IUPAC name for [Fe(NH3)4Cl2]NO3
Answers
Tetraamminedichloridoiron(3) nitrate
Related Questions
What are some differences between burning ethanol and burning hydrogen fuel?
Answers
Ethanol is proven to reduce combustion emissions. Hydrogen can produce more energy per pound of fuel.
What is combustion?A chemical reaction involving two or more chemicals, generally including oxygen, that produces light and heat in the form of a flame.
The pace which the reactants mix is fast, due in part to the structure of the chemical process and in part to the fact that more energy is created than can escape through into surrounding medium. Ethanol is proven to reduce combustion emissions. Hydrogen can produce more energy per pound of fuel.
Therefore, ethanol is proven to reduce combustion emissions. Hydrogen can produce more energy per pound of fuel.
To learn more about combustion, here:
https://brainly.com/question/14335621
#SPJ1
Help I’ll mark brainliest

Answers
Answer:
A and C
Explanation:
both stay the same in every experiment
(a) Explain why ammonia has a low boiling point
Answers
Explanation: Ammonia has pretty strong intermolecular forces because it can form hydrogen bonds, however it can't form as many hydrogen bonds per molecule as water and so its boiling point and melting point are lower.
As ammonia has less intermolecular force of attraction, it have usually low boiling point.
What is hydrogen bonding?Hydrogen bonding is not a covalent bond to a hydrogen atom, but rather a type of dipole-dipole attraction between molecules.
The attractive force between a hydrogen atom covalently bonded to a very electronegative atom such as an N, O, or F atom and another very electronegative atom causes it.
When a hydrogen atom bonded to an electronegative atom approaches another electronegative atom, strong intermolecular forces are created.
Simple molecules include hydrogen, ammonia, methane, and pure water. All of them have strong covalent bonds among both their atoms but much weaker intermolecular forces.
When one of these substances melts or boils, the weak intermolecular forces, not the strong covalent bonds, break.
Thus, ammonia usually have low boiling point.
For more details regarding hydrogen bonding, visit:
https://brainly.com/question/15099999
#SPJ2
ow many grams of dry nh4cl need to be added to 2.20 l of a 0.800 m solution of ammonia, nh3 , to prepare a buffer solution that has a ph of 8.56? kb for ammonia is 1.8×10−5 .
Answers
The amount of dry NH₄HCl needed to prepare a buffer solution that has a pH of 8.56 is 188.29 grams.
To prepare the buffer solution with a pH of 8.56, first, we need to calculate the concentration of NH₄⁺ ions using the Henderson-Hasselbalch equation:
pH = pKa + log([NH₄⁺]/[NH₃])
Since we have pH and [NH₃], we need to find pKa using Kb for ammonia:
Kb = 1.8 × 10⁻⁵
Kw = 1 × 10⁻¹⁴ (ion product of water)
Ka = Kw / Kb = 1 × 10⁻¹⁴ / 1.8 × 10⁻⁵ = 5.56 × 10⁻¹⁰
pKa = -log(Ka) = 9.26
Now, we can use the Henderson-Hasselbalch equation:
8.56 = 9.26 + log([NH₄⁺]/(0.800))
Rearranging to find [NH₄⁺]:
[NH₄⁺] = 0.800 * 10^(9.26 - 8.56) = 0.800 * 10^(0.70) = 1.6 M
Now, we can calculate the grams of NH₄Cl needed to achieve this concentration:
1.6 mol/L * 2.20 L = 3.52 mol of NH₄Cl
Finally, convert moles to grams using the molar mass of NH₄Cl (53.49 g/mol):
3.52 mol * 53.49 g/mol = 188.29 g
So, you need to add 188.29 grams of dry NH₄Cl to the 2.20 L of 0.800 M NH₃ solution to prepare the buffer with a pH of 8.56.
