Is Salt and pepper an example of a homogenous mixture?
Answers
Answer:
no it is a heterogeneous mixture because salt and pepper are not mixed uniformly.
Related Questions
Which element in the third period would you expect to have the larger atomic radius, sodium (Na) or Sulfur
Answers
look at image please

Answers
The mass of oxygen gas produced, given that 15.8 g of potassium permanganate is heated until no more oxygen gas is given off is 1.6 g
How do i determine the mass of oxygen produced?From the question given, the following data were obtained:
Mass of potassium permanganate = 15.8 gMass of remaining material after heating = 14.2 gMass of oxygen gas =?The mass of oxygen gas produced from the reaction can be obtained as follow:
Mass of potassium permanganate = Mass of remaining substance + mass of oxygen
Inputting the given parameters, we have:
15.8 = 14.2 + mass of oxygen
Collect like terms,
Mass of oxygen = 15.8 - 14.2
= 1.6 g
Thus, we can conclude that the mass of oxygen gas produced from the reaction is 1.6 g
Learn more about mass produced:
https://brainly.com/question/9526265
#SPJ1
Help please Where does your water come from? Your tap or a bottle is not a valid answer.
where the water is actually collected, where it comes from originally, and how it actually gets
delivered to our houses in Southern California
Answers
Answer:
-water is collected from nearby water holes and put in a water tower after it's clean
Answer:
Your water comes from rivers and streams.
Explanation:
The water is collected from rivers and stream and gets delivered from the storage tank to the pipes of our house
How do movement of rocks cause earthquakes?
Answers
Answer:
Well its actually the movement of tectonic plates under the earths surface that when the slide along each other, pull away from each other, or push up against each other they create earth quakes.
Explanation: yes.
PLEASE HELP!!
if 9. 45 moles of C2H2 are burned how many moles of O2 are needed?
Answers
To determine the number of moles of O2 needed to burn 9.45 moles of C2H2, we first need to write down the balanced chemical equation for the combustion of acetylene (C2H2):
2 C2H2 + 5 O2 → 4 CO2 + 2 H2O
From this equation, we can see that 5 moles of O2 are required to burn 2 moles of C2H2. To find out how many moles of O2 are needed for 9.45 moles of C2H2, we can use a simple proportion:
(5 moles O2 / 2 moles C2H2) = (x moles O2 / 9.45 moles C2H2)
To solve for x (moles of O2 needed), simply cross-multiply and divide:
x = (5 moles O2 * 9.45 moles C2H2) / 2 moles C2H2
x ≈ 23.63 moles O2
Therefore, approximately 23.63 moles of O2 are needed to burn 9.45 moles of C2H2.
#SPJ11
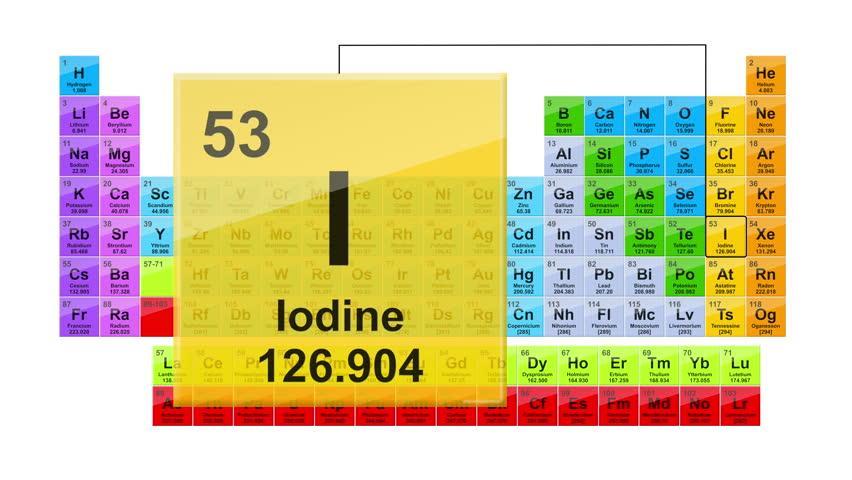
Which element is in Group 17 and has more than 50 protons but less than 75 protons?
Answers
Answer:
Iodine
Explanation:
Iodine is in group 17 on the periodic table (first picture attached). The second picture shows you in the top left-hand corner of Iodine's little square, there is the number 53. This is the atomic number, it is also the number of protons and electrons in an element.
