Isotopes (such as hydrogen-1, hydrogen-2, and hydrogen-3) are atoms of the same
element that differ in:
a. The number of neutrons in the nucleus
b. The mass number
c. The atomic number
d. The number of protons in the nucleus
e. The number of electrons
Answers
Answer:
a. The number of neutrons in the nucleus
Explanation:
Having more protons or electrons would change the charge of the atom making it an ion. Neutrons have no charge so the overall charge of the atom is not affected; neutrons also have a relative mass of 1 which is why there are different isotopes.
Hope this helps!
Related Questions
to what fraction of its original volume, vfinal/vinitial, must a 0.40−mole sample of ideal gas be compressed at constant temperature for δssys to be −4.3 j/k?
Answers
The problem involves calculating the change in entropy (δS) of an ideal gas during a constant temperature compression process. We can use the formula δSsys = -nRln(Vfinal/Vinitial) to solve for the ratio of final volume to initial volume (Vfinal/Vinitial). Then, the gas needs to be compressed to 0.0000174 times its original volume to achieve a δSsys of -4.3 J/K.
To determine the fraction of its original volume that a 0.40-mole sample of ideal gas must be compressed at constant temperature for δssys to be -4.3 J/K, we can use the formula: δssys = -nR ln(vfinal/vinitial)
where n is the number of moles of gas, R is the gas constant, and vfinal/vinitial is the ratio of final volume to initial volume.
Rearranging this formula, we get:
vfinal/vinitial = e^(-δssys/nR)
Plugging in the given values, we have:
vfinal/vinitial = e^(-(-4.3)/(0.40 mol x 8.31 J/(mol K)))
vfinal/vinitial = e^(13.5)
vfinal/vinitial = 57387.4
Therefore, the 0.40-mole sample of ideal gas must be compressed to 1/57387.4 or about 0.0000174 times its original volume to achieve a δssys of -4.3 J/K at constant temperature.
To learn more about entropy; https://brainly.com/question/15022152
#SPJ11
question content area what are its electron-pair and molecular geometries? what is the hybridization of the nitrogen atom? what orbitals on and overlap to form bonds between these elements? electron-pair geometry
Answers
Molecular geometry refers to how atoms are arranged within a molecule, typically in relation to a single center atom. Because the nitrogen is sp3 hybridized, it possesses four sp3 hybrid orbitals.
What are the definitions of the electron pair and molecule geometries?By taking into account both lone pairs and bond pairs, electron pair geometry can forecast the form of a molecule. By merely taking into account bond pairs, molecular geometry may predict the form of a molecule.
N2 sp2 hybridization is it possible?The nitrogen atom likewise hybridizes in the sp2 configuration, but unlike the carbon atom, it has a "lone pair" of remaining electrons that are not involved in the bonding. As a result, the geometry of nitrogen with three bonded ligands is trigonal pyramidal.
To know more about N2 sp2 hybridization visit:-
https://brainly.com/question/29607102
#SPJ4
How many representative particles are 9.01 moles of water? (Make sure to write you answer in scientific notation.)
PLEASE HELP!!!!
Answers
Answer:
54.26 × 10²³ representative particles
Explanation:
Given data:
Number of moles of water = 9.01 mol
Number of representative particle = ?
Solution:
one mole of any substance contain 6.022 ×10²³ representative particles.
9.01 mol × 6.022 ×10²³ representative particles
54.26 ×10²³ representative particles
The number 6.022 × 10²³ is called Avogadro number.
"It is the number of atoms , ions , molecules or representative particles in one gram atom of element, one gram molecules of compound and one gram ions of a substance "
For a hypothetical reaction of A --> B occurring in the cell, the ΔG is +3 kJ/mol and the ΔGo' is -2 kJ/mol for a reaction occurring at 25oC.
What is the ratio of [A]/[B] found in the cell?
Possible answers are:
0.13
2.01
5
7.5
Answers
The ratio of [A]/[B] found in the cell is 2.01. Option B is correct.
Given that the ΔG for a hypothetical reaction of A = B occurring in the cell is +3 kJ/mol and the ΔGo' is -2 kJ/mol for a reaction occurring at 25oC.
We are to find the ratio of [A]/[B] found in the cell.
To calculate the ratio of [A]/[B] found in the cell, we will make use of the Gibbs free energy equation that is given as follows:
ΔG = ΔGo' + RT ln([B]/[A])
whereΔG = Gibbs free energy of the reaction
ΔGo' = Standard Gibbs free energy of the reaction
R = Ideal gas constant = 8.314 J/mol
K = 0.008314 kJ/mol K
T = temperature in Kelvin
= 298 K [A] and [B] are the concentrations of the reactants A and product B, respectively.
The ratio of [A]/[B] can be obtained by rearranging the Gibbs free energy equation as follows:
ln([B]/[A]) = (ΔG - ΔGo') / RT[B]/[A]
= e^[ΔG - ΔGo') / RT]
Substitute the given values into the above equation as follows:
[B]/[A] = e⁵ / (0.008314 × 298)] = 2.01
Therefore, Option B is correct.
Learn more about reactants -
brainly.com/question/26283409
#SPJ11
If the mixture in question 1 is in a 3.0 Liter container at 34 °C, what mass (in grams)of oxygen is present?If oxygen was 0.472 atm
Answers
Answer
The mass (in grams) of oxygen present = 1.79 grams.
Explanation
Given
Volume, V = 3.0 L
Temperature, T = 34 °C = (34 + 273.15 K) = 307.15 K
Pressure, P = 0.472 atm
What to find:
The mass (in grams) of oxygen present.
Step-by-step solution:
Step 1: Calculate the moles of oxygen present.
Using the ideal gas law:
\(PV=nRT\)R is the molar gas constant = 0.0820574 L•atm/mol•K.
\(\begin{gathered} 0.472\text{ }atm\times3.0\text{ }L=n(0.0820574\text{ }L•atm/mol•K\times307.15\text{ }K) \\ \\ n=\frac{0.472\text{ }atm\times3.0\text{ }L}{0.0820574\text{ }L•atm/mol•K\times307.15\text{ }K} \\ \\ n=0.056\text{ }mol \end{gathered}\)The moles of oxygen present is 0.056 mol.
Step 2: Convert 0.056 mol oxygen to mass in grams.
The molar mass of oxygen gas = 31.998 g/mol
Using the mole formula below, the mass of oxygen can be calculated as follows:
