Answers
Answer:
The Law of Conservation of Mass dates from Antoine Lavoisier's 1789 discovery that mass is neither created nor destroyed in chemical reactions. In other words, the mass of any one element at the beginning of a reaction will equal the mass of that element at the end of the reaction.
Explanation:
Answer:
The law of conservation of mass states that mass cannot be created nor destroyed.
Explanation:
In terms of chemical equations, in a basic reaction, the amount of mass produced in the products must be the same as the amount of mass of the inital reactions. Therefore, we must balance chemical equations in order to fulfill law of conservation of mass. For example, if H2 + O2 -> H2O, you must add a 2 coefficient to balance the equation in order for the equation to obey the law of conservation of mass. Therefore, it must be 2H2 + O2 -> 2H2O. 4 hydrogens and 2 oxygens go in, and 2 water molecules come out (4 hydrogens, 2 oxygens).
Related Questions
3. The area indicated by the blue arrow is a stream. In which direction does this stream flow?
Explain.

Answers
The viscosity, which affects the amount of friction between water molecules, also plays a role. Despite the low viscosity of water, friction is still a problem. All moving fluids constantly lose energy as a result of rubbing against their surroundings. Water will move from high-energy regions to low-energy ones.
When it comes to confined aquifers, the situation becomes much more complicated, but we still need to understand how they function because they are significant water sources. demonstrates that even if the geological materials at the surface have very low permeability, there is always a water table. This aquifer will have its own "water table," which is actually called a potentiometric surface because it is a measure of the total potential energy of the water, wherever there is a confined aquifer, which is one that is separated from the surface by a confining layer. The potentiometric surface for the confined aquifer as a red dashed line. It represents the total energy that the water is under. inside the restricted aquifer. The water will rise to the water table if we drill a well into the unconfined aquifer. But if we drill a well into the confined aquifer through both the confining layer and the unconfined aquifer, the water will rise to the level of the potentiometric surface above the top of the confined aquifer. Due to the fact that the water rises above the aquifer's surface, this is referred to as an artesian well.
Learn more about potentiometric from here;
https://brainly.com/question/28033256
#SPJ1
Calculate the percent mass/mass of a solution that contains 3 g of circles and 75 g of distilled water.
Answers
answer and explanation
the percent mass %m/m of a solution is the mass of the solute divided by the mass of the solution x 100
therefore
%m/m = 3 g/ 78g x 100
= 3.85%
The chromatogram shows fluorescent peak data from a dye-terminating nucleotide sequencing reaction. The peaks are shown with shortest fragment on the left to longer fragments on the right. Select the DNA sequence that matches the data. O S-TGAAGCATTCATAT-3 O S-ACTTOGTAAGTATA-3 O S-ATATGAATGCTTCA-3 O S-TATACTTACGAAGT-3 O S-GTCCTACGGACGCG-3'
Answers
5'-GTCCTACGGACGCG-3' is the DNA sequence that matches the data given by shortest fragment on the left to longer fragments on the right.
What is chromatogram?Chromatography is a laboratory method used in chemical analysis to separate a mixture into its constituents. The combination is dissolved in a fluid solvent known as the mobile phase, which transports it through a system containing a substance known as the stationary phase. A chromatogram is the result of a chromatography run. It is a hardcopy or electronic file comprising the information generated during the chromatography run. Chromatography works on the premise of smearing molecules in a mixture onto a solid or surface, and a stable phase (fluid stationary phase) separates the components of a mixture while working with a mobile phase.
Here,
The DNA sequence 5'-GTCCTACGGACGCG-3' corresponds to the data provided by the smallest fragment on the left to the larger segments on the right.
To know more about chromatogram,
https://brainly.com/question/30086631
#SPJ4
Look at the attachment below.

Answers
Sally is wrong because copper is less electropositive than hydrogen, thus, can not displace hydrogen from dilute acids.