Hope this helped :)

Does a sharpener have kinetic energy ?
Answers
Answer:
No, of itself a pencil sharpener is not kinetic or potential energy. However, due to its position in a gravity field, a pencil sharpener on a desk does have potential energy.
Explanation:
an organic compound is composed of 49.31% carbon, 43.79% oxygen, and the rest hydrogen. the molar mass is 146.1 g/mol. what is the molecular formula of this compound?
Answers
An organic compound is composed of 49.31% carbon, 43.79% oxygen, and the rest hydrogen and if the molar mass is 146.1 g/mol then the molecular formula of this compound is C₆H₁₀O₄(Adipic acid).
What is Adipic acid?The chemical molecule with the formula (CH2)4(COOH)2 is termed as adipic acid or hexanedioic acid. It is the most significant dicarboxylic acid from an industrial standpoint; every year, around 2.5 billion kilograms of this white crystalline powder are manufactured, primarily as a precursor to the manufacture of nylon.
Molecular formula:A molecular compound's kind and quantity of atoms of each element are indicated by its molecular formula.
To know more about adipic acid visit
https://brainly.com/question/29601963
#SPJ4
what is the freezing point of a solution in which 2.50 grams of sodium chloride are added to 230.0 ml of water? you answered
Answers
The freezing point of the solution would be lower than the freezing point of pure water. To calculate the exact freezing point, we would need to know the initial temperature of the water and the concentration of the sodium chloride solution.
ΔTf = Kf × m
Where ΔTf is the change in freezing point, Kf is the freezing point depression constant for water (1.86 °C/m), and m is the molality of the solution (moles of solute per kg of solvent).
First, we need to calculate the molality of the solution:
m = (2.50 g / 58.44 g/mol) / (0.230 kg)
m = 0.180 mol/kg
Now we can calculate the change in freezing point:
ΔTf = 1.86 °C/m × 0.180 mol/kg
ΔTf = 0.3348 °C
This means that the freezing point of the solution would be lowered by approximately 0.3348 °C compared to pure water. To find the actual freezing point, we would need to subtract this value from the freezing point of water at the given initial temperature.
The freezing point of a solution depends on the molality of the solute (in this case, sodium chloride) in the solvent (water). To find the freezing point, we first need to calculate the molality.
1. Calculate moles of sodium chloride (NaCl):
Molecular weight of NaCl = 58.44 g/mol
Moles of NaCl = (2.50 g) / (58.44 g/mol) = 0.0428 mol
2. Convert 230.0 mL of water to kilograms:
Mass of water = (230.0 mL) * (1 g/mL) * (1 kg/1000 g) = 0.230 kg
3. Calculate molality:
Molality = moles of solute / mass of solvent (in kg)
Molality = (0.0428 mol) / (0.230 kg) = 0.186 mol/kg
4. Calculate freezing point depression:ΔTf = Kf × molality
For water, the freezing point depression constant (Kf) is 1.86 °C/mol/kg.
ΔTf = (1.86 °C/mol/kg) × (0.186 mol/kg) = 0.346 °C
5. Find the new freezing point:
Freezing point of pure water = 0 °C
Freezing point of the solution = 0 °C - 0.346 °C = -0.346 °C
The freezing point of the solution is approximately -0.346 °C.
Learn more about freezing here
https://brainly.com/question/3121416
#SPJ11
red gold is a gold‑copper alloy used to make jewelry. a piece of jewelry made of red gold weighs 9.67 g and has a volume of 0.620 cm3 . gold has a density of 19.3 g/cm3 and copper has a density of 8.96 g/cm3 . calculate the percentage by mass of each metal in the jewelry. assume the total volume of the jewelry is the sum of the volumes of the two metals it contains.
Answers
Red gold weighs 9.67 g and has a volume of 0.620 cm3 . gold has a density of 19.3 g/cm3 and copper has a density of 8.96 g/cm3 percentage by mass of each metal in the jewelry is 94,4732031% of gold and 5,5267969% of copper
Here given data is
Red gold weighs = 9.67 g and volume of gold = 0.620 cm3 and density of gold = 19.3 g/cm3 and density of copper = 8.96 g/cm3
So here we have to calculate percentage by mass = ?
