Limiting and Excess Reactants POGIL (Extension Questions)
Answers
Limiting reactants are the reagents that are used up first in a chemical reaction, and determine the amount of product that can be formed.
Excess reactants are reagents that, once the limiting reactant has been used up, are still present in the reaction mixture.
The limiting reactant is important because it is the reagent that limits the amount of product that can be produced. When excess reactants are present, they do not contribute to the amount of product that can be produced and are thus considered to be "excess" material.
This excess material can cause problems in a reaction, such as unwanted byproducts or the formation of side reactions. Therefore, it is important to carefully control the amounts of reactants that are used in a reaction to ensure that the desired product is formed in the maximum possible yield.
Know more about Chemical reaction here
https://brainly.com/question/29039149#
#SPJ11
Related Questions
Which of these is true about electrons? posses a positive electrical charge of one (+1) have a negative electrical charge of one (-1) indicates the number of protons in each atom equals the sum of protons plus neutrons in each atom
Answers
Answer:
have a negative electrical charge of one (-1)
Explanatio
Electrons have an electrical charge of negative one. When you think electron, always think -1
at the exact instant that a carbonated beverage is opened, it isSelect the correct answer below:A. unsaturated with carbon dioxideB. saturated with carbon dioxideC. supersaturated with carbon dioxideD.saturated with oxygen
Answers
At the exact instant that a carbonated beverage is opened, it is saturated with carbon dioxide. The carbon dioxide is dissolved in the liquid under high pressure, which maintains its solubility in the liquid.
When the bottle or can is opened, the pressure is released, and the carbon dioxide begins to come out of solution, forming bubbles. As the carbon dioxide leaves the liquid, the beverage becomes less saturated with the gas.
Carbonated beverages, such as soda, are made by dissolving carbon dioxide gas (CO2) under high pressure into a liquid, such as water. The pressure forces more gas to dissolve in the liquid than would normally be possible under normal atmospheric conditions. The dissolved carbon dioxide forms carbonic acid (H2CO3), which gives the drink a slightly acidic taste.
When the container of the carbonated beverage is opened, the pressure is released, and the carbon dioxide comes out of solution. The carbon dioxide gas forms bubbles that rise to the surface of the liquid and escape into the air. This process is called degassing, and it causes the drink to lose its fizziness and become flat.
The degree of carbonation in a beverage depends on several factors, such as the amount of carbon dioxide added, the temperature of the liquid, and the pressure at which it is stored. For example, colder liquids can hold more dissolved gas than warmer liquids, and higher pressures can force more gas into the liquid. Different types of carbonated beverages can also have different levels of carbonation, with some having more or less carbon dioxide than others.
Learn more about carbonation here:
https://brainly.com/question/19886129
#SPJ4
Substance A undergoes a first order reaction A → B with a half-life of 20 min at 25 °C. If the initial concentration of A in a sample is 1.6 M, what will be the concentration of A after 80 min? (A) 0.40 M(B) 0.20 M (C) 0.10 M (D) 0.050 M
Answers
0.10 M will be the concentration of A after 80 min.
We need to use the equation for first order reactions, which is: ln[A]t = -kt + ln[A]0, where [A]t is the concentration of A at time t, k is the rate constant, and [A]0 is the initial concentration of A.
We are given that the half-life of the reaction is 20 minutes, which means that k = ln2/20 = 0.03465 min^-1.
We can now use this value of k to find the concentration of A after 80 minutes:
ln[A]80 = -0.03465 x 80 + ln(1.6)
ln[A]80 = -2.772 + 0.470
ln[A]80 = -2.302
To get the concentration of A, we need to take the antilog of this value:
[A]80 = e^-2.302
[A]80 = 0.099 M
Therefore, the answer is (C) 0.10 M.
Substance A undergoes a first-order reaction A → B with a half-life of 20 minutes at 25 °C. The initial concentration of A is 1.6 M. To determine the concentration of A after 80 minutes, we can use the half-life concept. Since 80 minutes is equivalent to 4 half-lives (80 minutes / 20 minutes per half-life), we can calculate the concentration as follows:
1st half-life (20 min): 1.6 M / 2 = 0.8 M
2nd half-life (40 min): 0.8 M / 2 = 0.4 M
3rd half-life (60 min): 0.4 M / 2 = 0.2 M
4th half-life (80 min): 0.2 M / 2 = 0.1 M
Therefore, the concentration of A after 80 minutes will be 0.1 M (Option C).