The reactions to prepare copper (ii) chloride are:
the chlorination of copper sulfide at a high temperature
reaction of copper (ii) oxide with dilute hydrochloric acid
The equations of the given reactions are as follows:CuS + Cl₂ ---> CuCl₂ + SCuO + 2HCl ----> CuCl₂ + H₂O
What are reactive metals?Reactive metals are metals that readily give up their electrons to form positive ions.
Reactive metals displace hydrogen from dilute acids. They are found in group 1A and 2A of the periodic table. Copper is not a reactive metal and will not displace hydrogen from acids.
Learn more about reactive metals at: https://brainly.com/question/20273277
#SPJ1
Sally is wrong because copper chloride is not made from the reaction of copper and dilute hydrochloric acid.
2. Copper (ii) chloride can be prepared as follows:
reacting copper (ii) oxide with dilute hydrochloric acidsingle replacement reaction of copper sulfide and chlorine gas at a high temperature3. the equations of the reaction are:
CuO + 2HCl ----> CuCl₂ + H₂OCuS + Cl₂ ---> CuCl₂ + SWhat are single replacement reactions?Single replacement reactions are reactions in which a more reactive atom replaces another atom in a compound.
An example of a single replacement reaction is the reaction of chlorine gas with copper sulfide at high temperatures to form copper chloride.
Learn more about single replacement reaction at: https://brainly.com/question/20216315
#SPJ1
Describe how to prepare 400 grams of a 15% (mass/mass) aqueous solution of KBr.
Answers
Dissolve 60g of potassium bromide in 340g of water to produce 15% (mass/mass) aqueous solution of potassium bromide.
Here we have to prepare a total of 400 g of solution. Aqueous solution means the solvent we use here is water.
So to prepare 400 g of 15% aqueous solution of potassium bromide, we need to find out how many grams of potassium bromide need to be dissolved in water and how many grams of water must be used.
Here the weight percent is given, that is 15%
15/100 = weight of potassium bromide/ 400 g
0 .15 = weight of potassium bromide / 400
weight of potassium bromide needed = 0.15 × 400
= 60 g
So, we calculated the required amount of potassium bromide as 60 grams. The total weight of the solution to be made is 400 grams.
So amount of water required = 400 - 60
= 340 g
So we need to mix 60 grams of potassium bromide in 340 grams of water to get a 15% (mass/mass) aqueous solution.
For further information about preparing aqueous solutions, please refer
https://brainly.com/question/13684060
What is the percent composition of Fluorine (F) in the compound XeF6?
Od
26.258%
12.520%
110.76%
46.472%
Answers
The percent by mass of the fluorine in the compound is 46.472%.
What is the percent by mass?We know that the percent by mass has to do with the ratio of the total mass of the atom that is part of the compound and the total molar mass of the compound multiplied by one hundred.
The question in this case has demanded that we ought to obtain the mass percent of fluorine from the compound that we can be able to identify from the formula of the compound that is shown here as xenon hexa fluoride.
Mass of the compound can be obtained from; 131 + 6(19)
= 245 g/mol
The total mass of the fluorine atom in the compound is 114 g
Thus we have the use of; 114 /245 * 100/1
= 46.472%
The percent by mass is now gotten for the fluorine atom as 46.472%.
Learn more about percent by mass:https://brainly.com/question/5394922
#SPJ1
I have the smallest atomic number of all the
elements that have electrons in three different
energy levels. Who am I?
Answers
The element with the smallest atomic number is hydrogen
What is atomic number?
A chemical element's atomic number is its position in the periodic system, which places the elements in ascending order of the number of protons in their nuclei. As a result, the atomic number is also determined by the number of protons, which is always equal to the number of electrons in a neutral atom.
The charge number of an atomic nucleus is the chemical element's atomic number, also known as nuclear charge number. This is the number of protons present in the nucleus of each atom of that element, or the proton number, for conventional nuclei. The atomic number of common chemical elements can be used to uniquely identify certain elements.
To learn more about atom number refer to:
https://brainly.com/question/621740
#SPJ2
In which list are the three compounds above correctly listed in order of increasing boiling point? A) lowest b.p.... isopropanol < isobutane < acetone ...highest b.p. B) lowest b.p.... isobutane < acetone < isopropanol ...highest b.p. C) lowest b.p.... isobutane < isopropanol < acetone ...highest b.p. D) lowest b.p.... acetone < isobutane < isopropanol ...highest b.p. E) lowest b.p.... acetone < isopropanol < isobutane ...highest b.p.
Answers
Answer:
The correct answer is - option B - lowest b.p.... isobutane < acetone < isopropanol ...highest b.p.
Explanation:
Isobutane has lowest boiling point due to no hydrogen bonding and no diole to dipole interaction found in them. Isobutane only shows weak dispersion force.
Acetone has dipole dipole interaction but due to lack of Hydrogen bonding they have low boiling point than isopropanol but higher than isobutanol.
Isopropanol is the compound that has ability to form hydrogen bonding with other molecule its boiling point is maximum among all three.
Thus, the correct answer is - option B - lowest b.p.... isobutane < acetone < isopropanol ...highest b.p.
The police found 488.43 grams of chlorate of
potassium (KCIO) in the room. To kill someone with the
mixture, 420.45g of potassium chloride would be required.
Is there enough potassium chloride to
that Melanie killed the butler?
KCIO3 > KCI + O2?
Answers
The formed amount of KCl from the reaction is 297.12 g which is not enough to kill someone.
What is the limiting reagent?A limiting reagent can be defined as a reactant that is exhausted completely from the mixture at the completion of a chemical reaction.
When quantities of reactant are not taken in stoichiometry, the limiting reactants in the chemical reaction will decide the maximum amount of product.
Given, a balanced equation of the decomposition of KClO₃ is:
\(KClO_3\longrightarrow KCl +O_2\)
One mole of KClO₃ will produce one mole of KCl.
122.55 g of the KClO₃ will produce KCl = 74.55 g
Given, the amount of KClO₃ = 488.43 g
Then 488.43 g of KClO₃ will produce KCl = (74.55/122.55) × 488.43
= 297.12 grams
As given 420.45g of potassium chloride would be required to kill someone. Therefore, there is not enough potassium chloride that Melanie killed the butler.
Learn more about limiting reagents, here:
brainly.com/question/26905271
#SPJ1
1. The equation for the reaction between zinc and aqueous copper(II) sulfate is shown.
Zn + Cuso, → ZnSO, + Cu
Which statement is correct?
a. The oxidation state of the oxidising agent has changed from 0 to +2.
b. The oxidation state of the reducing agent has changed from 0 to +2.
c. The oxidation state of the reducing agent has changed from +2 to 0
d. This is not a redox reaction. The solution changes from colourless to blue.

Answers
Answer:
The oxidation state of the reducing agent has changed from 0 to +2.
Explanation:
reducing agent is anything that loses electron or gains oxygen
in this case, zinc
how may liters are in 0.8291moles of hexane (c6h14)?
Answers
In 0.8291 moles of hexane (\(C_6H_1_4\)) there are 20.8 liters in 0.8291 moles of hexane at room temperature and atmospheric pressure.
To determine the number of liters in 0.8291 moles of hexane (C6H14), we need to use the ideal gas law equation:
PV = nRT
where:
P = pressure (atm)
V = volume (L)
n = moles of gas
R = gas constant (0.0821 L*atm/mol*K)
T = temperature (K)
We need to rearrange this equation to solve for V:
V = nRT/P
First, we need to calculate the number of moles of hexane:
n = mass/molar mass
The molar mass of hexane (C6H14) is:
6(12.01 g/mol) + 14(1.01 g/mol) = 86.18 g/mol
n = 0.8291 moles
Next, we need to convert the temperature to Kelvin. Assuming room temperature (25°C or 298 K):
T = 298 K
Finally, we need to assume a pressure value. Let's assume atmospheric pressure (1 atm).