19.3x + 8.96y = 8.62
x + y = 0.475 this is the volume
x = 0,421947674 and y = 0,0530523256
So, 19.3 × 0,421947674 × 100% / 8.62 = 94,4732031% of gold
and 100-94,4732031 = 5,5267969% of copper
Know more about percentage by mass of metal
https://brainly.com/question/28607312
#SPJ4
Find the quantinum numbers n,m,l,s for the last of potassium layer pleasee help explain correctly all
Answers
Answer:
Quantum numbers of the outermost electron in potassium:
\(n = 4\).\(l = 1\).\(m_l = 0\).Either \(m_s = 1/2\).Explanation:
Refer to the electron configuration of a potassium atom. The outermost electron in a ground-state potassium atom is in the \(4s\) orbital (fourth \(s\) orbital.)
The quantum number \(n\) (the principal quantum number) specifies the main energy shell of an electron. This electron is in the fourth main energy shell (as seen in the number four in the orbital.) Hence, \(n = 4\) for this electron.
The quantum number \(l\) (the angular momentum quantum number) specifies the shape (\(s\), \(p\), \(d\), etc.) of an electron. \(l = 1\) for \(s\!\) orbitals (such as the one that contains this electron.
Quantum numbers \(n\) and \(l\) specify the shape of an orbital. On the other hand, the magnetic quantum number \(m_l\) specifies the orientation of these orbitals in space.
However, \(s\) orbitals are spherical. Regardless of the value of \(n\), the only possible \(m_l\) value for electrons in \(s\!\) orbitals is \(m_l = 0\).
The spin quantum number \(m_s\) distinguishes between the two electrons in an orbital. The two possible values of \(m_s \!\) are \((+1/2)\) and \((-1/2)\). Typically, the first electron in an orbital is assigned an upward (\(\uparrow\)) spin, which corresponds to \(m_s = (+1/2)\).
Do the ingredients changing into a cake in the oven a chemical reaction?
Answers
Answer:
When you bake a cake, the ingredients go through a chemical change. A chemical change occurs when the molecules that compose two or more substances are rearranged to form a new substance! When you start baking, you have a mixture of ingredients. The flour, egg, sugar,
Explanation:
Please help I'm struggling
What type of attractive force exists when two atoms form a temporary dipole or charge because of the movement of electrons?

Answers
Answer:
The correct option is A
Explanation:
The forces of attraction among the molecules or atoms of a substance are called inter molecular forces.
When two atoms gain temporary dipole and become polar for an instant the inter molecular forces of attraction between then are known as London dispersion forces.
London Dispersion Forces:
It is a type of inter molecular force that occurs when the electrons in two adjacent atoms are displaced in such a way that the atoms get some temporary dipoles, they attract each other through London dispersion forces. These inter molecular forces usually occur between non polar substances.
Answer: B intramolecular force
Explanation:
a solution contains 0.50 m acetic acid () and 0.50 m sodium acetate (). what are the major species in this solution?
Answers
The major species present in the given solution are acetic acid (CH3COOH), sodium ions (Na+), acetate ions (CH3COO-), and water (H2O).
The sodium acetate and acetic acid ions will be present in the solution in equal amounts because the solution includes 0.50 m of acetic acid and 0.50 m of sodium acetate.
Because it is a weak acid, acetic acid will partially dissociate in the solution to produce hydrogen ions (H+) and acetate ions.
The sodium acetate, on the other hand, will totally dissociate into sodium ions and acetate ions.
As a result, the acetic acid, sodium ions, acetate ions, and water are the main species in the solution, with the acetate ions being the most prevalent species.
Complete Question:
A solution contains 0.50 m of acetic acid (CH3COOH) and 0.50 m of sodium acetate (CH3COONa). What are the major species in this solution?
To learn more about acetic acid visit:
https://brainly.com/question/15231908
#SPJ4
Identify the type of reaction:
2Nal + F2 ---> 2NaF + I2
Synthesis
Decomposition
Single Replacement
Double Replacement
Combustion
Answers
When rocks break down or decompose, they can form
A.
soil.
B.
magma.
C.
bigger rocks.
D.
lava.
Answers
Answer:
C
Explanation:
because when rocks break down they can form and once they form they can make more and even bigger rocks hopes this helps.
Find the mean of the data.
The number of students who
have a cat in each class.
4, 1, 3, 9, 6, 3, 2, 4
Mean [?] cats
Answers
The mean of the data is 4.
The formula of the mean is
Sum of all the observation÷ Total number of observations
So, According to the formula :
Sum of all the observations= 4+1+3+9+6+3+2+4 = 32
The number of observations is 8
So,
32÷8
= 4
Hence, the mean of the data is 4.
To know more about Mean please refer to this link:
https://brainly.com/question/22871228?utm_source=android&utm_medium=share&utm_campaign=question
2 questions Help need asap help thank you so 80 points and branliest random answers are reported plus deleted questions are in picture it’s for science retaking a test

Answers
what is the leaving group when acetic anhydride is reacted with ethanol in a nucleophilic acyl substitution reaction?
Answers
BH3/THF with H2O2/NaOH is the leaving group when acetic anhydride is reacted with ethanol in a nucleophilic acyl substitution reaction
Corn and other plant resources are used to make ethanol, a sustainable fuel. In the United States, ethanol is widely used and is present in more than 98% of gasoline. E10 (10% ethanol, 90% gasoline) is the most widely used ethanol mix. The production of medicines, polymers, lacquers, polishes, plasticizers, and cosmetics all need ethanol. In medicine, ethanol is employed as a topical anti-infective and as an antidote for methanol or ethylene glycol overdose. More than two billion people consume ethanol (also known as ethyl alcohol) daily.
Learn more about ethanol here:
https://brainly.com/question/25002448
#SPJ4
How does nuclear fusion produce energy in a star?
Answers
Nuclear fusion in stars, such as our Sun, produces energy through the fusion of light atomic nuclei, mainly hydrogen, into heavier nuclei like helium. This fusion process releases a tremendous amount of energy.
Within the core of a star, where temperatures and pressures are extremely high, nuclear fusion takes place. The collisions between hydrogen atoms at such high temperatures provide the necessary energy to overcome electrostatic repulsion, enabling the fusion process.
In the proton-proton chain, the most common fusion process in stars, hydrogen nuclei combine to form helium nuclei through several reactions. The conversion of a small fraction of mass into energy, as described by Einstein's mass-energy equivalence, results in the release of energy in the form of gamma rays.
These high-energy photons interact with matter, gradually transforming into light and heat. This energy release sustains the star's stability by countering gravitational collapse and powers its luminosity for billions of years.
To know more about gamma rays click here: brainly.com/question/14847283
#SPJ11
Select all of the following that are products in the overall equation for aerobic respiration. (select more than one)
ATP (adenosine triphosphate)
NaOH (natrium hidroxide)
CO2 (carbon dioxide)
CO (carbon monoxide)
Answers
The products in the overall equation for aerobic respiration are ATP (adenosine triphosphate) and CO2 (carbon dioxide).
What is aerobic respiration?
Aerobic respiration is a metabolic process that uses oxygen to convert glucose into energy. During aerobic respiration, glucose (sugar) is broken down in the presence of oxygen (O2) to produce energy in the form of ATP (adenosine triphosphate). This process also produces carbon dioxide (CO2) and water (H2O). The energy produced by aerobic respiration is used to fuel cellular processes such as protein synthesis and muscle contractions. Aerobic respiration is the main form of respiration in humans and other animals.
The overall equation for aerobic respiration is C6H12O6 + 6O2 yields 6CO2 + 6H2O + energy (as ATP). ATP (adenosine triphosphate) and CO2 (carbon dioxide) are products in the overall equation for aerobic respiration. NaOH (natrium hydroxide) and CO (carbon monoxide) are not products in the equation.
Therefore, ATP (adenosine triphosphate) and CO2 (carbon dioxide) are the answers.
To learn more about aerobic respiration the link
https://brainly.com/question/12605249
#SPJ1
what happens if you don't disconnect the wires in a series circuit?
Answers
Answer:
the series circuit has one current so if you don't disconnect the wires nothing happens
but if you disconnect the wires in series circuit, the current stop and the current doesn't go to the end
Explanation:
(≧▽≦)hope it helps (≧▽≦)
Molecular formula of a certain ionic compound is XY2 and X is a metal. State group to which elements X and Y belong to in the periodic table. plzzz help
Answers
Answer: m Most likely Group2 metals and Group 17 non-ktta
Explanation:
A little more information is needed to be certain, but the likely answer is that X belongs to Group 2 and Y belongs to Group 17. Group 2 metals (Be, Mg, Ca, Sr, Ba, etc.) are all divalent. They gave rive up 2 electrons each to return to a full shell. Group 17 elements (e,g, F, Cl, Br, I, etc.) all require 1 electron to reach a full valence shell. That would make the proportion 1X to 2Y, or XY2. It is possible that a metal outside of Group 2 would also have a valency of 2. Iron(II) forms FeCl2, for example.
A filament bulb is labelled ‘2.5 V, 1.25 W'. How much energy will be transferred to it when it is connected to a 2.5 V battery for one minute?
Answers
To calculate the amount of energy transferred to the filament bulb, we can use the power, voltage, and time involved in the circuit. The given information states that the bulb is labeled as '2.5 V, 1.25 W'.
To start, we can use the formula P = IV, where P represents the power, I represents the current, and V represents the voltage. Since the resistance of the bulb is not provided, we cannot directly use the formula P = V² / R. However, we can derive the value of current using I = V / R, where R is the resistance.
By substituting I = V / R into the equation P = IV, we obtain P = V² / R. Now, we have the power value, which is 1.25 W. The bulb is connected to a 2.5 V battery, so we can use P = IV to find the current, I. Substituting P = 1.25 W and V = 2.5 V into the equation, we get I = P / V = 1.25 / 2.5 = 0.5 A.
Now that we know the current flowing through the bulb is 0.5 A, we can calculate the energy transferred using the formula E = Pt, where E represents energy, P represents power, and t represents time. Given that the bulb is connected for 1 minute (60 seconds), we can calculate E = Pt = 1.25 W × 60 s = 75 J (Joules).
Therefore, the amount of energy transferred to the filament bulb when it is connected to a 2.5 V battery for one minute is 75 Joules.
To Learn more about filament bulb. Click this!
brainly.com/question/22940744
#SPJ11
Put the following elements in order of increasing first ionization energy (lowest to highest): S, Ca, Mg,
CI
Answers
Answer: Ca, Mg, S, Cl
Explanation: general trends are IE increases as the number of electrons in the outermost shell, and decreases as the number of shells (and shielding effect) increases.
S outer 6, shells 3
Ca outer 2, shells 4
Mg outer 2, shells 3
Cl outer 7, shells 3
Ca has 2 outer, 4 shells —> lowest
others have 3shells
Mg 2outer, S 6, Cl 7
2 C2F4 → C4F8 is 0.0410 M−1 s −1 . We start with 0.105 mol C2F4 in a 4.00-liter container, with no C4F8 initially present. What will be the concentration of C2F4 after 3.00 hours ? Answer in units of M.
Answers
Answer:
After three hours, concentration of C₂F₄ is 0.00208M
Explanation:
As the units of the rate constant of the reaction are M⁻¹s⁻¹ we can know this reaction is of second order. Its integrated law is:
\(\frac{1}{[A]} =\frac{1}{[A]_0} +Kt\)
Where [A] and [A]₀ represents initial and final concentrations of the reactant (C₂F₄), K is rate constant (0.0410M⁻¹s⁻¹) and t is time.
3.00 hours are in seconds:
3 hours ₓ (3600 seconds / 1 hour) = 10800 seconds
Computing in the equation:
\(\frac{1}{[A]} =\frac{1}{[0.105mol / 4L]} +0.0410M^{-1}s^{-1}*10800s\\\frac{1}{[A]} = 480.9M^{-1}\\\\)
[A] = 0.00208M
After three hours, concentration of C₂F₄ is 0.00208Mif e°cell = 1.587 v and e°red of the cathode half–cell is 0.536 v, what is e°ox of the anode half–cell? s2o82-(aq) 2h (aq) 2i-(aq) 2hso4-(aq) i2(aq
Answers
E°ox of the anode half-cell is 1.051 V.
To determine the E°ox of the anode half-cell, we need to use the following equation: E°cell = E°cathode - E°anode. We are given E°cell (1.587 V) and E°red of the cathode half-cell (0.536 V). We can rearrange the equation to solve for E°ox of the anode half-cell: E°anode = E°cathode - E°cell.
Plugging the values into the equation, we get: E°anode = 0.536 V - 1.587 V = -1.051 V. However, since E°ox is the oxidation potential and we are given the reduction potential, we need to reverse the sign to obtain the oxidation potential. Thus, E°ox of the anode half-cell is 1.051 V.
To know more about half-cell visit:-
https://brainly.com/question/31522202
#SPJ11
At a given temperature, 0.500 mols of CO and 1.50 moles of water vapor are added to a 2.50 L vessel. When the reaction reaches equilibrium, the [CO2] and [H2] are 0.00775 M. Find the [CO] and the [H2O] at equilibrium. Calculate the Keq and predict the sign of ΔG.
Answers
The concentrations of the reaction's reactants and products must be equal at equilibrium. Following is a description of how CO and H2O react to generate CO2 and H2: CO + H2O <=> CO2 + H2 We can determine the equilibrium CO and H2O concentrations using the available data.
The starting concentrations of CO and H2O are 0.800 M and 0.800 M, respectively, due to the total moles of CO and H2O being 2.00 moles and the total volume being 2.50 L. The equilibrium expression may be used to compute the equilibrium concentrations of CO and H2O: K = [CO2][H2]/[CO][H2O] K = (0.00775)(0.00775)/[CO] may be used to derive the equilibrium constant given that [CO2] and [H2] are both equal to 0.00775 M.
[H2O] K = (0.00775)(0.00775)/[0.0455], when the equilibrium concentrations of CO and H2O are plugged in.[0.0455]. ][0.0455] K = 0.0020 From this, we can calculate the equilibrium concentrations of CO and H2O: [CO] = 0.0455 M [H2O] = 0.0455 M .
The standard free energy change (G°), which can be calculated using the formula G° = -RTlnK, may be used to estimate the sign of G for this reaction. Since K > 1, we may anticipate a spontaneous response, meaning that G will be negative.
Learn more about concentrations at:
https://brainly.com/question/10725862
#SPJ1
if 6 moles of a a compound produce 84 J of energy, what is the h reaction in j/mol
Answers
The enthalpy of the reaction is 14 J/mol.
The enthalpy of a reaction (ΔH) is the amount of energy transferred between a system and its surroundings during a chemical reaction at constant pressure, measured in joules per mole (J/mol). This value is important because it can tell us whether a reaction is exothermic or endothermic, as well as give us information about the strength of chemical bonds within the reactants and products.To calculate the enthalpy of a reaction, we need to know the amount of energy released or absorbed (Q) and the number of moles of the compound involved in the reaction (n). We can use the equation:
ΔH = Q/n
Given that 6 moles of a compound produce 84 J of energy, we can calculate the enthalpy of the reaction as follows:
ΔH = Q/n
ΔH = 84 J / 6 mol
ΔH = 14 J/mol
This means that for every mole of the compound involved in the reaction, 14 J of energy is transferred between the system and the surroundings. Since the value is positive, we can conclude that the reaction is endothermic, meaning that it requires an input of energy to occur.It is worth noting that the enthalpy of a reaction can depend on a number of factors, such as temperature, pressure, and the specific conditions under which the reaction occurs. As such, it is important to take these factors into account when calculating or predicting enthalpy values.
for such more questions on enthalpy
https://brainly.com/question/14047927
#SPJ8
1. How many atoms of P are in 5 moles of P4010?
2. 1.0 x 10^20 atoms of Fe is equivalent to how many moles of Fe?
3. How many moles of P4O10 are produced when we react 8 moles of phosphorus with oxygen?
4. How many atoms of hydrogen are contained in 100g of methane (CH4)
Answers
Answer:
1) Assuming you mean Phosphorous Pentoxide (P4O10) there are 6.02x10^23 x 4 (number of P in P4O10) x 5 moles = 1.38x10^25
2) Divide 1.0x10^20/6.02x10^23 = 1.7x10^-4 moles
3) The balanced equation would be 4P + 5O2 —-> P4O10. Starting with 8 moles of P 8/4 = 2. You generate 2 moles P4O10
4) CH4 has a molecular mass of 12+4 = 16. Starting with 100g/16g/mole = you have 6.25 moles of methane. As there are 4 atoms of hydrogen in each molecule of methane, you multiply 6.25 moles methane by 4 atoms H/molecule and get 25 moles of hydrogen or 1.505e25 atoms of hydrogen
Explanation: