Is it reasonable to expect that the atom model will change again in time?
Answers
Related Questions
A lab technician made an observation that during the winter it took longer for potassium nitrate to dissolve in water and during the summer the same process took a shorter time.
Can someone please help me create an hypothesis from this statement?
Answers
Hypothesis could be: "The temperature of the water affects the rate of dissolution of potassium nitrate, with higher temperatures leading to faster dissolution and lower temperatures leading to slower dissolution."
This hypothesis is based on the knowledge that temperature affects the solubility of solids in liquids, with higher temperatures generally leading to higher solubility.
In the case of potassium nitrate, it is likely that the colder water during the winter months reduces its solubility, making it take longer to dissolve, while warmer water during the summer months increases its solubility, making it dissolve faster.
To test this hypothesis, one could conduct an experiment in which the same amount of potassium nitrate is added to water at different temperatures (e.g. room temperature, warm water, and cold water) and the time taken for the potassium nitrate to dissolve is measured.
The results of this experiment could be used to either support or refute the hypothesis.
To know more about Hypothesis:
https://brainly.com/question/606806
#SPJ1
Please help I’ll give brainiest!

Answers
Answer:
Its the first answer
Explanation:
Which type of isomers are o-xylene, m-xylene, and p-xylene?
structural isomers
optical isomers
geometric isomers
Answers
o-xylene, m-xylene, and p-xylene are structural isomers.
o-xylene, m-xylene, and p-xylene are examples of aromatic isomers, also known as ortho-, meta-, and para-xylene, respectively. These isomers are classified as aromatic because they are part of a family of compounds known as aromatic hydrocarbons, which contain a ring of six carbon atoms bonded to each other in a specific way.
o-xylene, m-xylene, and p-xylene are also examples of structural isomers. Structural isomers are compounds that have the same molecular formula but differ in the arrangement of their atoms. In the case of o-xylene, m-xylene, and p-xylene, the atoms are arranged differently around the ring of six carbon atoms.
o-xylene, m-xylene, and p-xylene are not optical isomers, which are also known as enantiomers. Optical isomers are compounds that are mirror images of each other but are not superimposable. Optical isomers are important in medicinal chemistry because they can have different biological properties even though they have the same molecular formula and structure.
o-xylene, m-xylene, and p-xylene are also not geometric isomers. Geometric isomers are compounds that have the same molecular formula and the same arrangement of atoms, but differ in the orientation of their atoms or groups of atoms in space. Geometric isomers are often found in compounds with cis-trans double bonds or ring structures.
6) A student measures out 96.21 g of sulfur for an experiment. How many moles of Sulfur are in this
sample? (show your work!)
Answers
Atomic mass of Sulfur = 32g
32g of Sulfur is one mole.
1g of Sulfur is \( \frac{1}{32} moles \)
96.21g of Sulfur is \( \frac{96.21}{32} moles=> 3moles(appx) \)
Answer:
\(\boxed {\boxed {\sf 3 \ moles \ sulfur}}\)
Explanation:
To convert from grams to moles, the molar mass is used. This value is the number of grams in 1 mole of a substance and it can be found on the Periodic Table. Look for Sulfur or S.
Sulfur: 32.07 g/molWe use this as a ratio.
\(\frac {32.07 \ g \ S}{1 \ mo;\l\ S }\)
Multiply by the given number of grams.
\(96.21 \ g \ S*\frac {32.07 \ g \ S}{1 \ mo\l\ S }\)
Flip the fraction so the grams of sulfur cancel out.
\(96.21 \ g \ S*\frac {1 \ mol \ S}{32.07 \ g \ S }\)
\(96.21*\frac {1 \ mol \ S}{32.07 }\)
\(\frac {96.21 \ mol \ S}{32.07 }= 3 \ mol \ S\)
96.21 grams of sulfur is equal to 3 moles of sulfur.
What is the chemical formula for ammonium carbonate?
(NH4),CO
(NH, HCO
NH,(CO)
NH(CO)
Answers
How will the rate of reaction -A+B→AB change if A and gases increase 3-3 times? V=
Answers
The rate of the reaction will increase if the concentration of A and gases increase.
Rate of reactionsIf the concentration of reactant A and gas increases by 3 times, the rate of the reaction -A+B→AB will also increase.
This is because an increase in the concentration of reactant A means that there will be more A molecules colliding with B molecules, leading to an increase in the number of successful collisions and the formation of more AB product molecules.
Similarly, an increase in gas concentration will increase the number of gas molecules colliding with reactant molecules, which can also increase the rate of the reaction.
Therefore, the rate constant, k, of the reaction will also increase, resulting in an overall increase in the rate of the reaction.
More on rate of reactions can be found here: https://brainly.com/question/30546888
#SPJ1
True or false Cell division in Prokaryotes that form two genetically identical cells is know as fission.
Answers
Because Binary fission is the method by which prokaryotes produce new individuals that are genetically identical to the parent organism.
How does heat transfer work?
Answers
Answer:
Conduction is one of the three main ways that heat energy moves from place to place. The other two ways heat moves around are radiation and convection. Conduction is the process by which heat energy is transmitted through collisions between neighboring atoms or molecules
Explanation:
a molecule of an element is called what?
Answers
Answer:
a molecule of an element is called atomicity
a sealed flask initially contains pure nitrogen dioxide gas (no2). over time, the nitrogen dioxide forms dinitrogen tetroxide gas (n2o4). the graph below shows the relative amounts of (no2) and (n2o4) over time. what is true about the time indicated by the blue arrow? a. no2 molecules are no longer reacting to form n2o4 molecules. b. the reactant has been used up so the reaction can no longer proceed. c. the rate of the forward reaction (n2o4 formation) is equal to the rate of the reverse reaction (no2 formation). d. the activation energy required for the reaction to occur has been used up.
Answers
Based on the given information, the true statement about the time indicated by the blue arrow is: (c) The rate of the forward reaction (N₂O₄ formation) is equal to the rate of the reverse reaction (NO₂ formation).
The graph shows the relative amounts of NO₂ and N₂O₄ over time, and the point indicated by the blue arrow represents a state of equilibrium. In an equilibrium state, the forward and reverse reactions occur at equal rates.
The concentrations of NO₂ and N₂O₄ reach a constant value, indicating that the conversion of NO₂ to N₂O₄ and the conversion of N₂O₄ to NO₂ are occurring at the same rate.
Option a suggests that NO₂ molecules are no longer reacting, which is incorrect as the reaction is still ongoing at equilibrium. Option b suggests that the reactant has been completely used up, which is not the case in an equilibrium state. Option d refers to the activation energy, which is unrelated to the equilibrium state. Therefore, option c is the correct choice.
To know more about the blue arrow refer here :
https://brainly.com/question/31741705#
#SPJ11
How is energy transferred through the water cycle?
Please help
Answers
Answer:
The water cycle is driven primarily by the energy from the sun.
1.07 option 1 -- laboratory techniques lab report anybody got this lab filled out for me?
Answers
For this reason, chromatographic methods such as liquid, gas, or high-performance liquid chromatography are typically used in laboratories, with spectroscopic methods such as UV-visible spectroscopy, NMR, or MS being used for detection.
What purpose do laboratory methods serve?The use of laboratory methods is crucial to a researcher's career. These abilities are essential for carrying out various studies and examinations. One cannot use these strategies in projects or research without the necessary understanding. The majority of experiments require these procedures.
An lab report is what?A lab report describes an experiment and any findings that were made as a result of the experiment. The three major goals of a lab report are to: clearly explain the results of an experiment by giving data. talk about the outcomes.
What is the name of a lab report?Experimental reports, commonly referred to as "lab reports," are summaries of the writers' own empirical study. An experimental report should be viewed as a "narrative" of your research that guides the reader through your experiment.
learn more about the lab here
https://brainly.com/question/21164257
#SPJ4
b. What is the critical path? \[ \begin{array}{l} \text { B-E-G-H } \\ \text { A-D-F-H } \\ \text { A-D-G-H } \\ \text { A-C-F-H } \end{array} \] c. What is the expected project completion time? (Roun
Answers
b. The critical path refers to the longest sequence of activities from the start to the end of a project, considering the duration of each activity.
c. The total duration for the critical path activities is 18 days, which represents the expected project completion time.