\(\begin{gathered} Moles=\frac{Mass}{Molar\text{ }mass} \\ \\ \Rightarrow Mass=Moles\times Molar\text{ }mass \\ \\ Mass=0.056\text{ }mol\times31.998\text{ }g\text{/}mol \\ \\ Mass=1.791888\text{ }g\approx1.79\text{ }grams \end{gathered}\)The mass (in grams) of oxygen present = 1.79 grams.
Helpppp I will mark brainliest!!!

Answers
Answer:
Position
Explanation:
TEACH ME how to do question 2 through your explanations.
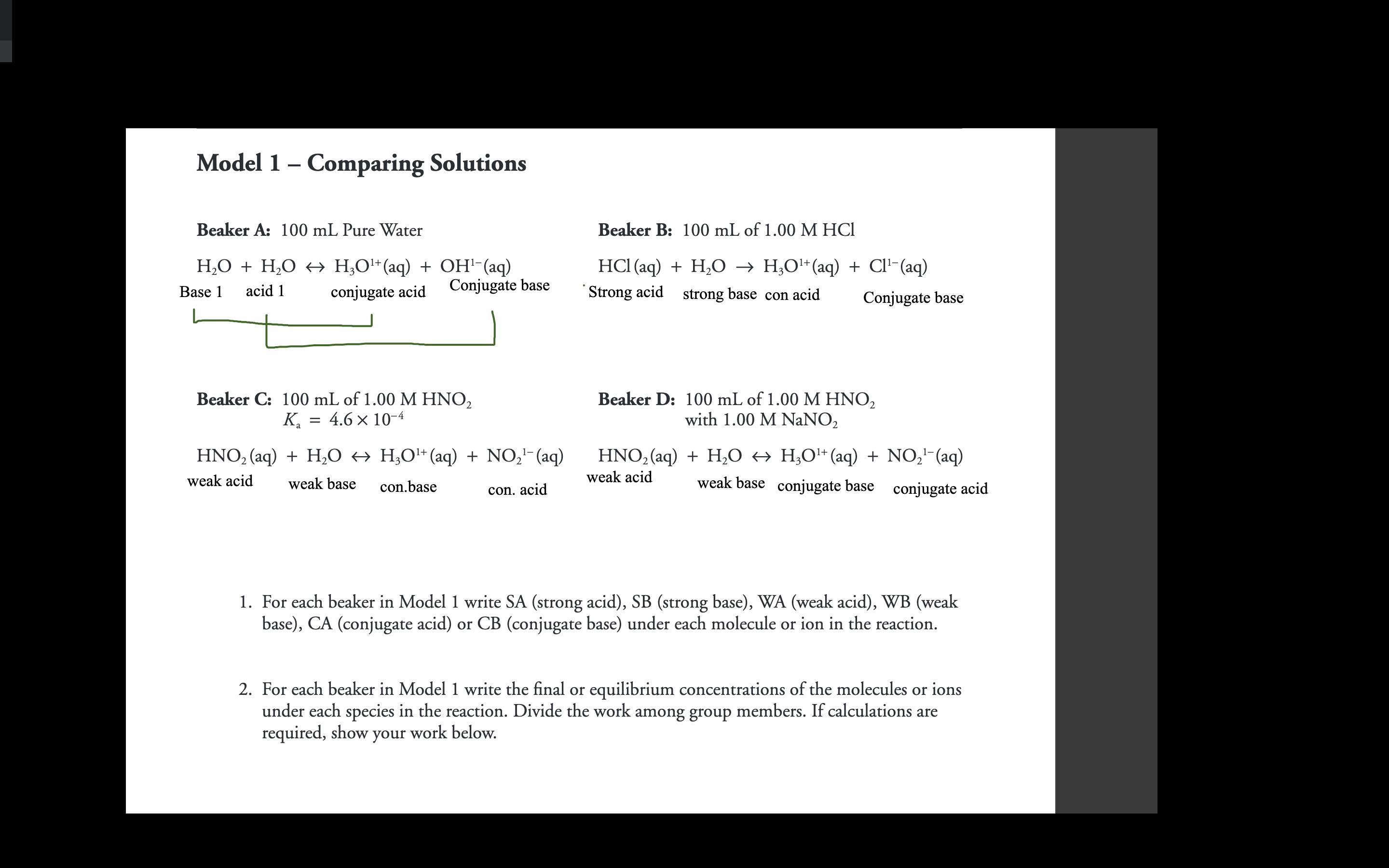
Answers
Answer:
Explanation:
The first two are easy:
H2O, H3O+ and OH- all exist in an equilibrium
[H3O+] = [OH-] = 10^-7 M
HCl is a strong acid so 1M of it will disassociate completely
[H3O+] = [Cl-] = 1 M
The third one is also an equilibrium as HNO2 is a weak acid. Ka is given so [HNO2] and [NO2-] can be calculated.
The fourth one is a buffer solution so its pH can be looked up to give the concentrations.
Answer:
Explanation:
H20 has pH of 7
1st reaction [H30+] = 10^-7M
[OH-] = 10^-7M
1M HCl has pH of 0
2nd reaction [H3O+] = 1M
[Cl-] = 1M
given Ka = 4.6x10^-3 = [H3O+]x[NO2-] / [HNO2]
and [HNO2] + [NO2-] = 1M
each concentration can be solved
beaker D is a buffer solution n requires more info 2 solve
Liquids with many free hydroxide ions (OH-) are called _________.
Answers
Answer:
hypothesis testing center of the year with 32 days
What type of reaction occurs between acetic acid and sodium bicarbonate in the Titration Lab?
Answers
Answer:
Explanation:
Titration is
when in a lab someone uses
a small pipe called a
pipette
and
drop by drop
they add chemical B (titrant) to chemical A
until something happens
its done drop by drop to find out what is the exact amount needed to cause a reaction
sciencedirectcom
Answer:
Sodium bicarbonate and acetic acid reacts to carbon dioxide, water and sodium acetate.