Learn more about Henderson-Hasselbalch equation here: https://brainly.com/question/26746644
#SPJ11
Based on their chemical structure, would phospholipids form a bilayer if they were in a hydrophobic sybstance like methane, rather than water? How would this affect membrane permeability?
Answers
Phospholipids are amphipathic molecules, meaning they have both hydrophobic and hydrophilic regions. In an aqueous environment like water, phospholipids arrange themselves into a bilayer structure, with the hydrophobic tails oriented inward and the hydrophilic heads facing outward towards the water.
In a hydrophobic substance like methane, phospholipids would not form a bilayer structure because there would be no polar environment for the hydrophilic heads to interact with. Instead, phospholipids would likely aggregate or create different structures to minimize their exposure to the hydrophobic environment.
Forming a bilayer structure is crucial for the function of cell membranes, as it provides a selectively permeable barrier. In a hydrophobic substance like methane, the absence of a bilayer structure would result in increased membrane permeability. The hydrophobic substance could penetrate and disrupt the lipid structure more efficiently, compromising the membrane's ability to regulate the passage of ions, molecules, and other substances.
Therefore, the absence of a polar environment like water would affect the formation of the phospholipid bilayer.
Learn more about amphipathic molecules here:
https://brainly.com/question/12070285
#SPJ 4
0.155 moles of c is reacted with 0.117 moles of o2 to form co and co2. $$ using the balanced chemical equation, calculate the moles of co2 which could be produced based upon the moles of the each reagent. based on the theoretical production of co2 which reagent, if either, is the limiting reagent for this problem?
Answers
The balanced chemical equation for the reaction between carbon (C) and oxygen (O2) to form carbon monoxide (CO) and carbon dioxide (CO2) is below and the moles of CO2 produced is 0.117.
C + O2 → CO + CO2
According to the equation, for every 1 mole of C, we need 1 mole of O2 to produce 1 mole of CO and 1 mole of CO2.
Given that we have 0.155 moles of C and 0.117 moles of O2, we can use the mole ratio from the balanced equation to determine how many moles of CO2 could be produced:
Moles of CO2 = Moles of C = 0.155 moles
Therefore, based on the moles of each reagent, we could produce a maximum of 0.155 moles of CO2.
To determine the limiting reagent, we need to calculate the amount of CO2 that would be produced if all of the limiting reagent were consumed. We can do this by comparing the amount of CO2 that would be produced by each reagent and identifying the one that produces the smaller amount:
Using C as the limiting reagent:
Moles of CO2 produced = Moles of C = 0.155 moles
Using O2 as the limiting reagent:
Moles of CO2 produced = Moles of O2 × (1 mole CO2 / 1 mole O2) = 0.117 moles × (1 mole CO2 / 1 mole O2) = 0.117 moles
Since the amount of CO2 produced by the reaction with C is greater than the amount produced by the reaction with O2, we can conclude that O2 is the limiting reagent for this problem. Therefore, only 0.117 moles of CO2 could be produced based on the available amount of O2, and any excess C would be left over after the reaction.
Learn more about Balanced chemical equations here:
brainly.com/question/28294176
#SPJ4
_____ energy has the total amount of
kinetic energy contained in all the
particles of a substance.
A: Thermal
B: Sound
C: Chemical
D: Electric
Answers
Answer: Thermal Energy
Explanation:
Which of the following is a propagation step in the free radical chlorination of methane?
∙CH3 + Cl2 → CH3Cl + Cl∙
∙CH3 + Cl∙ → CH3Cl
∙CH3 + ∙CH3 → CH3CH3
Cl2 → ∙Cl + ∙Cl
Answers
The propagation step in the free radical chlorination of methane is:
∙CH₃ + Cl∙ → CH₃Cl
In the free radical chlorination of methane, the propagation step is a crucial part of the overall reaction mechanism. It involves the interaction between a methyl radical (∙CH₃) and a chlorine radical (Cl∙), resulting in the formation of chloromethane (CH₃Cl).
During the propagation step, the methyl radical (∙CH₃) and chlorine radical (Cl∙) combine to produce chloromethane (CH₃Cl). This reaction occurs through the abstraction of a hydrogen atom from methane by the chlorine radical, forming a new C-Cl bond and generating a new methyl radical. The overall reaction can be represented as follows:
∙CH₃ + Cl∙ → CH₃Cl
Learn more about chlorine and methane from the link given below.
https://brainly.com/question/2409985
#SPJ4
what is diffusion ,??
Answers
Diffusion is the net movement of anything generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential.
The process by which a solid is converted to a gas is called _______
a) liquefaction.
b) vaporization.
c) sublimation.
d) condensation.
Answers
The process by which a solid is converted to a gas is called _______
Answer :c) sublimationExplanation.Liquefaction : It is the process by which the substance in the gaseous state is converted to the liquid state.
For example : Oxygen
Vaporization : It is the process by which the substance from the liquid or solid state is converted to the gaseous state.
For example: Wet clothes drying in the sun.
Condensation : It is the process in which the gaseous water is converted into liquid water. For example - Morning dew on the grass.
Answer:
c) sublimation.
Explanation:
The process by which a solid is converted to a gas is called sublimation. So the answer is (c).
Liquefaction is the process of converting a gas to a liquid. Vaporization is the process of converting a liquid to a gas. Condensation is the process of converting a gas to a liquid.Inorganic compounds which are found in the earth are called: minerals vitamins synthetics cement
Answers
Answer:
Minerals
Explanation:
I looked it up on google. Thanks.
The total number of calcium atoms in the expression 3 cos 2 shown in the equation 3 CaCl 2 +2Na 3 PO 4 Ca(PO 4 ) 2 +6 NaCl is:

Answers
Answer:
\(C\)Explanation:
Here, we want to calculate the percentage composition of the compound formed when oxygen reacts with iron
We have the equation of reaction as follows:
\(4Fe_{(s)}\text{ + }3O_{2(g)}\text{ }\rightarrow\text{ }2Fe_2O_{3(s)}\)The compound formed is Fe2O3
Now, let us get its percentage composition
The molar mass of the compound is 160 g/mol
The atomic mass of iron is 56 amu
The atomic mass of oxygen is 16 amu
Now, let us get the percentage composition:
\(\begin{gathered} \text{Iron = }\frac{2\times56}{160}\text{ }\times\text{ 100 \% = }70\text{ \%} \\ \\ \text{Oxygen = }\frac{3\times16}{160}\text{ = 30\% } \end{gathered}\)The closest here is thus the option C
is the valency of carbon 2 or 4
Answers
Answer:
4
explanation:Carbon has four valence electrons and here a valence of carbon is four.
Answer:
4
Explanation:
needs four electrons to complete its orbit
Al +CI = Al CI3
how do i solve this
Answers
Al + Cl2 —> AlCl3
If you are requesting for it to be balanced, it would be:
2 Al + 3 Cl2 —> 2 AlCl3
quick answer plz............An oxide was prepared by combining 15.3g of an element “X” with 13.6g of oxygen. What is the simplest formula for the oxide? (At. Mass X = 27, O = 16)
A. XO3
B. X2O3
C. X2O5
D. XO
Answers
Answered it coz of you
Any doubts just ping me
follow meh

Answer:
the answer is B

what is the minimum mass of caco3 required to establish equilibrium at a certain temperature in a 6.50-l container if the equilibrium constant (kc) is 0.50 for the decomposition reaction of caco3 at that temperature
Answers
the reaction of the equilibrium for caco3 was found to be: CaCO 3(s)⇌CaO(s)+CO 2(g); K c=0.005 mole/litre A 3.25 g
what is calcium carbonate (caco3) ?
CaCO3 is the chemical formula for calcium carbonate. It is found in rocks as the minerals calcite and aragonite (most notably as limestone, a form of sedimentary rock composed primarily of calcite) and is the primary component of eggshells, gastropod shells, shellfish skeletons, and pearls. Calcareous refers to things that contain or resemble calcium carbonate. The active element in agricultural lime is calcium carbonate, which is formed when calcium ions in hard water combine with carbonate ions to form limescale. It is used in medicine as a calcium supplement or as an antacid, but excessive ingestion can be harmful, causing hypercalcemia and digestive problems.
the reaction of the equilibrium for caco3 was found to be: CaCO 3(s)⇌CaO(s)+CO 2(g); K c=0.005 mole/litre A 3.25 g
To learn more about calcium carbonate follow the given link: https://brainly.com/question/12024964
#SPJ4
Reactant A illustrates an example of which of the following

Answers
Answer:
But TVs de dog was nru
Explanation:
What is a variable? A. Something that must be kept constant in an experiment. B. A value or characteristic that can take different values. C. A statement that explains a phenomena and can be tested. D. An educated guess used in an experiment.
Answers
Answer:
The answer is B, a value or characteristic that can take different values
Explanation:
ex.) 1s + 3
s is the variable
Variables are the varying or unchangeable data or factors in an experimental design. It can be defined as a characteristic that can have different values. Thus, option B is correct.
What is the meaning of variables?Variables are experimental characteristics that may be consistent or can be changeable. In an experimental design, the variables are used to analyze the cause and effect.
The variables of an experiment can be dependent, independent, or controlled. The varying factors in an experiment to analyze are called independent variables.
The factors that depend and respond to the effect of the independent variables are called dependent variables. These all values are contrasted to the control group with a fixed value.
Therefore, in option B, the variables are the characteristics with the same or different values.
Learn more about variables here:
https://brainly.com/question/13760390
#SPJ2
A certain five cent coin contains 5.00 g of nickel. What fraction of the nickel atoms' electrons, removed and placed 1.12 m above it, would support the weight of this coin
Answers
The given mass of nickel in the 5-cent coin is 5.00 g. To find out what fraction of the nickel atoms' electrons, removed and placed 1.12 m above it, would support the weight of this coin, we can use the concept of Coulomb's law and gravitational force.
Let's begin:
Mass of the coin = 5.00 g = 0.00500 kg
The mass of one nickel atom is 58.6934 u.
Therefore, the number of atoms present in the coin is:
Number of nickel atoms = 0.00500 kg / (58.6934 g/mol) x (1 mol / 6.022 x 10²³ atoms)
= 4.96 x 10²¹ atoms
Charge on an electron = 1.602 x 10⁻¹⁹ C
The force due to gravity acting on the coin is given by the formula:
Fg = m x g
where m is the mass of the coin and
g is the acceleration due to gravity = 9.8 m/s²
Fg = 0.00500 kg x 9.8 m/s²
= 4.90 x 10⁻² N
The force of attraction between an electron and a nickel atom can be calculated using Coulomb's law.
F = (k x q₁ x q₂) / r²
where k = 9 x 10⁹ Nm²/C² is the Coulomb constant
q₁ and q₂ are the charges on the electron and the nickel nucleus, respectively.
They can be calculated as follows:
q₁ = -1.602 x 10⁻¹⁹ C (since the electron is negatively charged)
q₂ = 28 x 1.602 x 10⁻¹⁹ C (since a nickel nucleus has 28 protons)
The distance between the electron and the nickel nucleus is given as r = 1.12 m.
Therefore, the force of attraction between them is:
F = (9 x 10⁹ Nm²/C²) x (-1.602 x 10⁻¹⁹ C) x (28 x 1.602 x 10⁻¹⁹ C) / (1.12 m)²
F = -5.13 x 10⁻¹¹ N
The negative sign indicates that the force is attractive.
To find out how many electrons are required to support the weight of the coin, we need to divide the weight of the coin by the force acting on each electron:
N = Fg / F
= (4.90 x 10⁻² N) / (5.13 x 10⁻¹¹ N)
N = 9.54 x 10⁹ electrons
The total number of electrons in the nickel atoms present in the coin is given by the formula:
Number of electrons = Number of atoms x Number of electrons per atom
Number of electrons per nickel atom = 28 (since a nickel atom has 28 electrons)
Therefore,
Number of electrons = 4.96 x 10²¹ x 28
= 1.39 x 10²³ electrons
The fraction of electrons that would support the weight of the coin is given by dividing N by the total number of electrons:
fraction = N / (Number of atoms x Number of electrons per atom)
fraction = (9.54 x 10⁹) / (4.96 x 10²¹ x 28)
fraction = 6.51 x 10⁻¹³
Therefore, the fraction of the nickel atoms' electrons, removed and placed 1.12 m above it, that would support the weight of this coin is 6.51 x 10⁻¹³.
To know more about Coulomb's law
https://brainly.com/question/506926
#SPJ11
what reaction type is the followong reaction
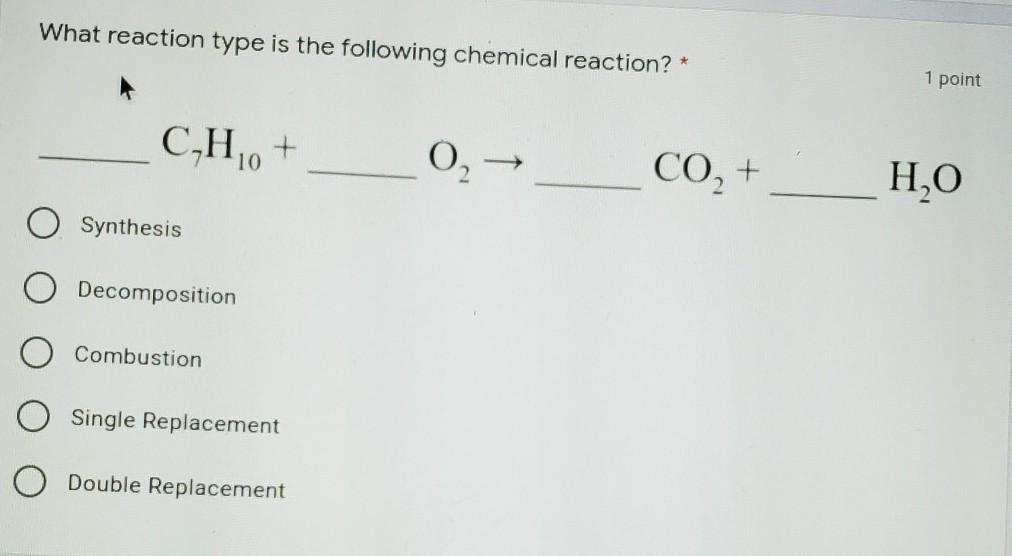
Answers
Answer:
its the fourth one please mark as brainlest
Explanation:
4. Diffrection Jtudies involving X-rays, electrons or Neutrons pire informetion about crystillographic properties of Solids. Compare these three techniques with reference to particle energies and types of information that ean be obtained. Which technique is most appropriate for studying surface Crystallography? Which technigue is used to determine mapnetic structure?
Answers
Neutron diffraction, on the other hand, is commonly used to determine magnetic structures in materials due to the interaction of neutrons with magnetic moments.
X-ray diffraction, electron diffraction, and neutron diffraction are commonly used techniques to study the crystallographic properties of solids.
Here's a comparison of these three techniques in terms of particle energies and types of information obtained:
X-ray Diffraction:Particle Energy:
X-rays have high energy in the electromagnetic spectrum.
Information Obtained:
X-ray diffraction provides information about the arrangement of atoms within a crystal lattice, crystal symmetry, interatomic distances, and crystal plane orientations.
It is widely used for structural determination of crystalline materials.
Surface Crystallography:
X-ray diffraction is suitable for studying the bulk crystal structure and is less appropriate for surface crystallography due to the low penetration depth of X-rays in materials.
Electron Diffraction:Particle Energy:
Electrons can have high energies (e.g., in electron microscopy) or low energies (e.g., in electron diffraction experiments).
Information Obtained:
Electron diffraction can provide detailed structural information about crystals, including crystal symmetry, lattice parameters, and atomic arrangement.
It is particularly useful for studying small crystals, nanostructures, and thin films.
Surface Crystallography:
Electron diffraction can be used to study surface crystallography, as electrons can penetrate the surface layers of materials.
Neutron Diffraction:Particle Energy:
Neutrons have intermediate energy levels.
Information Obtained:
Neutron diffraction provides information about atomic positions, magnetic moments, and magnetic structures of materials.
It is particularly sensitive to light elements and can be used to determine the positions of hydrogen atoms in crystal structures.
Surface Crystallography:
Neutron diffraction is less commonly used for surface crystallography due to the limited penetration depth of neutrons in materials.
For studying surface crystallography, electron diffraction is generally the most appropriate technique due to its ability to penetrate surface layers and provide detailed structural information at the nanoscale.
Neutron diffraction, on the other hand, is commonly used to determine magnetic structures in materials due to the interaction of neutrons with magnetic moments.
Learn more about Neutron diffraction from this link:
https://brainly.com/question/31324813
#SPJ11
Balance equation Co2(CO3)3(s)=Co2O3(s)+CoO2(g)
Answers
Answer:
answer
Explanation:
explination
Which of the following materials would probably be used as a conductor of electric current in a computer chip?
A rubber
B. glass
c. plastic
D. gold
Answers
Answer:
gold is metal which is a very good conductor for electric current and most chips are made out of it.
Answer:
The answer is D. gold
Explanation:
draw the structure of the organobromide that can be used to prepare the following gilman reagent.
Answers
A lithium and copper reagent complex, R2CuLi, where R is an alkyl or aryl, is referred to as a Gilman reagent.
The structure of organobromide or bromomethane is CH3Br. The structure is given above.
The production of carbon-carbon bonds in organic synthesis is accomplished using the Gilman reagent, also known as organocopper compounds. These substances are named after American scientist Henry Gilman, who first described them in the 1930s.
These chemicals are advantageous because they react with organic halides to exchange the halide group for a R group, unlike similar Grignard reagents and organolithium reagents.
Two steps can be used to manufacture the Gilman reagent: First, by mixing alkyl halide in pentane solvent with lithium metal powder. Secondly, by mixing alkyllithium in tetrahydrofuran at 78 °C with copper(I) bromide.
To know about reagent
https://brainly.com/question/28196410
#SPJ4

What is the mass of a bar of zinc measuring 2.5 cm by 4.0cm by 6.0cm if the density of zinc is 7.62cm
D=
M=
V=
Answers
The mass of the sample is the product of its volume and density. The mass of the zinc sample is 457.2 g.
What is density?
Density of a sample is its measure of mass per unit volume. It tells how much denser the substance is in a given volume. The volume of the substance is the space occupied by it.
Volume of a solid is the product of its length, breadth and height.
Given the dimensions of Zn sample is 2.5, 4 and 6 cm.
volume = 2.5 × 4 × 6 cm
= 60 cm³
Density = 7.62 g/cm³.
Mass of the sample is the product of its volume and density. Thus, mass of zinc sample is:
mass of Zn = volume × density
= 7.62 g/cm³ × 60 cm³
= 457.2 g.
Therefore, the mass of the Zn sample is 457.2 g.
To find more on density, refer here:
https://brainly.com/question/15164682
#SPJ1
1. Based on the Data Table, what mass of magnesium is contained in your compound? Show all calculations.
31.634 – 31.064 = 0.57
2. Based on the Data Table, what mass of oxygen is contained in your compound? Show all calculations.
3. Compare the mass of the Mg ribbon with the mass of the magnesium oxide. How can you account for the change in mass between the two?
4. Now that you have the mass of magnesium and oxygen in your compound, you can find moles of each element in the compound and you can determine your experimental empirical formula. Show all calculations and your empirical formula below.
5. What is the known formula for magnesium oxide? Compare the known formula to the empirical formula you determined in question 4. Are they the same or no?

Answers
Answer:
Answer 1: The mass of magnesium contained in the compound is 0.57g, which can be determined by subtracting the mass of the crucible and lid (31.064g) from the mass of the crucible, lid, and magnesium ribbon (31.634g).
Answer 2: The mass of oxygen contained in the compound is 1.39g, which can be determined by subtracting the mass of the crucible, lid, and magnesium oxide (31.970g) from the mass of the crucible and lid (31.064g).
Answer 3: The change in mass between the two can be accounted for by the reaction of the magnesium with oxygen to form magnesium oxide.
Answer 4: The number of moles of magnesium is 0.0995 (2.39/24) and the number of moles of oxygen is 0.0868 (1.39/16). Dividing the moles of each element by the smallest amount of moles (0.0868) results in a simplest ratio of 1:1. Therefore, the empirical formula of magnesium oxide is MgO.
Answer 5: The known formula for magnesium oxide is MgO, which is the same as the empirical formula determined in question 4.
if ∆h = 498 kj and ∆s = 319 j/k, the spontaneity of the reaction depends on temperature. above what temperature will the reaction be spontaneous?
Answers
The temperature at which a reaction becomes spontaneous depends on the change in enthalpy and entropy, which can be calculated using the equation ΔG = ΔH - TΔS. In this case, a temperature above 1564 Kelvin is required for the reaction to be spontaneous.
The spontaneity of a reaction is determined by the change in free energy, which is calculated using the equation ΔG = ΔH - TΔS, where ΔG is the change in free energy, ΔH is the change in enthalpy, T is the temperature in Kelvin, and ΔS is the change in entropy.
If ΔG is negative, the reaction is spontaneous. Therefore, we need to find the temperature at which ΔG becomes negative.
Given ΔH = 498 kJ and ΔS = 319 J/K, we can plug these values into the equation and solve for T:
ΔG = ΔH - TΔS
-ΔG = TΔS - ΔH
T = (ΔH/ΔS)
T = (498 kJ / 319 J/K)
T = 1564 K
Therefore, the reaction will be spontaneous above a temperature of 1564 Kelvin.
In summary, the temperature at which a reaction becomes spontaneous depends on the change in enthalpy and entropy, which can be calculated using the equation ΔG = ΔH - TΔS. In this case, a temperature above 1564 Kelvin is required for the reaction to be spontaneous.
To know more about spontaneous , refer
https://brainly.com/question/4248860
#SPJ11
I just need the answers for a test

Answers
Answer:
This chemical equation represents Photosynthesis. 6CO2 means there are 6 molecules of carbon dioxide. 6H20 means there are 6 water molecules. Then when sunlight is added, Photosynthesis occurs giving you the products of Glucose (6CH12O6) and Oxygen (6O2).
Explanation:
PLEASE HELP
Which chemical reaction supports the Law of Conservation of Mass?

Answers
Answer:A
Explanation:
What type of compound is formed by the transfer of electrons from one atom to another?
1) Hydrogen Compound
2) Coordinate Covalent Compound
3) Ionic Compound
4) Covalent Compound
5) None of the above
Answers
Answer:
ionic compound
Explanation:
ionic bonding
In ionic bonding, electrons are completely transferred from one atom to another. In ionic bonding, electrons are completely transferred from one atom to another. In the process of either losing or gaining negatively charged electrons, the reacting atoms form ions.
pls vote brainliest it would rlly help me out ❤❤❤