The critical path is a term used in project management to refer to the sequence of activities that determines the minimum time required to complete a project. It is the longest path from the start to the end of the project, considering the duration of each activity. The critical path helps identify the activities that must be closely monitored and managed to ensure the project is completed on time.
Learn more about project completion time
https://brainly.com/question/32318500
#SPJ11
15. How many moles of CaCl are in 250. mL of 3.00 M of CaCl solution?
a. 750. mol
b. 1.33 mol
C. 83.3 mol
d. 0.750 mol
e. 3.00 mol
Answers
Answer d
How do I balance this?

Answers
Answer: 4NH3 + 5O2 —-> 4NO + 6H2O
Explanation: You balance it by making sure each have the same amount on each side.
There is 4 nitrogen on each side.
There is 12 hydrogen on each side (4*3) and (6*2).
There is 10 oxygen on each side (5*2) and (4+6)
give at least five insights that you learn from this lesson, as your way of recognizing the uniqueness of earth as a planet life
Answers
Answer and Explanation:
The lesson about the uniqueness of the earth allowed me to realize that the earth is the only planet that has drinking water and oxygen, thus allowing the existence of life on the planet and showing that we would not fully survive on another planet.
The lesson also shows that the earth is the only planet where rotation is capable of causing seasonal differences in different parts of the globe. This is important for several things, including food production and plant growth.
Another factor that shows the uniqueness of the land is its favorable temperature for life, allowing us to have water and to be able to live outdoors, for example. This factor is worrying, since the holes in the ozone layer have caused changes in this temperature.
I was also able to learn about the gravity of the earth, which is the only thing that allows us to do all the activities we do and allows us not to be launched into space. In addition, gravity has a strong influence on seas and agriculture.
Finally, I was able to learn about the tectonic plates, which provide the movement of our crust and influence in several geological processes.
help me please i don’t understand this

Answers
There you go :) just reword it and try make it into your own words like just switch some sentences around
Which statement best describes how forces are important to chemistry?
A. Forces are transferred when atoms form ions.
B. Forces are released during chemical reactions.
C. Forces determine the extent of a reaction.
D. Forces hold atoms and molecules together.
Answers
Answer:
D
Explanation:
Balance the following equation using the change in oxidation numbered method Ag + H2S+ O2 —> Ag2 S + H2O
Answers
Answer
Explanation
In the oxidation number method of balancing chemical equations, you determine the oxidation numbers of all atoms. Then you multiply the atoms that have changed by small whole numbers. You are making the total loss of electrons equal to the total gain of electrons. Then you balance the rest of the atoms.
The given equation is:
Ag + H₂S + O₂ —> Ag₂S + H₂O
Step 1. Identify the atoms that change oxidation number.
Left hand side: Ag = 0, H = +1, S = -2, O = 0
Right hand side: Ag = +1, S = -2, H = +1, and O = -2
The changes in oxidation number are:
Ag: 0 → +1; Change = +1
O: 0 → -2; Change = -2
Step 2. Equalize the changes in oxidation number.
Each Ag atom has lost one electron, and each O atom has gained two electrons.
O: 0 → -2; Change = -2,
What current (in a) is required to plate out 2. 96 g of nickel from a solution of ni2 in 27. 12 minutes?
Answers
The current required to plate out 2.96 g of nickel from the solution of Ni²⁺ in 27.12 minutes is 5.95 A
Balanced equationWe'll begin by writing the balanced equation showing the number of faraday required to plate nickel. This is given below
Ni²⁺ + 2e —> Ni
Molar mass of Ni = 59 g/mol
Mass of Ni from the balanced equation = 1 × 59 = 59 g
Number of faraday = 2 F
1 faraday = 96500 C
2 faraday = 2 × 96500 = 193000 C
SUMMARY
From the balanced equation above,
59 g of Nickel was deposited by 193000 C of electricity
How to determine the quantity of electricityFrom the balanced equation above,
59 g of Nickel was deposited by 193000 C of electricity
Therefore,
2.96 g of Nickel will be deposited by = (2.96 × 193000) / 59 = 9682.71 C of electricity
How to determine the current Quantity of electricity (Q) = 9682.71 CTime (t) = 27.12 mins = 27.12 × 60 = 1627.2 sCurrent (I) =?I = Q / t
I = 9682.71 / 1627.2
I = 5.95 A
Learn more about Faraday's law:
https://brainly.com/question/24795850
The current required to plate out 2. 96 g of nickel from a solution of ni2 in 27. 12 minutes is 5.95 ampere.
What is current?Current is a charged particle moving through an electrical conductor.
The equation is \(\rm Ni^2^+ + 2e = Ni\)
step 1: calculate the mass of Ni
Molar mass of Ni is 59 g/mol
59 × 1 = 59 g
Step2: calculate the number of Faraday
no. of Faraday is 2F
1 Faraday = 96500 C
then, 2 Faraday is equal to 2 × 96500 = 193000 C
Step 3: calculate the quantity of electricity
59 g of Nickel is deposited by, 193000 C of electricity
So, for 2.96 g of nickel
\(\dfrac{2. 96\;g \times 193000}{59} = 9682.71 \;C\)
Step4: calculate the current:
Electricity Q = 9682.71 C
Time = \(27.12 \times 60 = 1627.2 \;s\)
To find current
\(I = \dfrac{Q}{t} \\\\I = \dfrac{9682.71 }{1627.2}\\I = 5.95 A\)
Thus, the current required is 5.95 ampere.
Learn more about current
https://brainly.com/question/13076734
#SPJ4
Get It? Consider How might the formation of smog be affected if more nitrogen oxides and other pollutants are added to the air?
Answers
If more nitrogen oxides (NOx) and other pollutants are added to the air, it would likely have a significant impact on the formation of smog. Smog is primarily formed when certain pollutants react in the presence of sunlight.
The two main types of smog are:
Photochemical Smog: This type of smog forms in urban areas with high traffic and industrial emissions. It is characterized by a brownish haze and is primarily composed of nitrogen oxides, volatile organic compounds (VOCs), and sunlight.
When nitrogen oxides and VOCs are released into the atmosphere, they undergo chemical reactions in the presence of sunlight, leading to the formation of ground-level ozone, a key component of photochemical smog.
If more nitrogen oxides and other pollutants are added to the air, the concentration of nitrogen oxides and VOCs would increase. As a result, more of these pollutants would be available for reactions in the presence of sunlight, leading to greater formation of ground-level ozone and exacerbating the formation of photochemical smog.
This would contribute to poor air quality and respiratory issues for individuals exposed to the smog.
Industrial Smog: Industrial smog, also known as sulfur smog, is primarily caused by the combustion of fossil fuels, particularly coal, which releases sulfur dioxide (SO2) into the atmosphere.
If more nitrogen oxides and other pollutants are added to the air, it may not directly affect the formation of industrial smog since it is primarily driven by sulfur dioxide emissions. However, the overall air pollution levels would increase, leading to a deterioration in air quality and potential health effects.
In summary, the addition of more nitrogen oxides and other pollutants to the air would likely intensify the formation of photochemical smog, characterized by increased ground-level ozone concentrations.
It would also contribute to overall air pollution, even though it may not directly impact industrial smog unless it involves the release of sulfur dioxide. Reducing emissions of nitrogen oxides and other pollutants is crucial in mitigating smog formation and improving air quality.
For more question on pollutants click on
https://brainly.com/question/22517940
#SPJ11
Find the empirical formula for a compound with 92.25 % Cand 7.75 % H.
Please help. I’m really struggling. No wrong answers please, if you don’t know it please don’t answer, I’m already failing the class.
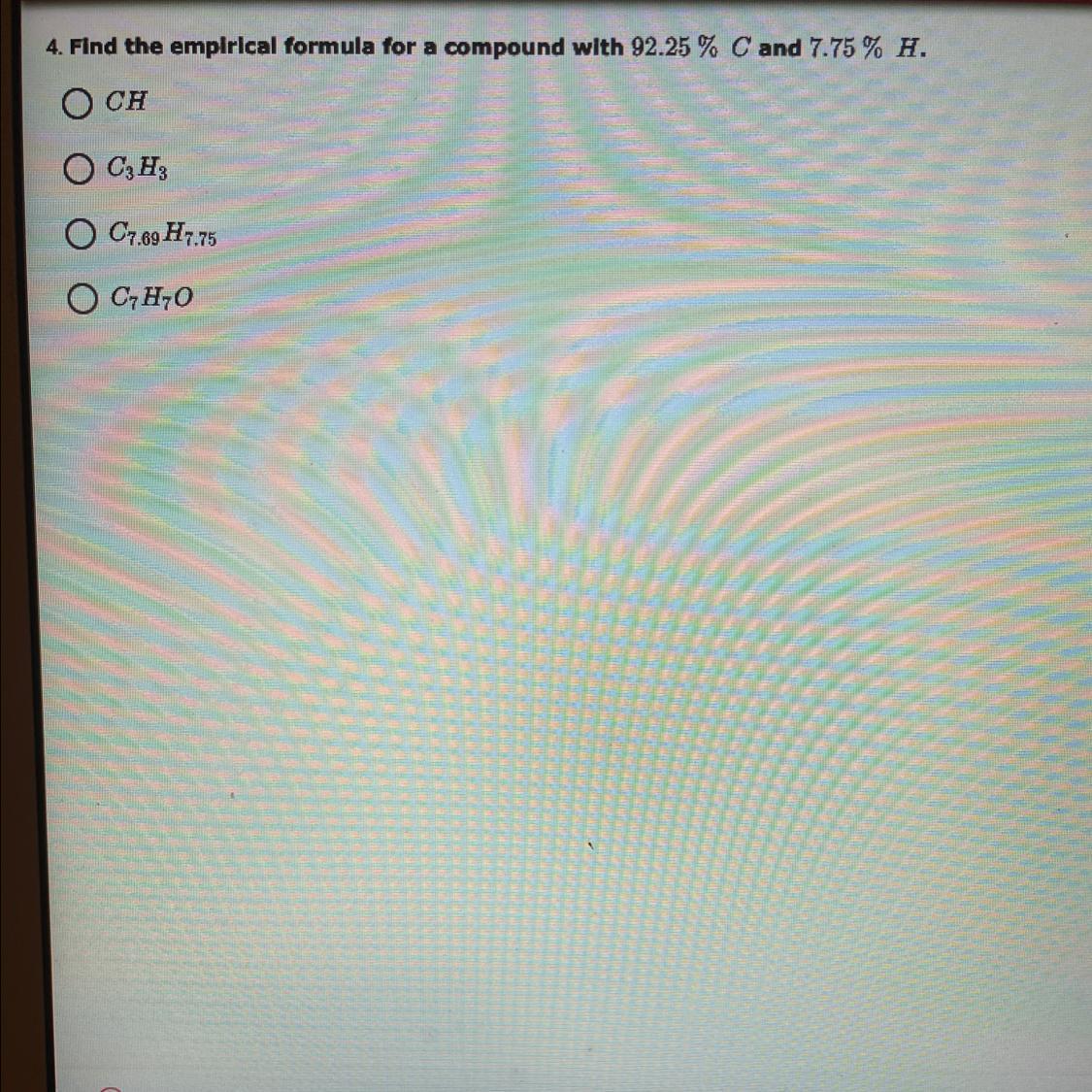
Answers
Answer:CH
Explanation:it’s simply has the answer in the question. It’s just throwing you off with the percentages.
How many milliliters of water will it take to fill a 2 L bottle that already contains 1.87 L of water?
Answers
Answer:
0.00013
Explanation:
I'm pretty sure. I first subtracted 1.87 from 2. I got 0.13. The conversion from mL to L is 0.001. I multiplied 0.13 and 0.001 and got 0.00013 mL.
A solution of 5 m hci is already made. you need to dilute the solution to 1 m hci. the 1 m hci solution is _____
options :slower, stronger, weaker, faster ,
Answers
1M HCl solution is weaker
What is the molarity of a solution that has 3.5 moles of NaOH dissolved in 8.50 liters of solution?
Answers
Answer
Molarity (C) = 0.41 mol/L
Explanation
Given:
Number of moles = 3.5 moles
Volume = 8.50 L
Required: Molarity of the solution
Solution
To solve for molarity, you can use the following equation
\(C\text{ = }\frac{n}{V}\)Where C is the concentration or molarity, n is the number of moles and V is the volume of the solution.
C = 3.5 mol/8.50 L
C = 0.41 mol/L
You collect a sample of gases from an indoor pool area. the sample contains air and water vapor. the total pressure is 100.18 kilopascals, and the partial pressure of the water vapor is 3.36 kilopascals. what is the partial pressure of the air in the sample?
Answers
Answer:
Explanation:
The partial pressure of the air in the sample can be found using the formula for Dalton's law of partial pressures, which states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the individual gases:
Total pressure = partial pressure of gas 1 + partial pressure of gas 2 + ...
In this case, we have two gases: air and water vapor. We know the total pressure of the mixture and the partial pressure of the water vapor, and we want to find the partial pressure of the air. We can set up the equation as follows:
Total pressure = partial pressure of air + partial pressure of water vapor
Substituting the given values:
100.18 kPa = partial pressure of air + 3.36 kPa
Solving for the partial pressure of air:
partial pressure of air = 100.18 kPa - 3.36 kPa
partial pressure of air = 96.82 kPa
Therefore, the partial pressure of the air in the sample is 96.82 kilopascals.
What is the most important reason for using hydrates in fire extinguishers?
-They keep fire extinguishers dry during shipping and storage.
-They make fire extinguishers more affordable for household use.
-They create foams that have high water content to help extinguish fires.
-They create high pressure in the cylinder to quickly force out the fire r*tardant.
Answers
The importance of the use of hydrates in fire extinguishers are;
-They keep fire extinguishers dry during shipping and storage.
-They create foams that have high water content to help extinguish fires.
They create high pressure in the cylinder to quickly force out the fire r*tardant.
What is a fire extinguisher?We know that a fire extinguisher has to do with any device that has been made in such a way that the device can be used to eliminate a flame that is burning. We all know that fore can be very destructive. This implies that it is important to be able to put out the fire so that it does not cause big problems.
The fire extinguisher is composed of certain chemical substances that are able to react together quickly and then be able to quench the flame of the reaction that is going on in the system.
Learn more about fire extinguishers:https://brainly.com/question/8822808
#SPJ1
practice naming ionic compounds containing polyatomic ions. aluminum nitrite al no3 ammonium sulfate calcium phosphate ammonium nitrate
Answers
To name ionic compounds containing polyatomic ions, you first need to identify the cation (positive ion) and the anion (negative ion). The cation is usually a metal ion, while the anion is a polyatomic ion made up of two or more atoms bonded together with a charge.
Let's start with the first compound, aluminum nitrite. The cation here is aluminum, which has a charge of +3. The anion is nitrite, which has a charge of -1. To name this compound, you simply combine the names of the two ions. So, aluminum nitrite would be written as Al(NO3)3.
Moving on to calcium phosphate. The cation here is calcium, which has a charge of +2. The anion is phosphate, which has a charge of -3. To name this compound, you need to balance the charges by using subscripts. So, calcium phosphate would be written as Ca3(PO4)2. Lastly, we have ammonium nitrate. The cation here is ammonium, which has a charge of +1. The anion is nitrate, which has a charge of -1. To name this compound, you once again combine the names of the two ions. So, ammonium nitrate would be written as NH4NO3.
To know more about ionic visit :-
https://brainly.com/question/29523788
#SPJ11
32. Barium sulfate, BaSO4, is a white crystalline solid that is insoluble in water. It is used by doctors to diagnose
problems with the digestive system. Barium hydroxide, Ba(OH)2, is also a white crystalline solid and is used in
wastewater treatment.
How many more oxygen atoms are represented in the formula for barium sulfate than in the formula for barium
hydroxide?
Answers
Answer: sawcon
Explanation:
the answer is sawcon
How many molecules of water are there in 1. 222 grams of water
Answers
1.22 grams of water has 5.0 x10^22 H2O water molecules.
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds. There is no guarantee that the term will include ions that meet this criterion, depending on the context.
Calculate the number of moles of water = mass / molar mass of water
Moles = 1.2 g/ 18 g/mol = 0.083 mol H2O
1 mole of any substance = 6.02 x 10^23 H2O molecules (Avogadro's number)
Resolution:
Molecules = 0.083 moles (6.02 x 10^23 H2O molecules/mole)
= 0.5 x 10^23 or 5.0 x10^22 H2O molecules
answer:
Number of molecules = 5.0 x 10^22 H2O molecules
Learn more about Molecules
brainly.com/question/14130817
#SPJ4