Explanation:
Sodium bicarbonate and acetic acid reacts to carbon dioxide, water and sodium acetate. The solid baking soda was placed in liquid vinegar producing carbon dioxide gas, which is evident because of the formation of bubbles in the foaming mixture.
Help!!! 1) True or False - The small flag indicating wind direction on a station weather plot points in the direction the wind is going. 2) How much of the sky is covered in clouds if the weather station is completely filled in?
Answers
The statement is False
If the weather station is completely filled in, the sky is considered to have 8/8 cloud cover or 80/100 cloud cover, indicating that the entire sky is covered in clouds.
The small flag indicating wind direction on a station weather plot points in the direction from which the wind is coming. This convention is followed to ensure consistency and to avoid confusion. By pointing in the direction from which the wind is coming, it allows observers to easily understand the wind's direction relative to the station's location.
The amount of the sky covered in clouds can be determined by using a system called the cloud cover code, which is represented in eighths or octas. Each octa represents one-eighth of the sky. So, if the weather station is completely filled in, it means the sky is completely covered in clouds, which corresponds to eight octas or 8/8 cloud cover.
Cloud cover is often reported in terms of tenths as well. In this case, each tenth represents 1/10th of the sky. To convert from octas to tenths, we multiply the octas by 10. Therefore, if the sky is completely filled in with clouds (8 octas), it would be equivalent to 8/8 or 80/100 cloud cover, which is 80% of the sky covered in clouds.
for more question on weather
https://brainly.com/question/29709289
#SPJ8
calculate the total volume of gas (at 127 ∘c ∘ c and 747 mmhg m m h g ) produced by the complete decomposition of 1.44 kg k g of ammonium nitrate.
Answers
The total volume of gas produced by the complete decomposition of 1.44 kg k g of ammonium nitrate is 33.5 L.
The decomposition reaction of ammonium nitrate is given by:
NH4NO3(s) → N2(g) + 2H2O(g)
From the balanced chemical equation, we can see that 1 mole of ammonium nitrate produces 1 mole of nitrogen gas and 2 moles of water vapor. The molar mass of NH4NO3 is 80.04 g/mol, so 1.44 kg of NH4NO3 is equal to 18 moles.
To find the volume of gas produced, we can use the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin.
First, we need to convert the temperature from Celsius to Kelvin:
T = 127°C + 273.15 = 400.15 K
Next, we need to convert the pressure from mmHg to atm:
747 mmHg / 760 mmHg/atm = 0.981 atm
Now we can plug in the values and solve for V:
V = nRT/P = (1 mole N2)(0.08206 L·atm/mol·K)(400.15 K)/0.981 atm
= 33.5 L
Therefore, the total volume of gas produced by the complete decomposition of 1.44 kg of ammonium nitrate at 127°C and 747 mmHg is 33.5 L.
For more questions on ammonium nitrate:
https://brainly.com/question/13678113
#SPJ11
The total volume of gas produced by the complete decomposition of 1.44 kg of ammonium nitrate at 127°C and 747 mmHg is 960.4 L.
Explanation: To solve this problem, we need to use the ideal gas law, PV=nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin. We can first find the number of moles of gas produced by calculating the amount of ammonium nitrate in moles (1.44 kg divided by the molar mass of NH4NO3), then multiplying by the stoichiometric ratio of gas produced per mole of ammonium nitrate (2 moles of gas per mole of NH4NO3).
Next, we can use the given temperature and pressure to convert the number of moles of gas into volume using the ideal gas law. It's important to note that the given temperature is in Celsius, so we need to convert it to Kelvin by adding 273.15. After plugging in the values and solving for V, we get a total volume of 960.4 L.
Learn more about ammonium nitrate here :
brainly.com/question/13678113
#SPJ11
mass of 2 into 10 to power 21 number of atoms of an element is 0.4 gram what is the mass of 0.5 mole of the elements
Answers
The mass of 0.5 mole of the element is approximately 6.025 grams.
To calculate the mass of 0.5 mole of the element, we need to know the molar mass of the element.
Given that the mass of 2 x 10^21 atoms of the element is 0.4 grams, we can use this information to find the molar mass.
The number of atoms in 1 mole of any substance is given by Avogadro's number, which is approximately 6.022 x 10^23 atoms/mol.