P = 1 atm
Now we can plug in the values and solve for V:
V = (0.8291 mol)(0.0821 L*atm/mol*K)(298 K)/(1 atm)
V = 20.8 L
Therefore, there are 20.8 liters in 0.8291 moles of hexane at room temperature and atmospheric pressure.
For more details regarding ideal gas law, visit:
https://brainly.com/question/28257995
#SPJ1
Show the complete ionic equation and net ionic equation for all the equations below, then state whether or not a precipitate (insoluble compound) will form. To receive full credit, you must show ALL your work.
Cacl2(aq) + K2co3(aq) + -------->
Bacl2(aq) + MgSO4(aq) + -------->
AgNO3(aq) + Kl(aq) →
Nacl(aq) + (NH4)2Cro4(aq) →
Answers
Answer:
(a): Precipitate of calcium carbonate will form.
(b): Precipitate of barium sulfate will form.
(c): Precipitate of silver iodide will form.
(d): Precipitate of sodium chromate will form.
Explanation:
Complete ionic equation is defined as the equation in which all the substances that are strong electrolytes present in an aqueous state and are represented in the form of ions.
Net ionic equation is defined as the equations in which spectator ions are not included.
Spectator ions are the ones that are present equally on the reactant and product sides. They do not participate in the reaction.
(a):
The balanced molecular equation is:
\(CaCl_2(aq)+K_2CO_3(aq)\rightarrow 2KCl(aq)+CaCO_3(s)\)
The complete ionic equation follows:
\(Ca^{2+}(aq)+2Cl^-(aq)+2K^+(aq)+CO_3^{2-}(aq)\rightarrow 2K^+(aq)+2Cl^-(aq)+CaCO_3(s)\)
As potassium and chloride ions are present on both sides of the reaction. Thus, they are considered spectator ions.
The net ionic equation follows:
\(Ca^{2+}(aq)+CO_3^{2-}(aq)\rightarrow CaCO_3(s)\)
Precipitate of calcium carbonate will form.
(b)
The balanced molecular equation is:
\(BaCl_2(aq)+MgSO_4(aq)\rightarrow MgCl_2(aq)+BaSO_4(s)\)
The complete ionic equation follows:
\(Ba^{2+}(aq)+2Cl^-(aq)+Mg^{2+}(aq)+SO_4^{2-}(aq)\rightarrow Mg^{2+}(aq)+2Cl^-(aq)+BaSO_4(s)\)
As magnesium and chloride ions are present on both sides of the reaction. Thus, they are considered spectator ions.
The net ionic equation follows:
\(Ba^{2+}(aq)+SO_4^{2-}(aq)\rightarrow BaSO_4(s)\)
Precipitate of barium sulfate will form.
(c):
The balanced molecular equation is:
\(AgNO_3(aq)+KI(aq)\rightarrow KNO_3(aq)+AgI(s)\)
The complete ionic equation follows:
\(Ag^{+}(aq)+NO_3^-(aq)+K^+(aq)+I^{-}(aq)\rightarrow K^+(aq)+NO_3^-(aq)+AgI(s)\)
As potassium and nitrate ions are present on both sides of the reaction. Thus, they are considered spectator ions.
The net ionic equation follows:
\(Ag^{+}(aq)+I^{-}(aq)\rightarrow AgI(s)\)
Precipitate of silver iodide will form.
(d):
The balanced molecular equation is:
\(2NaCl(aq)+(NH_4)_2CrO_4(aq)\rightarrow 2NH_4Cl(aq)+Na_2CrO_4(s)\)
The complete ionic equation follows:
\(2Na^{+}(aq)+2Cl^-(aq)+2NH_4^+(aq)+CrO_4^{2-}(aq)\rightarrow 2NH_4^+(aq)+2Cl^-(aq)+Na_2CrO_4(s)\)
As ammonium and chloride ions are present on both sides of the reaction. Thus, they are considered spectator ions.
The net ionic equation follows:
\(2Na^{+}(aq)+CrO_4^{2-}(aq)\rightarrow Na_2CrO_4(s)\)
Precipitate of sodium chromate will form.
A stock solution is 2.5 M K2Cr2O7.How many cm3 of this solution you need to dilute to make 50cm3 of 0.05M K2Cr2O7
Answers
The volume needed to dilute a stock solution diluted to make 50cm3 of 0.05M K2Cr2O7 is 1cm³.
How to calculate volume?The volume needed to dilute a solution can be calculated by using the following formula:
M1V1 = M2V2
Where;
M1 = initial molarityM2 = final molarityV1 = initial volumeV2 = final volumeAccording to this question, a stock solution needs to be diluted to make 50cm3 of 0.05M K2Cr2O7. The volume needed can be calculated as follows:
2.5 × V1 = 50 × 0.05
2.5V1 = 2.5
V1 = 2.5/2.5
V1 = 1cm³
Therefore, the volume needed to dilute a stock solution diluted to make 50cm3 of 0.05M K2Cr2O7 is 1cm³.
Learn more about volume at: https://brainly.com/question/27304546
#SPJ1
In using the Haber process in the formation of ammonia, what mass of hydrogen is needed to produce 51.0 grams of ammonia? 3 H₂(g) + N2 (g) → 2 NH3(g).
Answers
The mass of hydrogen needed to produce 51.0 grams of ammonia is ≈ 9.07 grams.
To determine the mass of hydrogen required to produce 51.0 grams of ammonia (NH3) using the Haber process, we need to calculate the stoichiometric ratio between hydrogen and ammonia.
From the balanced chemical equation:
3 H₂(g) + N₂(g) → 2 NH₃(g)
We can see that for every 3 moles of hydrogen (H₂), we obtain 2 moles of ammonia (NH₃).
First, we need to convert the given mass of ammonia (51.0 grams) to moles. The molar mass of NH₃ is 17.03 g/mol.
Number of moles of NH₃ = Mass / Molar mass
= 51.0 g / 17.03 g/mol
≈ 2.995 moles
Next, using the stoichiometric ratio, we can calculate the moles of hydrogen required.
Moles of H₂ = (Moles of NH₃ × Coefficient of H₂) / Coefficient of NH₃
= (2.995 moles × 3) / 2
≈ 4.493 moles
Finally, we can convert the moles of hydrogen to mass using the molar mass of hydrogen (2.02 g/mol).
Mass of H₂ = Moles × Molar mass
= 4.493 moles × 2.02 g/mol
≈ 9.07 grams
Therefore, approximately 9.07 grams of hydrogen is needed to produce 51.0 grams of ammonia in the Haber process.
Know more about the mass of hydrogen here:
https://brainly.com/question/14083730
#SPJ8
2.59 Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (a) Ga and F, (b) Li and H, (c) Al and I, (d) K and S.
Answers
Answer:
(a) GaF3, gallium(III) fluoride
(b) LiH, lithium hydride
(c) AlI3, aluminum(III) iodide
(d) K2S, potassium sulfide
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
Which element is most likely to form a negative 2 ion? a Oxygen b Magnesium c Beryllium d Nitrogen
Answers
Magnesium is 2 plus and beryllium 2 plus too and nitrogen is 3 minus.
Answer:
a
Explanation:
The number of moles in 4.27×10^22 particles of silver is
Answers
Answer:
\(mol=0.0709mol\)
Explanation:
Hello,
In this since one mole equals 6.022x10²³ particles of silver by means of the Avogadro's number, we can compute the moles in 4.27x10²² particles as shown below:
\(mol=4.27x10^{22}particles*\frac{1mol}{6.022x10^{23}particles}\\\\mol=0.0709mol\)
Best regards.
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers
Answer:
Volume of the gass will decrease by three times of the original volume
Explanation:
Volume is inversly propotional to the pressure applied on it.
Answer:
it is decreased to one third of its original volume
Explanation:
How much heat is released when 245 g of steam cools from 106.0°C to -4.5°C
Answers
ANSWER
The amount of heat released is -54, 957.175J
EXPLANATION
Given that;
\(\begin{gathered} \text{ The mass of the steam is 245g} \\ \text{ The final temperature of the steam is -4.5}\degree C \\ \text{ The initial temperature of the steam is 106.0}\degree C \end{gathered}\)To find the amount of energy released, then apply the below formula
\(\text{ q = mc \lparen}\theta2\text{ - }\theta1)\)Recall, that the specific heat capacity of steam (c) is 2.03 J/g degrees Celcius
\(\begin{gathered} \text{ q = 245 }\times\text{ 2.03 \lparen-4.5 - 106\rparen} \\ \text{ q = 245 }\times\text{ 2.03 \lparen -110.5\rparen} \\ \text{ q = -54,957.175J} \end{gathered}\)Therefore, the amount of heat released is -54, 957.175J
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia.
N2(g)+3H2(g)⟶2NH3(g)
Assume 0.290 mol N2 and 0.954 mol H2 are present initially.
After complete reaction, how many moles of ammonia are produced?
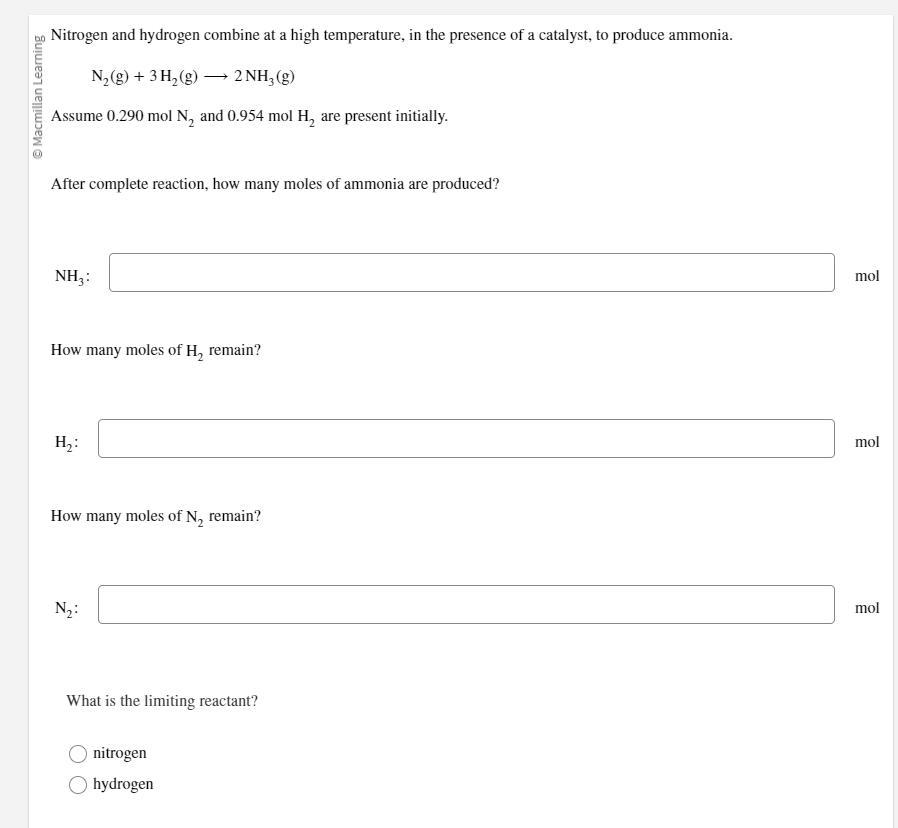
Answers
Answer:
17 reactions
Explanation:
1s2 2s2 2p6 3s2 3p6 4s2 3d 10 4p6 5s2 4d7 element electron configuration?
Answers
Answer:
antimony
Explanation:
The representation of the electrons in the shell is called electronic configuration.
The correct answer is antimony
The shell is as follows:-
SPDFThese are the shells in which electrons are filled.
The total number of electrons is 2+2+6+2+6+2+10+6+2+7= 45
After calculating the electrons, the total number of electrons is 45 which is the atomic number of antimony.
Hence, antimony is the correct option.
For more information, refer to the link:-
https://brainly.com/question/15804584
What is the percent yield for the reaction below when 364 g SO2 and 42.0 g
02 produce 408 g SO3?
2SO₂(g) + O₂(g) → 2SO3(g)
A. 89.7%
B. 97.1%
C. 51.5%
D. 100%
Answers
C. 51.5% is the percent yield for the reaction below when 364 g SO2 and 42.0 g O2 produce 408 g SO3
How is percentage yield calculated?The actual yield is determined by calculating the quantity of the product created. We can estimate the percentage yield by dividing the actual yield by the theoretical value. The percentage yield is the difference between the quantity of product that was actually created and the maximum calculated yield.
2SO₂(g) + O₂(g) → 2SO3(g)
SO₂ mass => 364g
O₂ mass => 42g
SO3 mass => 408g
Theoretical yield of SO3:
mole of O₂ => 42g/32g => 1.31
according to the chemical equation:
1 mole of oxygen => 2 moles of SO3
1.31 mole O2 => x moles of SO3
x=> 2 x 1.31 => 2.62
Hence, the mass of SO3 => 2.62 x 80.06 => 209.75g
The percentage of yield => Actual yield / theoretical yield x 100
=> 209.75/408 x 100 => 51.45 => 51.5%
What differs the theoretical yield from the actual yield?The theoretical yield is the yield that is derived using a balanced chemical reaction. What you actually acquire from a chemical reaction is the actual yield.
Learn more about percentage yield here:
brainly.com/question/29767432
#SPJ1
g A chemist combines 59.9 mL of 0.282 M potassium bromide with 15.4 mL of 0.512 M silver nitrate. (a) How many grams of silver bromide will precipitate
Answers
Answer:
\(m_{AgBr}=1.48gAgBr\)
Explanation:
Hello,
In this case, the undergoing chemical reaction is:
\(KBr(aq)+AgNO_3(aq)\rightarrow AgBr(s)+KNO_3(aq)\)
Thus, since the potassium bromide and silver nitrate are in a 1:1 mole ratio, the first step is to identify the limiting reactant, by considering the reacting volumes of reactants in order to compute the available moles of potassium bromide and the moles of potassium bromide consumed by the 15.4 mL of 0.512-M solution of silver nitrate:
\(n_{KBr}=0.0599L*0.282\frac{molKBr}{L} =0.0169molKBr\\\\n_{KBr}^{consumed}=0.0154L*0.512\frac{molAgNO_3}{L} *\frac{1molKBr}{1molAgNO_3}=0.00788molKBr\)
In such a way, since less moles are consumed than available, we infer that silver nitrate is the limiting reactant, for which the resulting grams of silver bromide (molar mass 187.8 g/mol) result:
\(m_{AgBr}=0.00788molAgNO_3*\frac{1molAgBr}{1molAgNO_3} *\frac{187.8gAgBr}{1molAgBr} \\\\m_{AgBr}=1.48gAgBr\)
Best regards.
2. 4.6gof X is burnt completelyto produce 6.2g of X oxide (X,O). M (0) = 16 gmol ¹. Calculate the amount of oxygen that reacted in this experiment. [2 MARKS]
[ii] calculate the mass of 1 mole of x.[2mark]
[iii] predict and give a reason explaining the reaction of x2o in water.[1mark]
Answers
As per the given data, 1.6 grams of oxygen reacted in this experiment.
To calculate the amount of oxygen that reacted in the experiment, we need to determine the difference in the mass of X oxide (X,O) formed and the mass of X initially used.
Given:
Mass of X = 4.6 g
Mass of X oxide (X,O) = 6.2 g
To find the amount of oxygen that reacted:
Mass of oxygen = Mass of X oxide - Mass of X
= 6.2 g - 4.6 g
= 1.6 g
Therefore, 1.6 grams of oxygen reacted in this experiment.
Calculate the mass of 1 mole of X:
Given that the mass of X is 4.6 g, we can calculate the molar mass of X by dividing the mass by the number of moles:
Molar mass of X = Mass of X / Number of moles of X
Molar mass of X = 4.6 g / 0.1 mol
Molar mass of X = 46 g/mol
Therefore, the mass of 1 mole of X is 46 grams.
Thus, the answer is 46 grams.
For more details regarding moles, visit:
https://brainly.com/question/30885025
#SPJ1
Velocity is
is a given
O distance, time
O speed, displacement
O speed, direction
Answers
Write the electronic configuration of the following elements and specify their number of Valence electrons. 1H^+; 17Cl^-; 25Mn^2+; 29Cu^+
Answers
29 Cu⁺. We have to place 29 electrons and then we will substract 1 electron because of the positive charge.
The electron configuration of Cu should be:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁹
2 + 2 + 6 + 2 + 6 + 2 + 9 = 29
Then Copper has one anomalous thing. The energy difference between the 3d and 4s orbitals is very low. It's an exception, but one electron jumps from the subshell 4s to the subshell 3d. In that way it has the subshell 3d that is lower in energy filled, and it is more stable. So the electron configuration of 29 Cu is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s¹
To write the electron configuration of 29 Cu+ we take off one electron from the 4s orbital. S
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰
It has 18 valence electrons (3s² 3p⁶ 3d¹⁰).
What is the molar mass of CH4?(C = 12.01 g/mol, H = 1.008 g/mol)A. 1 g/molB. 4 g/molC. 13.02 g/molD. 16.04 g/molEnterYea

Answers
Answer:
\(D\text{ : 16.04 g/mol}\)Explanation:
Here, we want to get the molar mass of the given molecule
To get this, we have to add up the atomic mass unit of the individual constituent elements, using the multiples where necessary
Thus, we have it that:
\(\text{ 12.01 + 4(1.008) = 12.01 + 4.032 = 16.042 g/mol}\)How many moles of
Cts are needed to make
15.5 moles of CO₂? How
much O2 will be needed?
0₂
C₂Hs +50₂3CO₂ + 4H₂O
Answers
1. The number of mole of C₃H₈ needed to make 15.5 moles of CO₂ is 5.2 moles
2. The number of moles of O₂ needed is 25.8 moles
1. How do I determine the number of mole of C₃H₈ needed?The number of mole of C₃H₈ needed can be obtained as illustrasted below:
C₃H₈ + 5O₂ -> 3CO₂ + 4H₂O
From the balanced equation above,
3 moles of CO₂ was obtained from 1 mole of C₃H₈
Therefore,
15.5 moles of CO₂ will be obtain from = (15.5 × 1) / 3 = 5.2 moles of C₃H₈
Thus, number of mole of C₃H₈ needed is 5.2 moles
2. How do I determine the number of mole of O₂ needed?We can obtain the number of mole of O₂ needed as follow:
C₃H₈ + 5O₂ -> 3CO₂ + 4H₂O
From the balanced equation above,
3 moles of CO₂ was obtained from 5 moles of O₂
Therefore,
15.5 moles of CO₂ will be obtain from = (15.5 × 5) / 3 = 25.8 moles of O₂
Thus, number of mole of O₂ needed is 25.8 moles
Learn more about number of mole:
https://brainly.com/question/23350512
#SPJ1
Complete question:
How many moles of C₃H₈ are needed to make
15.5 moles of CO₂? How much O₂ will be needed?
C₃H₈ + 5O₂ -> 3CO₂ + 4H₂O
Some objects can store more thermal energy than others.
A. True
b. False
Answers
Answer:
A. True
Explanation:
Explanation:
The transfer of thermal energy can occur by conduction, convection, or radiation. Some materials can store more energy than others.