To know more about First order reaction visit:
https://brainly.com/question/31141406
#SPJ11
identify which substance has the lowest vapor pressure at 25c and 1.00 atm external (atmospheric) pressure
Answers
The one having lowest vapour pressure at `25^C` is a : The vapour pressure of water is least because of strong intermolecular H-bonding in water molecules.
The pressure by a vapour when it is in equilibrium with a substance's liquid, solid, or both forms—i.e., when the conditions permit the substance to exist in both of these phases or in all three—is known as vapour pressure. The measurement of a substance's propensity to change into a gaseous or vapour state, vapour pressure increases with temperature. The temperature at which the pressure equals the vapour pressure existing at the liquid's surface is known as the boiling point of a liquid. When conditions allow for the creation of vapour inside a liquid, the process is referred to as boiling. It follows that the more molecules that evaporate at a given temperature, the higher the vapor pressure that results.
To learn more about vapor pressure,
https://brainly.com/question/26127294
#SPJ4
determine the value of kp for the following reaction: 4hcl(g)+o2(g)⇌2cl2(g)+2h2o(g)
Answers
The equilibrium constant at a temperature of 25°C is Kp = 28.18. This value can be expressed to three significant digits as Kp = 28.2.
What exactly is balance?A state of balance between conflicting forces or influences is known as equilibrium. It is a principle that is applied in a variety of fields, including as physics, chemistry, and economics.
The following equation can be used to determine the equilibrium constant for the specified reaction:
Kp = [Cl₂]²[H₂O]² / [HCl]⁴[O₂]
The partial pressures of the gases can be used to determine Kp on the assumption that the gas is ideal.
Kp = (PCl₂)²(PH₂O)²/ (PHCl)⁴(PO₂)
Kp is easily calculable if the partial pressures of each gas are known. Finding the equilibrium constants at a given temperature is important because no pressures are provided in this scenario.
According to the information in the thermochemical table, Kp = 28.18 is the equilibrium constant at 25°C. 3 significant figures can be used to express this amount Kp = 28.2.
To learn more about equilibrium
brainly.com/question/18849238
#SPJ4
what mass (in grams) of nh3 must be dissolved in 475 g of methanol (solvent) to make 0.147 m solution
Answers
The 1.504 g of \(NH_3\) must be dissolved in 475 g of methanol to make a 0.147 M solution.
To solve this problem, we can use the formula:
molarity = moles of solute/liters of solution
First, we need to convert the mass of methanol to liters:
475 g / 0.7918 g/mL = 600.1 mL = 0.6001 L
Now we can use the formula to find the moles of \(NH_3\) needed:
0.147 M = moles of \(NH_3\) / 0.6001 L
moles of \(NH_3\) = 0.147 M × 0.6001 L = 0.08827 moles
Finally, we can use the molar mass \(NH_3\) to find the mass of \(NH_3\) needed:
mass of \(NH_3\) = moles of \(NH_3\) × molar mass of \(NH_3\)
mass of \(NH_3\) = 0.08827 moles × 17.03 g/mol = 1.504 g
To know more about methanol, here
brainly.com/question/24077457
#SPJ1
What is the correct formula for calcium dihydrogen phosphate?
A) CaH2PO4
B) Ca(H2PO4)2
C) Ca(H2HPO4)2
D) Ca2H2PO4
Answers
Answer:
B) Ca(H2PO4)2
Explanation:
To solve this we must be knowing each and every concept related to chemical formula. Therefore, the correct option is option B among all the given options.
What is chemical formula?The chemical formula is indeed a means of expressing information about just the chemical proportion of atoms that comprise a certain chemical compound as well as molecule through the use of chemical element numbers and symbols.
Furthermore, the molecular formula does not contain any words and does not serve as a chemical name. However, it just indicates basic chemical structures and is not the same as the entire chemical structural formula. The correct formula for calcium dihydrogen phosphate is Ca(H\(_2\)PO\(_4\))\(_2\).