First, we calculate the molar mass of the element using the given information:
Molar mass = Mass of 2 x 10^21 atoms / Number of moles of 2 x 10^21 atoms
Molar mass = 0.4 g / (2 x 10^21 atoms / (6.022 x 10^23 atoms/mol))
Molar mass ≈ 0.4 g / (3.32 x 10^-2 mol)
Molar mass ≈ 12.05 g/mol
Now that we know the molar mass of the element is approximately 12.05 g/mol, we can calculate the mass of 0.5 mole of the element:
Mass = Molar mass x Number of moles
Mass = 12.05 g/mol x 0.5 mol
Mass = 6.025 grams
for more such questions on element
https://brainly.com/question/28376204
#SPJ8
A sample of gas has a volume of 215 cm3 at 23.5 °C and 3 atm. What will the volume of the gas be at STP
Answers
Answer:
165.3 cm^3
Explanation: hope this is correct!!
P1 * V1 / T1 = P2 * V2 / T2
P1 = 84.6 kPa
V1 = 215 cm³
T1 = 23.5°C = 23.5 + 273 K = 296.5 K
At STP:
P2 = 101.3 kPa
V2 = ?
T2 = 273 K
Except for ________ and ________, the occurrences of trace mineral deficiencies and toxicities are rare. a. iodine; selenium b. iodine; iron c. copper; chromium d. iron; copper
Answers
Except for iron and copper, the occurrences of trace mineral deficiencies and toxicities are rare. Trace minerals are required by the body in small quantities for various physiological functions. Iron is essential for the formation of hemoglobin in red blood cells, while copper is required for the formation of various enzymes that play a role in energy metabolism, connective tissue formation, and neurotransmitter synthesis. The answer to the question is option D,
Deficiencies in these trace minerals can lead to anemia, fatigue, weakness, and impaired immune function. Toxicity, on the other hand, can occur when these minerals are consumed in excess amounts. Excessive iron intake can lead to liver damage, joint pain, and diabetes, while copper toxicity can cause gastrointestinal distress, liver damage, and neurological symptoms.
However, deficiencies and toxicities of other trace minerals such as iodine, selenium, copper, and chromium are relatively rare. Iodine deficiency can lead to hypothyroidism, goiter, and mental disorder, while selenium deficiency can cause muscle weakness, cardiomyopathy, and thyroid dysfunction. Copper deficiency can cause anemia, neutropenia, and bone abnormalities, while chromium deficiency can lead to impaired glucose metabolism and increased risk of diabetes.
In conclusion, while deficiencies and toxicities of trace minerals can occur, it is important to ensure adequate intake of all trace minerals through a balanced diet or supplements to prevent these conditions. It is also essential to avoid excessive intake of trace minerals to prevent toxicity. Option D.
For more such questions on hemoglobin
https://brainly.com/question/4577862
#SPJ11
Part D Questions and Problems
Answer the following questions in the space provided.
18. Classify each substance as an element or a compound.
a. water a.
b. oxygen b.
c. table salt c.
d. sucrose d.
e. gold e.

Answers
Answer:
There are 120 possible ways.To calculate the number of permutations here, where order is important and repetition is not allowed, we use the following formula:Number of permutations = n! / (n - r)! = 5!/0! = 12345 / 1 (note: "!" means "factorial" and 0! equals 1) = 120/1 = 120.For a complete list see below. Let a,b,c,d & e represent the 5 players and their order determine their position:{a,b,c,d,e}
Explanation:
During an action potential, Na
+
ions move into the cell at a rate of about 5×10
−7
mol/m
2
⋅s. - Part A How much power must be produced by the "active Na
+
pumping" system to produce this flow against a +25mV potential difference? Assume that the axon is 10 cm long and 20μm in diameter. Express your answer using one significant figure.
Answers
The power required by the "active Na⁺ pumping" system to produce this flow against the +25 mV potential difference is approximately 4 × 10⁻¹⁷ W.
To calculate the power required by the "active Na⁺ pumping" system, we need to consider the current (rate of ion movement) and the potential difference across the axon. Power is given by the equation:
Power = Current × Voltage
Given:
Current (I) = 5 × 10⁻⁷ mol/(m²·s)
Voltage (V) = +25 mV = +25 × 10⁻³ V (since 1 mV = 10⁻³ V)
To determine the power, we need to convert the current to amperes (A) and multiply it by the voltage:
I (in A) = Current × elementary charge (e)
e = 1.6 × 10⁻¹⁹ C (charge of an electron)
Now we can calculate the power:
Power = I × V
First, let's convert the current from mol/(m²·s) to A/m²:
I (in A/m²) = Current (in mol/(m²·s)) × Avogadro's number (Nₐ) / time (s)
Nₐ = 6.022 × 10²³ mol⁻¹ (Avogadro's number)
Now, we can calculate the power:
Power = I (in A/m²) × V (in V)
Note: We assume the axon is a cylinder with a circular cross-section.
Given:
Length of axon (L) = 10 cm = 0.1 m
Diameter of axon (d) = 20 μm = 20 × 10⁻⁶ m
To calculate the cross-sectional area (A) of the axon, we use the formula for the area of a circle:
A = π × (d/2)²
Now, we can calculate the power:
Power = I (in A/m²) × V (in V) × A (in m²)
Substituting the given values:
A = π × (20 × 10⁻⁶ / 2)² = π × 100 × 10⁻¹² m²
Power = (5 × 10⁻⁷ A/m²) × (25 × 10⁻³ V) × (π × 100 × 10⁻¹² m²)
Simplifying the expression:
Power ≈ 4 × 10⁻¹⁷ W
Rounding to one significant figure, the power required by the "active Na⁺ pumping" system to produce this flow against the +25 mV potential difference is approximately 4 × 10⁻¹⁷ W.
To know more about potential difference , visit:
https://brainly.com/question/23716417
#SPJ11
2) A half-cell containing Br and Br- is connected to a half-cell containing Mg+2 and Mg to form a voltaic cell. Determine the proper direction for each half cell reaction to assure a spontaneous, complete cell reaction. Find the value of the complete cell electrode potential (E°).
Answers
A half cell is one of the two electrodes in a galvanic cell or simple battery.
Find the value of the complete cell electrode potential (E°)?
Look up the standard reduction potentials for Br and Mg. I find the following:
Mg2+ + 2e- ==> Mg(s) Eº = -2.38 V
Br2(l) + 2e- ==> 2Br-(aq) Eº = 1.07 V
Accordingly, Br2 will be reduced and Mg(s) will be oxidized in order to have a positive potential.
Br2 + 2e- ==> 2Br-
Mg ==> Mg2+ + 2e-
------------------------------------
Br2 + Mg ==> Mg2+ + 2Br- Eº = 1.07 + 2.38 = 3.45 V
To learn more about half cell refers to:
https://brainly.com/question/24002834
#SPJ1
Which of the following extremophiles has evolved in conditions
of extreme drought or extreme salt, respectively?
Group of answer choices
halophile; xerophile
xerophile; thermophile
xerophile; psychrop
Answers
The extremophile that has evolved in conditions of extreme drought is the xerophile, while the extremophile that has evolved in conditions of extreme salt is the halophile.
Extremophiles are organisms that thrive in extreme environments, where most other life forms cannot survive. They have developed unique adaptations to withstand and thrive in these extreme conditions. Two types of extremophiles specifically adapted to different extreme environments are xerophiles and halophiles.
Xerophiles are extremophiles that have evolved to survive in conditions of extreme drought. They are adapted to environments with very low water availability or high water stress. These organisms have developed mechanisms to prevent water loss, such as efficient water retention and protection of cellular structures. Xerophiles can be found in desert environments and other arid regions.
On the other hand, halophiles are extremophiles that have evolved to live in conditions of extreme salt concentration. They are adapted to environments with high salinity, such as salt flats, salt lakes, and hypersaline environments. Halophiles have specialized adaptations to cope with the osmotic stress caused by high salt concentrations. They have enzymes and transport proteins that function in high-salt environments and can maintain osmotic balance within their cells.
In summary, xerophiles have evolved in conditions of extreme drought, while halophiles have evolved in conditions of extreme salt. These extremophiles showcase remarkable adaptations to thrive in their respective harsh environments.
To learn more about Extremophiles click here: brainly.com/question/30627815
#SPJ11
) A compound has a molar mass of 180.15 g/mol. Given the following percent composition, calculate the molecular formula: A) CH3O2 B) CH2O C) C3H6O3 D) C6H12O6 E) none of the above Answer: D
Answers
Given percent composition: 40.00% C, 6.71% H and 53.29% O.Molar mass of compound = 180.15 g/mol.
To find the molecular formula of a compound, we need to find its empirical formula first.Empirical formula shows the simplest whole-number ratio of atoms present in a compound. It can be calculated using the percent composition of a compound as follows:Convert the percent composition of each element into its mass composition (mass percent).For this, assume a 100 g sample of the compound, so the percentages represent the masses of each element in the sample.Convert the mass of each element into its moles by dividing the mass by the molar mass of the element.
Divide each of the mole values by the smallest of them to get the simplest whole-number ratio of moles.Write the empirical formula of the compound using the mole ratios of the elements.Now that we have the empirical formula, we can find the molecular formula of the compound using its molar mass.We can do this by comparing the molar mass of the empirical formula to the given molar mass of the compound. If the two masses are different, then we need to multiply the empirical formula by an integer to get the molecular formula whose molar mass is equal to the given molar mass.
learn more about empirical formula
https://brainly.com/question/1603500
#SPJ11
a 30.5-g sample of ca contains how many calcium atoms?
Answers
Answer:
Explanation:
To find the number of calcium atoms in a 30.5-g sample, we need to use the molar mass of calcium and Avogadro's number.
The molar mass of calcium (Ca) is approximately 40.08 g/mol.
First, let's find the number of moles of calcium in the 30.5-g sample:
moles = (mass of the sample) / (molar mass of Ca)
moles = 30.5 g / 40.08 g/mol ≈ 0.7607 moles
Now, we will use Avogadro's number (6.022 x 10^23 atoms/mol) to find the number of calcium atoms in the sample:
number of atoms = (number of moles) × (Avogadro's number)
number of atoms = 0.7607 moles × 6.022 x 10^23 atoms/mol ≈ 4.58 x 10^23 atoms
So, the 30.5-g sample of calcium contains approximately 4.58 x 10^23 calcium atoms.
Why do you think there’s so much controversy around vaccines?
Answers
How many atoms are there in molecule of glucose (CH12O6)
Answers
Answer:
One molecule of glucose has 6 atoms, as you can see in carbon's index. ( C6 ). In 20 molecules there will be 6⋅20 carbon atoms.
Explanation:
what are the basic elements of environment
Answers
Answer:
the basic elements of environment are lithosphere, hydrosphere, atmosphere and biosphere.
Explanation:
C219
COMPLETE
-241.82
What is the enthalpy of reaction for the
decomposition of NO2(g)?
H2O(g)
NO2(g)
33.84
2NO2(g) → N2(g) + 2O2(g)
11 kJ
This reaction is
Answers
Answer:
a
Explanation:
CHEMISTRY 50 POINTS!
Which of the following explains the VSEPR geometry of a water molecule?
A) It is tetrahedral because there are four bonded pairs around oxygen.
B) It is bent because there are four bonded pairs around oxygen.
C) It is tetrahedral because there are two bonded pairs and two lone pairs around oxygen.
D) It is bent because there are two bonded pairs and two lone pairs around oxygen.
Answers
Answer: D
Explanation:
Water is comprised of 2 Hydrogen atoms and 1 Oxygen atom. This gives us a total of 8 valence electrons to form bonds with.
Because Hydrogen is in first row of elements it can only hold a total of two electrons. This means each Hydrogen holds 2 electrons.
But that means there are four more electrons. VSEPR theory is basically how electron repulsion causes atoms to arrange in different ways.
There will be four electrons left on the oxygen because they can not go anywhere else. This will cause the hydrogen molecules to move away from the lone electrons and cause a bent geometry rather than a straight-line geometry.
Metal
Nickel
Silver
Lead
Mercury
Density (g/cm)
8.9
10.5
11.35
13.55
Mr. Keans's class is doing a lab to determine the identity of an unknown metal. Use the table to help determine
the identity of a metal that has a mass of 89 grams and occupies 10 cm of space.
