Look at the graph. Which part of the line shows a time
when the object was not moving?
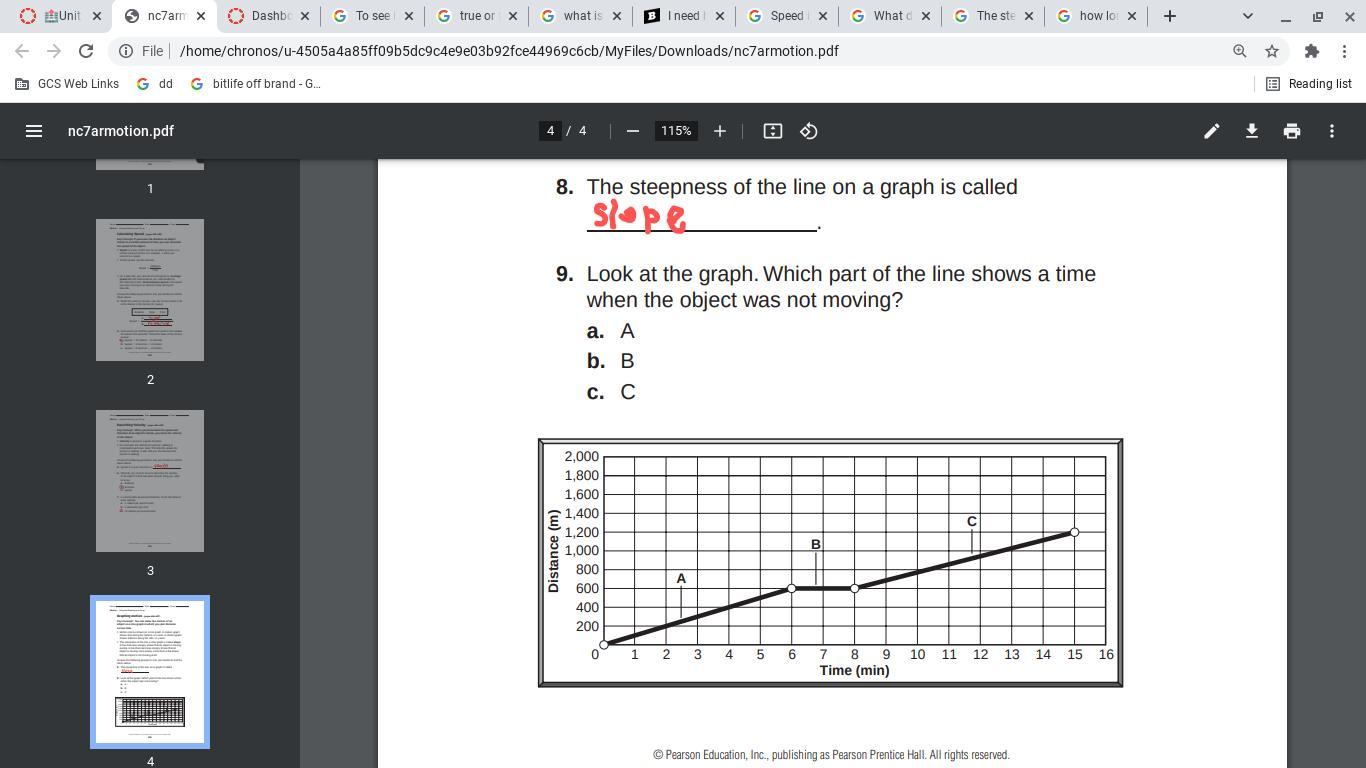
Answers
For the period of B, the line didn’t go up or down, it stayed still.
this shows that time was still passing but the car didn’t move.
Related Questions
Which of the following words states what the arrow means in a chemical equation. A. Destroys B. Produces C. Yields D. Both b and c
Answers
Answer:
Terms in this set (26) What do the formulas, arrow, and plus signs in a chemical equation tell you? Elements and compounds involved in the reaction, the arrow means "yields" and points to products, and the plus sign indicate two or more reactants or products.
Explanation:
using the equation e=(hcrh)(1n2)=(−2.18×10−18j)(1n2)e=(hcrh)(1n2)=(−2.18×10−18j)(1n2) calculate the energy of an electron in the hydrogen atom when n=2n=2 .