Therefore, the correct option is option B among all the given options.
To know more about chemical formula, here:
https://brainly.com/question/29031056
#SPJ2
what special equipment did niels bohr use to develop his atomic model?
Answers
Neil Bohr used the fluorescent screen and an alpha particle detector to study the structure of an atom.
What is an atom?
An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
The atom as a whole is neutral and stable due to presence of oppositely charged particles.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ1
PLEASE HELP WITH THESE THREE (30 POINTS)
1) How is the number of neutrons in the nucleus of an atom calculated?
A. Add the number of elections- and protons+ together
B. Subtract the number of elections- from protons+
C. Subtract the number of protons+ from the atomic mass number
D. Add the atomic mass number to the number of elections-
2) Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), and Francium (Fr) are in the same group on the periodic table. Why? *
A. They have the same size.
B. They react with each other.
C. They have similar properties.
D. They have the same number of protons
3) Which of the following elements would be a transition metal?
A. Ca
B. Au
C. Rb
D. Fr
Answers
2. C
3. A
i had the test with the same exact questions
Which statement provides the best example of a scientific conclusion?
O A. Graphite can be used to make pencils.
O B. Diamond is mined in South Africa
O C. Diamond and graphite are both composed of carbon.
D. Diamond jewelry is prettier than graphite jewelry.
Answers
Answer:
c
Explanation:
In science, there is always processing of an item or sample to get an end product. So if the diamond is mined; there is a processing stage used to come out with the final product as Diamond through carbon in the heating process
Hi can you help me please 
Answers
(d) The equation can be written in particulate form. Using the following key, draw the correct products above their formulas.
Key
H = O
O
For F-=
HF(aq)
+
OH(aq)
F (aq)
+
H2O()
Answers
The complete reaction equation, using the lowest number of coefficients possible is; HF(aq) + OH^-(aq) ----> F^-(aq) + H2O(l)
The reaction equation here shows the reaction between hydrogen fluoride and the the hydroxide ion from a base. This is a neutralization reaction as shown.
The reaction can fully be represented as;
HF(aq) + OH^-(aq) ----> F^-(aq) + H2O(l)
This is the full reaction equation using the lowest number of coefficients possible in the reaction equation.
Learn more: https://brainly.com/question/12108425
Which of the following is NOT and example of a mixture?*
air
salt and pepper
water
Answers
Answer:
water because the other two were formed through a chemical process
soluble fiber is described as ""viscous"" because it:
Answers
soluble fiber is described as "viscous" because it forms a gel-like substance when it comes into contact with liquids. This gel-like consistency is due to its ability to absorb water and create a thick, sticky gel in the digestive tract. The viscosity of soluble fiber helps to slow down digestion, regulate blood sugar levels, lower cholesterol levels, and promote a feeling of fullness.
soluble fiber is a type of dietary fiber that dissolves in water to form a gel-like substance. This gel-like consistency is what makes it described as "viscous." When soluble fiber comes into contact with liquids, it absorbs water and forms a thick, sticky gel in the digestive tract.
This unique property of soluble fiber is due to its chemical structure. Soluble fiber is made up of long chains of sugar molecules that are soluble in water. These sugar molecules have the ability to attract and bind with water molecules, forming a gel-like substance.
The viscosity of soluble fiber plays an important role in its health benefits. The gel-like consistency of soluble fiber slows down the digestion and absorption of nutrients in the digestive tract. This slow digestion helps to regulate blood sugar levels, lower cholesterol levels, and promote a feeling of fullness, which can aid in weight management.
Learn more:About soluble fiber here:
https://brainly.com/question/30044994
#SPJ11
Soluble fiber is described as "viscous" because it forms a gel-like substance when mixed with water. This gel-like substance slows down the digestive process and increases feelings of fullness, making it an important part of a healthy diet.
Soluble fiber is a type of fiber that dissolves in water to form a gel-like substance. This type of fiber is found in many plant-based foods, including fruits, vegetables, legumes, and grains.What are the benefits of soluble fiber?Soluble fiber is known to provide several health benefits, including:Lowering cholesterol levels: Soluble fiber can help lower LDL cholesterol levels by reducing the absorption of cholesterol in the bloodstream. Controlling blood sugar: Soluble fiber slows down the absorption of sugar into the bloodstream, helping to stabilize blood sugar levels.
