moments following the Big Bang. These particles merged when the cosmos cooled and expanded to create hydrogen atoms, which subsequently fused to create the first stars. Helium, carbon, oxygen, and other atoms that make up the universe we see today were converted into heavier elements through nuclear fusion in these stars.
To know more about Hydrogen, visit:
https://brainly.com/question/28557068
#SPJ4