Answers
The total energy of an electron is the sum of its kinetic and potential energy. The kinetic energy of the electron in the nth orbit is The potential energy of the electron is found in the formula for electrostatic potential energy. The charges will be on Nucleus (+Ze) and on electron (-e)
Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton.
When n=2 in the hydrogen atom, we can use the equation e=(hcrh)(1n2)=(−2.18×10−18j)(1n2)e=(hcrh)(1n2)=(−2.18×10−18j)(1n2) to calculate the energy of an electron.
Plugging in n=2, we get e=(hcrh)(1n2)=(−2.18×10−18j)(1n2) = (−2.18×10−18j)(1ln2) = −(2.18×10−18j)(0.693)
Solving this equation gives us e = -1.51 × 10^-18 joules.
Therefore, the energy of an electron in the hydrogen atom when n=2 is -1.51 × 10^-18 joules.
To know more about the hydrogen atom https://brainly.com/question/29695801
#SPJ11
how many grams of phosphorus are in 50-gram sample of aluminum phosphate
Answers
There are approximately 12.7 grams of phosphorus in a 50-gram sample of aluminum phosphate.
To determine the number of grams of phosphorus in a 50-gram sample of aluminum phosphate, we need to know the molar mass and the chemical formula of aluminum phosphate.
The chemical formula for aluminum phosphate is AlPO4. It indicates that each molecule of aluminum phosphate contains one aluminum atom (Al), one phosphorus atom (P), and four oxygen atoms (O).
To calculate the molar mass of aluminum phosphate, we can add up the atomic masses of its constituent elements based on their stoichiometric ratios:
Molar mass of AlPO4 = (molar mass of Al) + (molar mass of P) + (4 * molar mass of O)
Using the periodic table, we can find the atomic masses of the elements:
Molar mass of Al = 26.98 g/mol
Molar mass of P = 30.97 g/mol
Molar mass of O = 16.00 g/mol
Now, let's calculate the molar mass of aluminum phosphate:
Molar mass of AlPO4 = (26.98 g/mol) + (30.97 g/mol) + (4 * 16.00 g/mol)
= 121.95 g/mol
The molar mass of aluminum phosphate is 121.95 g/mol.
To determine the number of grams of phosphorus in a 50-gram sample of aluminum phosphate, we need to calculate the mass fraction of phosphorus in the compound. The mass fraction is the ratio of the molar mass of phosphorus to the molar mass of aluminum phosphate.
Mass fraction of phosphorus = (molar mass of P) / (molar mass of AlPO4)
= (30.97 g/mol) / (121.95 g/mol)
≈ 0.254
Multiplying the mass fraction by the mass of the sample gives us the grams of phosphorus:
Grams of phosphorus = (mass fraction of phosphorus) * (mass of the sample)
= 0.254 * 50 g
≈ 12.7 g
For more such questions on aluminum phosphate visit:
https://brainly.com/question/15072110
#SPJ8
A flask contains helium and nitrogen
gases. The partial pressures of each are
77 mm Hg and 87 mm Hg, respectively.
What is the total pressure in the flask in
mm Hg?
Answers
total pressure
=77 mmHg+87 mmHg
=164 mmHg
Answer: 164 mm Hg
Explanation: 7 mmHg+87 mmHg
=164 mmHg
Substances formed as a result of a chemical reaction are calledImmersive Reader (1 Point) products reactants catalysts precipitates
Answers
Answer: substances formed as a result of chemical reaction are called PRODUCTS.
Explanation:
A chemical reaction can be defined as a reaction in which substances known as the REACTANTS makes or breaks it's bonds to form another substance usually referred to as the PRODUCT. Usually, a new substance which is referred to as the product that is formed is different from the original reacting substances. Some of the characteristics of a chemical reaction include:
--> colour change,
--> heat production,
--> precipitation
The rate at which these chemical reaction occurs depends on some factors such as pressure, temperature and concentration of the reactants
There are different examples of chemical reaction which includes:
--> Rusting of iron: this occurs when iron and oxygen combines to form a mixture of iron oxides, which is the product of the chemical reaction.
--> The burning of magnesium in air: in this Chemical reaction, magnesium reacts with oxygen to form magnesium oxide which is the product of the chemical reaction.
2Mg(s) + O2(g) △→ 2MgO(s)
--> Production of table salt: this is formed from the reaction of sodium and chloride to give sodium chloride as the product of the chemical reaction.
Therefore in a chemical reaction, no atom is destroyed or created but a new PRODUCT is formed from reactants.
How old is a bone if it presently contains 0.3125g of C-14, but it was estimated to have originally contained 80.000g of C-14?
Answers
Half-life of C-14 is 5730 years
The age of the bone is the time taken to decay carbon -14 from a mass of 80 g to 0.3125 g. The decay time is 46209 years. That is the age of the bone.
What is carbon dating?Carbon -14 isotope is radioactive and undergoes nuclear decay by the emission of charged particles. The age of a fossil sample which contains carbon -14 can be determined using the time of decay.
Half life of C-14 = 5730 years
decay constant k = 0.693 / 5730 = 0.00012 yr⁻¹
Initial amount of C-14 = 80 g
final amount = 0.3125 g
Then the time taken to decay 80 g to 0.3125 g is t = 1/k ln (80/0.312)
t = ln (80/0.312) / (0.00012 yr⁻¹)
= 46209 years.
Therefore, the age of the bone is 46209 years.
Find more on carbon dating:
https://brainly.com/question/3753282
#SPJ2
HELLO
can you give me some facts about the energy Energy Transformation Of burning wood and where you found your info I need it for a project I will give brainliest tysm
Answers
Burning wood is an exothermic reaction. This reaction is a chemical change. It is exothermic because its reactions release heat to the surroundings. It is a chemical change because it is not reversible and produces things like ash, smoke, heat, light, and more.
Joelle is a manager at a construction company, and she is interested in the chemistry behind the materials they use. She has begun studying the materials used to fill walls. She knows that to keep the temperature inside a room steady the material must be a thermal insulator, and she predicts that materials should not be acidic or else they would dissolve too easily in water.
Which of these is a molecular ingredient that could be used in a wall-filling material ?
C27H36N2O10
Na6Ba6
NeNa
HCl
Answers
Answer:
a
Explanation:
A molecular ingredient that could be used in a wall-filling material is C₂₇H₃₆N₂O₁₀, is not a good thermal insulator, hence option A is correct.
The big molecule C₂₇H₃₆N₂O₁₀ is a poor thermal insulator. Since NeNa is a relatively reactive chemical, it would probably dissolve in water far too quickly. The acidic molecule HCl would dissolve far too quickly in water.
Not a good thermal insulator is C₂₇H₃₆N₂O₁₀. It is a big molecule composed of a lot of hydrogen atoms. Since hydrogen atoms are excellent heat conductors, they would be ineffective in stopping heat from passing through a material used to fill a wall.
Thus, the option (A) C₂₇H₃₆N₂O₁₀ is correct.
Learn more about thermal insulator, here:
https://brainly.com/question/23134662
#SPJ6
study this chemical reaction: (aq)(s)(aq)(s) then, write balanced half-reactions describing the oxidation and reduction that happen in this reaction.
Answers
In order to write balanced half-reactions for the oxidation and reduction that occur in a chemical reaction, we first need to identify the species being oxidized and reduced. The (aq) and (s) labels indicate the states of the reactants and products.
Let's assume the reactants are A(aq) and B(s), and the products are C(aq) and D(s).
The oxidation half-reaction involves the species being oxidized, which loses electrons. The reduction half-reaction involves the species being reduced, which gains electrons.
To balance the half-reactions, we need to ensure that the number of atoms on both sides of the equation is the same and that the charges are balanced.
For example, if A is being oxidized and B is being reduced, the balanced half-reactions could be:
Oxidation half-reaction:
A(aq) → C(aq) + e-
Reduction half-reaction:
B(s) + e- → D(s)
So, the oxidation half-reaction is A(aq) → C(aq) + e- and the reduction half-reaction is B(s) + e- → D(s). To write the balanced half-reactions, we need to ensure that the number of atoms on both sides of the equation is the same and that the charges are balanced. In the oxidation half-reaction, A(aq) is being oxidized and loses an electron (e-). The resulting product is C(aq). On the other hand, in the reduction half-reaction, B(s) is being reduced and gains an electron (e-). The resulting product is D(s). By balancing the atoms and charges, we can write the balanced half-reactions as follows:
Oxidation half-reaction: A(aq) → C(aq) + e-
Reduction half-reaction: B(s) + e- → D(s)
The balanced half-reactions for the oxidation and reduction in the chemical reaction are A(aq) → C(aq) + e- and B(s) + e- → D(s), respectively.
To learn more about balanced half-reactions visit:
brainly.com/question/14339821
#SPJ11
Which is a saturated fatty acid?
Answers
Answer:
The saturated fatty acids are derived from both animal fats and plant oils. Rich sources of dietary saturated fatty acids include butter fat, meat fat, and tropical oils (palm oil, coconut oil, and palm kernel oil). Saturated fatty acids are straight-chain organic acids with an even number of carbon atoms (Table 2)
A ample of chloroform i found to contain 10. 1 g of carbon, 89. 6 g of chlorine, and 0. 85 g of hydrogen. If a econd ample of chloroform i found to contain 35. 6 g of carbon, what i the total ma of chloroform in the econd ample?
Answers
So, the empirical formula will be CHCl3.An ideal anesthetic was originally sought after via the use of the organic chemical chloroform.This substance has a 120.98 g/mol molar mass.
What is chloroform chemical formula?So, the empirical formula will be CHCl3.An ideal anesthetic was originally sought after via the use of the organic chemical chloroform.In 1831, it was initially made.The substance has the formula CHCl3.It is a thick, colorless liquid that has a sweet scent that is widely produced.Compounds of the elements carbon, hydrogen, and chlorine have the empirical formula C2 HCl.The molar mass of this chemical is 120.98 g/mol.It is frequently referred to as "chloroform" because of the molecule's molecular similarity to formic acid (CHOOH) [4].Chloroform can degrade and create dangerous chemicals like phosgene and hydrogen chloride while not being easily flammable.To learn more about chloroform refer
https://brainly.com/question/17380113
#SPJ4
number five on the worksheet

Answers
Answer: 15466 J
Explanation: The image quality is low, but I am assuming that you have 100.0mL of water at 0.0C and it is being raised to 37.0C.
You use the following formula:
(Temperature change)(Heat capacity)(Grams of Water) = J heat
So, you would substitute values like this:
(37)(4.18)(100) = J heat
We get 100g of water because 100 mL of water is equivalent to 100g of water. 37 is the change in temperature.
This equation evaluates to 15466 J
2. If you put 156. 32g barium hydroxide into this reaction, how much aluminium hydroxide can be
produced?
Answers
When 156.32 g of barium hydroxide is reacted, approximately 142.34 g of aluminum hydroxide can be produced, based on the balanced chemical equation and stoichiometry.
To determine the amount of aluminum hydroxide that can be produced when 156.32 g of barium hydroxide is reacted, we need to consider the balanced chemical equation for the reaction and use stoichiometry.
The balanced chemical equation for the reaction is:
Ba(OH)2 + 2AlCl3 → 2Al(OH)3 + 3BaCl2
From the balanced equation, we can see that for every 1 mole of Ba(OH)2, 2 moles of Al(OH)3 are produced.
First, we need to calculate the number of moles of barium hydroxide (Ba(OH)2) in 156.32 g:
Molar mass of Ba(OH)2 = (137.33 g/mol + 2(16.00 g/mol + 1.01 g/mol)) = 171.34 g/mol
Moles of Ba(OH)2 = mass / molar mass = 156.32 g / 171.34 g/mol = 0.911 mol
Now, using the stoichiometry of the balanced equation, we can determine the moles of aluminum hydroxide (Al(OH)3) produced:
Moles of Al(OH)3 = 2 × Moles of Ba(OH)2 = 2 × 0.911 mol = 1.822 mol
Finally, to convert the moles of aluminum hydroxide to grams, we need to multiply by the molar mass of Al(OH)3:
Molar mass of Al(OH)3 = (26.98 g/mol + 3(16.00 g/mol + 1.01 g/mol)) = 78.00 g/mol
Mass of Al(OH)3 = Moles of Al(OH)3 × molar mass = 1.822 mol × 78.00 g/mol = 142.34 g
Therefore, when 156.32 g of barium hydroxide is reacted, approximately 142.34 g of aluminum hydroxide can be produced.
For more such questions on barium hydroxide visit;
https://brainly.com/question/29344018
#SPJ8
You have a 9.1 ppm sodium ion (Na', 22.990 g/mol) aqueous solution. Express this in uM (umol/L).
Answers
The concentration of 9.1 parts per million of sodium in the solution is equal to 395.8 μM (molarity).
A ppm (parts per million) and M (molarity) are units of concentration. To convert ppm to μM we need to remember that:
\( 1 ppm = \frac{mg}{L} \)
\( 1 M = \frac{mol}{L} \)
Molar mass Na = 22.990 g/mol
Now, we can convert ppm to M as follows:
\([Na^{+}] = 9.1 \frac{mg}{L}*\frac{1 g}{1000 mg}*\frac{1 mol}{22.990 g}*\frac{10^{6} \mu mol}{1 mol} = 395.8 \mu mol/L\)
Therefore, the concentration of sodium in the solution is 395.8 μmol/L.
You can see another example of ppm conversion to molarity here: https://brainly.com/question/13565240?referrer=searchResults
I hope it helps you!
Which level of ecological organization includes both biotic and abiotic factors
Answers
The answer is Ecosystem
Answer:
ecosystem
Explanation:
i took it on edge 2021 and got 100
Which of the following radioactive emissions is the least penetrating?
alpha particles
gamma rays
beta particles
Answers
Answer:
alpha particles
Explanation:
alpha particles the least penetrating but potentially most damaging and gamma rays the most penetrating. A beta particle, also called beta ray or beta radiation, is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay.
sana po makatulong
#keeponlearning
#Godbless
Explain how food is transformed to energy for our bodies.
Answers
Why do you think that tsunamis occur most commonly in the Pacific Ocean?
Answers
How does the vapor pressure of mercury affect the pressure reading of the manometer?
Answers
URGENT:
Do you switch charges for ionic or covalent bonds when naming them?
Answers
Answer:
ionic
Explanation:
an element has two naturally occurring isotopes. one is 120.9 amu and a relative abundance of 57.4% and the other has a mass of 122.9042 amu. what is the atomic mass of this element
Answers
The atomic mass of the element is approximately 122.6 amu.
To calculate the atomic mass of the element, we need to consider the weighted average of the masses of its naturally occurring isotopes, taking into account their relative abundances.
Given:
Isotope 1 mass (m1) = 120.9 amu
Isotope 1 relative abundance (a1) = 57.4%
Isotope 2 mass (m2) = 122.9042 amu
To calculate the atomic mass (M) of the element:
M = (m1 * a1 + m2 * a2) / 100
Substituting the given values:
M = (120.9 amu * 57.4% + 122.9042 amu * (100% - 57.4%)) / 100
M = (69.6276 amu + 52.9726 amu) / 100
M = 122.6002 amu / 100
M ≈ 1.226002 amu
Therefore, the atomic mass of the element is approximately 122.6 amu.
Learn more about atomic mass, here:
https://brainly.com/question/29117302
#SPJ4
What is the ratio for O2 to CO2

Answers
Answer:
O2• The rates of CO2 uptake and O2 evolution exhibit nearly identical responses to CO2 pressure within each species while the absolute rate of CO2 uptake appears to be slightly higher than the rate of O2 evolution at all CO2 pressures, giving a C02/02 exchange ratio of 1.04 to 1.14.
HELPPPP!!! I need the CORRRECT answer
1.Which pair of properties describes the elements in Group 18?
a) They are gasseous at room tempature and chemically stable
b) they have 8 valence electrons and are stable
c) they are chemically stable and liquid at room tempature
d) they are magnetic and boil at room tempature
Answers
Answer: c
Explanation:
In what way does the structural formula of a compound differ from its molecular formula?.
Answers
In addition to providing the same information just like its molecular formula, a compound's structural formula also reveals the atoms' connections inside the molecule.
What components make up a molecular formula?The number of atoms from each element in a single chemical molecule is specified by the molecular formula. It displays the precise number of atoms in a molecule. For instance, the chemical structure of propane is C4H10. The provided compound contains 10 hydrogen atoms and 4 carbon atoms according to this formula.
Which formula is indeed a molecular formula?Empirical formulae reveal the simplest or the most reduced ratio of components in a compound while molecular formulas reveal how many atoms from each element are present in a complex. The empirical formula and the molecular formula of a substance are identical if the molecular formula could be decreased further.
To know more about molecular formula visit:
https://brainly.com/question/13058832
#SPJ4
Starch is a carbohydrate consisting of glucose polymers. The caloric value for glucose is3.9 kcal/g. You eat a potato that weighs 174 g. Assume that 92% of the total mass of apotato is starch. Determine (a) how many kcal, and how many kJ of energy were in thepotato you ate. 1 cal (gram calorie) = 4.184 joules. Show all your work

Answers
We are told that starch consists of glucose polymer, so we can assume that the caloric value of starch will be equal to the caloric value of glucose, that is, 3.9kcal/g.
Now to determine the kcal and kJ there were in the potato we must calculate the mass of starch present in that potato. We are told that it is 92% starch, therefore the mass of starch in the potato will be:
\(gStarch=174g\times\frac{92\%}{100\%}=160.gStarch\)We have that in the potato there are 160.08 grams of starch. By multiplying it by the caloric value we will have the kcal that were in the potato, assuming that the rest of the ingredients do not contribute caloric value, or it is insignificant.
\(\text{kcal of potato}=160g\times3.9\frac{kcal}{g}=624\text{kcal}\)To calculate the kJ we must make the conversion using the relationship that 1 cal is equal to 4.184 joules:
\(\text{kJ of potato}=624kcal\times\frac{1000cal}{kcal}\times\frac{4.184J}{1cal}\times\frac{1kJ}{1000J}=2612kJ\)In the potato, there were 624 kcal of energy or 2612kJ of energy.
Is nabro3 ionic or molecular?
Answers
NaBrO₃ (sodium bromate) is an ionic compound. It consists of a metal (sodium, Na) and a polyatomic ion (bromate, BrO₃⁻).
Ionic compounds are compounds composed of positive and negative ions held together by electrostatic forces. They are formed through the transfer of electrons between atoms, resulting in the formation of positively charged cations and negatively charged anions.
In an ionic compound, the cations are typically metal ions, while the anions are usually nonmetal ions or polyatomic ions. The transfer of electrons occurs from the metal atom(s) to the nonmetal atom(s), resulting in the formation of a crystal lattice structure.
Learn more about ionic compounds, here:
https://brainly.com/question/30420333
#SPJ6
Why are metallic and non-metallic minerals and fossil fuels thought of as non-renewable resources?
Answers
They are though of non renewable because the earth had them to begin with but once they're gone there is no getting them back.
Answer:I believe this is due to the fact that they aren't living things, and discovering them might be difficult at times. They don't grow back, don't get replaced, and don't get renewed.
Explanation:
a 20.0ml sample of a 0.10 m solution of agno3 needs to have the silver ions precipitated out. how many ml of 0.10 m solution of bacl2 would need to be used to precipate out all the silver as agcl?
Answers
You would need 0.002 mol / 0.10 mol/L = 0.020 L = 20.0 ml of the 0.10 m solution of BaCl2 to precipitate out all the silver as AgCl.
To precipitate all the silver ions as AgCl, you need to determine the stoichiometric ratio between AgNO3 and BaCl2. The balanced equation for the reaction is
AgNO3 + BaCl2 -> AgCl + Ba(NO3)2.
The stoichiometric ratio between AgNO3 and AgCl is 1:1.
Therefore, the number of moles of AgNO3 in the 20.0 ml sample is 0.10 mol/L x 0.020 L
= 0.002 mol.
Since the stoichiometric ratio is 1:1, you would need an equal number of moles of BaCl2 to precipitate all the silver ions. The concentration of BaCl2 is also 0.10 mol/L.
Therefore, you would need 0.002 mol / 0.10 mol/L = 0.020 L = 20.0 ml of the 0.10 m solution of BaCl2 to precipitate out all the silver as AgCl.
To know more about solution visit:-
https://brainly.com/question/1616939
#SPJ11
True or False?
To increase acceleration of a object, you reduce its mass or increase the applied force.
Answers
I used a stick of (vegan) butter as our example of thinking about how temperature changes the properties of something when it is wamer or cooler.
1. What is something else you could use to describe how temperature affects the properties of when it is warmer or cooler? How does this change whether it is warm or cold. Describe it.