Promoting feelings of fullness: Soluble fiber absorbs water and expands in the stomach, promoting feelings of fullness and reducing appetite. Improving digestion: Soluble fiber slows down the digestive process, allowing for more efficient absorption of nutrients. Preventing constipation: Soluble fiber adds bulk to stool and helps prevent constipation.How does soluble fiber form a gel-like substance?Soluble fiber forms a gel-like substance when it absorbs water. As it travels through the digestive system, it attracts water and expands in size. This expansion creates a thick, gel-like substance that slows down the digestive process and promotes feelings of fullness.
Learn more about Soluble fiber here:https://brainly.com/question/17146044
#SPJ11
Calculate the mass of carbon and the mass of hydrogen in 82.0 g of propane.
Answers
Mass of the carbon is 7.44 g while the mass of the hydrogen is 0.4 g.
What is the mass of the carbon?We have to know that we can be able to obtain the masses of each of the atoms that can be found in the molecule that is called propane. You have to note that propane is a hydrocarbon and this means that the only two atoms there are carbon and hydrogen.
We then have;
Number of moles of the propane = mass/molar mass = 82.0 g/44 g/mol
= 1.86 moles
Number of moles of the carbon = 1.86 moles/3 = 0.62 moles
Mass of carbon = 0.62 mol * 12 g/mol = 7.44 g
Number of moles of hydrogen = 1.62/8 = 0.2 moles
Mass of the hydrogen = 0.2 moles * 2 g/mol = 0.4 g
Learn more about moles:https://brainly.com/question/26416088
#SPJ1
which of the following statement(s) are true about the bonding in ccl4
A. The C-Cl bonds are ionic, and it is ionic.
B. It has polar covalent bonds, and it is nonpolar.
C. It has covalent bonds, and it is nonpolar.
D. It has polar covalent bonds, and it is polar.
E. It has covalent bonds, and it is polar.
Answers
The correct statement regarding the bonding in CCl4 is It has covalent bonds, and it is nonpolar. CCl4, or carbon tetrachloride, consists of a central carbon atom bonded to four chlorine atoms.
Each carbon-chlorine bond is a covalent bond, meaning the electrons are shared between the carbon and chlorine atoms. However, due to the difference in electronegativity between carbon and chlorine, the bonds are polar covalent. Polar covalent bonds arise when there is an unequal sharing of electrons between atoms with different electronegativities. In the case of CCl4, the chlorine atoms are more electronegative than carbon, causing the electrons to be pulled slightly towards.
The chlorine atoms, creating partial negative charges on the chlorine atoms and a partial positive charge on the carbon atom. Despite the polar covalent bonds, the molecule as a whole is nonpolar because the chlorine atoms are arranged symmetrically around the central carbon atom, resulting in a tetrahedral molecular geometry with equal electron distribution. The dipole moments of the polar bonds cancel each other out, leading to a nonpolar molecule.
Learn more about chlorine atoms here
https://brainly.com/question/30143031
#SPJ11
2. If I have an unknown volume of gas held at a temperature of 115. K in a container with a pressure of 60.0 kPa with 1.50 mol of particles. If by increasing the temperature to 225. K, decreasing the pressure to 30.0 kPa, and allowing some gas to escape until 1.00 moles remain causes the volume of the gas to be 29.0 liters, how many liters of gas did I start with?
Answers
The initial volume of the gas was approximately 14.5 liters. The molar volume of a gas expresses the volume occupied by 1 mole of that respective gas under certain temperature and pressure conditions. This answer supports our expectation from Charles law.
To find the initial volume, we can use the Ideal Gas Law formula, which is PV = nRT. Here, P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant (8.314 J/mol·K), and T is the temperature in Kelvin. Step 1, Convert the given pressure from kPa to Pa by multiplying by 1000. So, 60.0 kPa * 1000 = 60000 Pa. Step 2, Plug the values into the Ideal Gas Law formula: 60000 Pa * V = 1.50 mol * 8.314 J/mol·K * 115 K. Step 3, Solve for V: V = 1.50 mol * 8.314 J/mol·K * 115 K / 60000 Pa. Step 4, Calculate the volume: V ≈ 14.5 liters.