O Nickel
O Silver
O Lead
O Mercury
Answers
Answer:
\(\boxed {\boxed {\sf A. \ Nickel}}\)
Explanation:
First, we must find the density of the unknown metal.
Density is found by dividing the mass by the volume.
\(d=\frac{m}{v}\)
The mass of the metal is 89 grams and the density is 10 cubic centimeters.
\(m= 89 \ g \\d= 10 \ cm^3\)
Substitute the values into the formula.
\(d=\frac{89 \ g}{10 \ cm^3}\)
Divide.
\(d= 8.9 \ g/cm^3\)
Now we know the density and can identify the unknown metal.
Nickel: 8.9 g/cm³Silver: 10.5 g/cm³Lead: 11.35 g/cm³Mercury: 13.55 g/cm³The density matches nickel's density. Therefore, this metal must be nickel.
Identify all resonance forms of the enolate formed, and indicate whether or not a substantial amount of starting ketone will be present together with the enolate at equilibrium. (select all that apply)
Answers
At equilibrium, a significant quantity of starting ketone will be present with the enolate in the first case.
What is ketone?Ketones are substances produced by the liver. It is formed when there is insufficient insulin in the body to turn sugar into energy.
What happens in scenario two where the formation of possible enolate ions from the cyclohexan-1, 4-di.ketone by abstraction of acidic proton by suing sodium ethoxide base occurs?In this case a substantial amount of ketone will exist along side the enolate at equilibrium.
Learn more about ketones at;
https://brainly.com/question/27425066
#SPJ1
15 POINTS!! does anyone know the answer to this? is it A?
GIVING BRAINLIEST TO THE BEST EXPLANATION!!

Answers
Answer:
b.2
Explanation:
Answer:
Mole fraction of water(H₂O) is 0.5 and the mole fraction of CH₃OH(Methanol) is 0.5.
Explanation:
Greetings !
The molecular weight of CH₃OH(Methanol)=32g/mol
The number of moles of CH₃OH=
\( \frac{128g}{32g/mol} \)
=4moles
The molecular weight of water H₂O =18g/mol
The number of moles of=
\( \frac{72g}{18g/mol} \)
=4moles
Total number of moles in the solution =4mol + 4mol
=8mol
Mole fraction Methanol CH₃OH=
\( \frac{4mol}{8mol} \)
=0.5mol
Hope it helps!
Phenyl magnesium bromide is used as a Grignard reagent in organic synthesis. Determine its empirical and molecular formula if its molar mass is 181.313 g/mol
and it contains 39.7458 % C, 2.77956 % H, 13.4050 % Mg, and 44.0697 % Br
Answers
The molecular formula is C₆H₅MgBr.
The molecular formulation is an expression that defines the number of atoms of each element in a single molecule of a compound. It indicates the actual variety of every atom in a molecule.
39.7458 % C, means 39.7458 of C
⇒ 39.7458 of C × 1 mol c/12 g C = 3.31215 mol C/0.55153 = 6 mol C
⇒ 2.77956 g H × 1 mol H/1 g H = 2.77956 mol H = 5 mol
⇒ 13.4050 g Hg × 1 mol Hg/ 24.305 g Hg = 0.55153 mol Hg = 1 mol Hg
⇒ 44.067 g Br × 1 mo Br/79.904 = 0.55153 mol Hg = 1 mol Br
Empirical formula = ( C₆H₅MgBr)ₓ
Molecular Formula = (181.313g/mol) / 180 g/mol
X = 1
Hence, molecular formula = C₆H₅MgBr
Learn more about molecular formulas here:-https://brainly.com/question/26388921
#SPJ9
You place 10 grams of a salt into water and want it to dissolve. All of the following
will cause a salt to dissolve faster except for which one?
using a smaller container
Ostirring the mixture
raising the temperature
grinding up the salt before placing it in the water
Previous Page
Next Page
Page 1 of 5
Answers
Answer:
grinding up the salt
Explanation:
this is just what I think so take with a grain of salt (pun unintended) if its finer then it should dissolve faster because there is less volume per grain to undergo diffusion