So, you started with approximately 14.5 liters of gas. Finally, we can use the fact that the initial number of moles of particles was 1.50, and that only 1.00 moles remained after some gas escaped, to calculate the initial volume of the gas, so the correct number is 14.5 liters.
To know more about gas visit:
https://brainly.com/question/14812509
#SPJ11
How do I calculate the moles of calcium chloride with a mass of 1. 11 g
Answers
You need to know the molar mass of calcium chloride in order to calculate the moles of calcium chloride with a mass of 1.11 g. The total atomic masses of all the atoms in a single compound molecule make up the molar mass.
According to its chemical formula, calcium chloride is composed of two chlorine atoms (atomic mass = 35.45 g/mol) and one calcium atom (atomic mass = 40.08 g/mol). As a result, calcium chloride has the following molar mass:
CaCl2 has a molar mass of 110.98 g/mol (40.08 g/mol + 2 x 35.45 g/mol).
The number of moles of calcium chloride having a mass of 1.11 g may be determined using the molar mass of calcium chloride, which we already know. The following is the formula to get the number of moles:
Molar mass divided by mass equals a mole.Substituting the values we know, we get:
moles of CaCl2 = 1.11 g / 110.98 g/mol = 0.01 mol
learn more about moles of calcium chloride here:
https://brainly.com/question/12641371
#SPJ4
If 5.0 g of potassium chlorate (KClO3) is decomposed, what volume of oxygen gas is produced at STP?
Answers
Answer:
1.37dm³
Explanation:
To solve this problem, let us write the reaction expression:
2KClO₃ → 2KCl + 3O₂
Now, mass of KClO₃ is 5g, let us find the number of moles;
Number of moles = \(\frac{mass}{molar mass}\)
Molar mass of KClO₃ = 39 + 35.5 + 3(16) = 122.5g/mol
Now;
Number of moles = \(\frac{5}{122.5}\) = 0.04mole
So;
2 moles of KClO₃ will produce 3 moles of oxygen gas
0.04 mole of KClO₃ will produce \(\frac{3 x 0.04} {2}\) = 0.06moles
At STP;
1 mole of gas has a volume of 22.4dm³
0.06 mole of oxygen gas will have a volume of 22.4 x 0.06 = 1.37dm³
check the correct statements about carbon dioxide. phase diagram select one or more: a. at -50oc and 1 atm, carbon dioxide is in the gaseous state. b. at 1 atm carbon dioxide sublimes as it warms above the melting point. c. at -40oc and 8 atm, carbon dioxide is in the liquid state. d. as the temperature drops from -30oc to a minus -80oc at 10 atm, carbon dioxide freeze
Answers
Under increased pressure, the freezing point decreases, so carbon dioxide can potentially freeze at lower temperatures than its normal freezing point. Therefore, the correct statements are a. and b.
Based on the given statements, the correct statements about carbon dioxide (CO2) and its phase diagram are:
a. At -50°C and 1 atm, carbon dioxide is in the gaseous state.
b. At 1 atm, carbon dioxide sublimes as it warms above the melting point.
Explanation:
a. At -50°C and 1 atm, carbon dioxide exists as a gas. This is because the temperature is below the critical point of carbon dioxide (-56.6°C) but above its sublimation point (-78.5°C). Therefore, it remains in the gaseous state.
b. At 1 atm, carbon dioxide sublimes as it warms above the melting point. The normal melting point of carbon dioxide is -78.5°C, and at atmospheric pressure, it directly transitions from the solid state (dry ice) to the gaseous state without passing through the liquid state. This process is called sublimation.
The other statements are incorrect:
c. At -40°C and 8 atm, carbon dioxide is not in the liquid state. At this temperature and pressure, carbon dioxide is in the solid state (dry ice) or a supercritical fluid state, depending on the conditions.
d. As the temperature drops from -30°C to -80°C at 10 atm, carbon dioxide does not freeze. Carbon dioxide freezes at a lower temperature than -80°C at atmospheric pressure (1 atm). However, under increased pressure, the freezing point decreases, so carbon dioxide can potentially freeze at lower temperatures than its normal freezing point.
Therefore, the correct statements are a. and b.
Learn more about carbon dioxide:
https://brainly.com/question/31957044
#SPJ4
10 To draw a perfect circle, you'll need: A A protractor B. A sextant С A compass D A spirograph 10 of 10
Answers
Answer:
B
Explanation:
Two quick google searches later I grace you with the google has bestowed upon me
........,,,,,,?!!mm........
Answers
Answer:
añadir agua a la mezcla para disolver la sal. Después la arena se separa del agua salada por filtración. Finalmente, el agua salada se deja evaporar.
Explanation:
:u
Some cleansers may contain bromate salts as oxidizing agents. These salts will react with iodide ion under the conditions we are using according to the reaction
BrO3- + 6H+ + 6I- -> 3I2 + Br- + 3H2O
What percentage by weight of KBrO3 would a cleanser have to contain in order to produce an amount of iodine equivalent to that produced by an equal weight of cleanser containing 0.50% NaOCl by weight? (Hint: Start with the amount of NaOCl in a 100-g sample of cleanser and convert it to an equivalent mass of KBrO3).
Answers
The cleanser would need to contain approximately 1.52% KBrO3 by weight to produce an amount of iodine equivalent to that produced by an equal weight of cleanser containing 0.50% NaOCl by weight.
To solve this problem, we need to first calculate the amount of NaOCl in a 100-g sample of the cleanser, and then find the equivalent mass of KBrO3 that would produce the same amount of iodine.
Let's assume that the cleanser contains x% KBrO3 by weight. Then the mass of KBrO3 in a 100-g sample of the cleanser would be:
mass of KBrO3 = (x/100) * 100 g = x g
To find the amount of iodine produced by this amount of KBrO3, we need to convert the mass of KBrO3 to moles, and then use the stoichiometry of the reaction to calculate the moles of iodine produced:
mass of KBrO3 (g) → moles of KBrO3 → moles of I2 → mass of I2 (g)
x g KBrO3 → (x/MW(KBrO3)) mol KBrO3 → (3/1) * (x/MW(KBrO3)) mol I2 → (127/MW(I2)) * (3/1) * (x/MW(KBrO3)) g I2
where MW(KBrO3) and MW(I2) are the molecular weights of KBrO3 and I2, respectively.
Now we can set up the equation:
(127/MW(I2)) * (3/1) * (x/MW(KBrO3)) g I2 = (0.50/100) * 100 g NaOCl
Simplifying and solving for x, we get:
x = (0.50/100) * 100 * MW(KBrO3) * MW(I2) / (127 * 3)
x ≈ 1.52
Therefore, the cleanser would need to contain approximately 1.52% KBrO3 by weight to produce an amount of iodine equivalent to that produced by an equal weight of cleanser containing 0.50% NaOCl by weight.
To learn more about weight of cleanser here:
https://brainly.com/question/30113262
#SPJ11
This diagram shows a ball rolling from left to right along a frictionless surface. In which sections will the ball be subjected to a balanced force?
A. Sections 2 and 5
B. Sections 2 and 3
C. Sections 1 and 4
D. Sections 2, 3, and 5
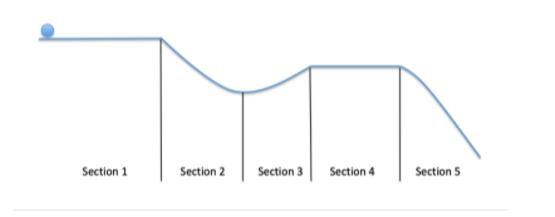
Answers
In sections 1 and 4 the ball is subjected to a balanced force. Hence, option C is correct.
What is the frictionless surface?A Frictionless surface refers to that kind of surface where the force acting on any object which makes it difficult for the object to slide is almost zero or negligible, i.e., no resistance between surface or substance allowing the object to slide and move freely without any friction.
In sections 1 and 4 the ball is subjected to a balanced force. Hence, option C is correct.
Learn more about frictionless surfaces here:
https://brainly.com/question/12911000
#SPJ2
Answer:1 and
Explanation:
Which product of the electron transport chain reenters the krebs cycle? a. atp b. coa c. nad d. nadh e. pyruvate
Answers
The product of the electron transport chain that reenters the krebs cycle is NAD. Details about electron transport chain can be found below.
What is cellular respiration?Cellular respiration is the process by which living organisms break down food molecules in their cells.
The process of cellular respiration involves the following stages:
GlycolysisKrebs cycleElectron transport chainThe electron transport chain is a series of reactions that involve the transfer of electrons from one molecule to another. NAD+ is one of the electron acceptor and products of the ETC that reenters the kreb cycle.
Learn more about electron transport chain at: https://brainly.com/question/24372542
#SPJ4
HNO3 ions in an aqueous solution. A forms or B. does not form
Answers
Answer:
A. forms, I used his answer and it was wrong so forms is the answer...
How many moles of sand (SiO2) are in 30 g of sand?
Answers
0.50 mol SiO₂
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Atomic Structure
Reading a Periodic TableUsing Dimensional AnalysisExplanation:Step 1: Define
30 g SiO₂ (sand)
Step 2: Identify Conversions
Molar Mass of Si - 28.09 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of SiO₂ - 28.09 + 2(16.00) = 60.09 g/mol
Step 3: Convert
Set up: \(\displaystyle 30 \ g \ SiO_2(\frac{1 \ mol \ SiO_2}{60.09 \ g \ SiO_2})\)Multiply/Divide: \(\displaystyle 0.499251 \ mol \ SiO_2\)Step 4: Check
Follow sig figs and round. We are given 2 sig figs.
0.499251 mol SiO₂ ≈ 0.50 mol SiO₂
Answer:
\(\boxed {\boxed {\sf About \ 0.5 \ moles \ of \ SiO_2}}\)
Explanation:
To convert from grams to moles, we must use the molar mass.
1. Molar Mass
Use the Periodic Table to find the masses of the individual elements (silicon and oxygen) in sand.
Silicon (Si): 28.085 g/mol Oxygen (O): 15.999 g/molExamine the formula for sand: SiO₂. There is a subscript of 2 after oxygen, so there must be 2 oxygen atoms. Multiply oxygen's mass by 2 and add silicon's mass to find the molar mass of sand.
SiO₂: 2(15.999 g/mol) + 28.085 g/mol= g/mol2. Calculate Moles
Use the molar mass as ratio.
\(\frac{60.083 \ g \ SiO_2}{1 \ mol \ SiO_2}\)
Multiply by the given number of grams (30)
\(30 \ g \ SiO_2 * \frac{60.083 \ g \ SiO_2}{1 \ mol \ SiO_2}\)
Flip the fraction so the grams of sand will cancel.
\(30 \ g \ SiO_2 *\frac{1 \ mol \ SiO_2}{60.083 \ g \ SiO_2}\)
\(30 *\frac{1 \ mol \ SiO_2}{60.083 }\)
\(\frac{30 \ mol \ SiO_2}{60.083 }\)
\(0.499309289 \ mol \ SiO_2\)
3. Round
The original measurement of grams has 1 signfiicant figure. We must round our answer to 1 sig fig.
For the answer we found, that is the tenth place. The 9 in the hundredth tells us to round the 4 to a 5.
\(\approx 0.5 \ mol \ SiO_2\)
There are about 0.5 moles of SiO₂ in 30 grams.
The part of the atom the determines what element it is is the
Answers
At 35.0°C and 3.00 atm pressure, a gas has a volume of 1.40 L. What pressure does the gas have at 0.00°C and a volume of 0.950 L? Which equation should you use? P subscript 2 equals StartFraction P subscript 1 V subscript 1 T subscript 2 over T subscript 1 V subscript 2 EndFraction. P subscript 2 equals StartFraction T subscript 1 V subscript 2 over P subscript 1 V subscript 1 T subscript 2 EndFraction. P subscript 2 StartFraction equals V subscript 1 V subscript 2 over T subscript 1 T subscript 2 EndFraction P subscript 1.
Answers
Answer:
a
3.92
Explanation:
Can someone please help me with this I need help asap

Answers
Answer:
???
Explanation: