In Vancouver where the air pressure is 98kPa, a child is given a 2.7 L ballon. The family then drives to Banff which is 1386 m above sea level and the air pressure is only 85 kPa. If the temperature is the same in both places, what is the volume of the baloon in Banff?.
Answers
The volume of the balloon in Banff is 3.113 liters.
According to the equation of state for ideal gases, the pressure (\(P\)), in kilopascals, is inversely proportional to the volume (\(V\)), in liters. The final volume of the balloon is found by the following relationship:
\(V_{2} = \frac{P_{1}\cdot V_{1}}{P_{2}}\) (1)
Where:
\(V_{1}\) - Initial volume, in liters.\(V_{2}\) - Final volume, in liters.\(P_{1}\) - Initial pressure, in kilopascals.\(P_{2}\) - Final pressure, in kilopascals.If we know that \(P_{1} = 98\,kPa\), \(P_{2} = 85\,kPa\) and \(V_{1} = 2.7\,L\), then the final volume of the balloon is:
\(V_{2} = 2.7\,L\times \frac{98\,kPa}{85\,kPa}\)
\(V_{2} = 3.113\,L\)
The volume of the balloon in Banff is 3.113 liters.
We kindly invite to check this question on ideal gases: https://brainly.com/question/16211117
Related Questions
identify the compound in the following group that is most soluble in water. pentanoic acid, hexane, 1-octanol
Answers
a. Butanone is the most soluble in water because it can form hydrogen bonds with water molecules due to its carbonyl group.
b. Ethanoic acid (acetic acid) is the most soluble in water because it can form hydrogen bonds with water molecules and it also has a small hydrophobic tail.
a. Butanone is the compound that is most soluble in water among the group of butanone, butanoic acid, and butane. This is because butanone is a polar compound with a dipole moment, which allows it to form hydrogen bonds with water molecules, resulting in better solubility in water. Butanoic acid, on the other hand, is a weak acid and can form hydrogen bonds with water molecules through its -COOH functional group, but the hydrophobic hydrocarbon tail makes it less soluble in water than butanone.
b. Ethanoic acid (acetic acid) is the compound that is most soluble in water among the group of ethanoic acid, hexanoic acid, and octanoic acid. Ethanoic acid is a polar compound with a hydrogen bond-donating -OH group, which can form hydrogen bonds with water molecules, resulting in better solubility in water. Hexanoic acid and octanoic acid are larger and have longer hydrocarbon tails, which makes them less soluble in water than ethanoic acid.
To learn about hydrogen bonds
https://brainly.com/question/30885458
#SPJ4
Full Question: Identify the compound in each group that is most soluble in water. Explain.
a. butanone, butanoic acid, butane
b. ethanoic acid (acetic acid), hexanoic acid, octanoic acid
Select the correct answer. Clive finds that the water coming out of the faucet is a little muddy. He concludes that the color in the water is due to the mining activity in the nearby forest. Clive shares his conclusion with his science class. His classmates question his conclusion, but Clive has no data to back up his conclusion. Which step in the scientific investigation did he miss? A. observing B. communicating results C. testing the hypothesis D. creating a hypothesis
Answers
Answer:
A. observing
Explanation:
There are various steps that are involved in a scientific investigation. Observation is the first step of the scientific investigation. In this step, a close examination is laid on the investigation. Observation is collected with the senses and the information is gathered.
In the given excerpt, Clive missed the observation part of the scientific investigation. All the other three steps were followed by Clive. He was not able to observe the situation and so was not able to provide the answers to the questions asked by his classmates.
Glass allows______ into a greenhouse

Answers
Answer: Sunlight
Explanation:
According to Goog|e, "In addition to assisting in the development of heat within the structure, glass in greenhouses assists in the management of light within the greenhouse. The glass allows light to be transmitted from the sun to the plants within the structure, allowing the plants to grow properly."
I hope this helps!
In the illustration, which solute will dissolve first?
00.0
00.0
tanka
tank
Solute in Tanks A and B will dissolve at equal rates.
Solute in Tonk Awill dissolve first
Soluten Tork B wil dissolves

Answers
The first to dissolve is the solute in tank B. The amount of a substance that dissolves in a specific solvent concentration at a specific temperature is known as its solubility.
Which phase of the dissolving process is the first?The introduction of a solute to a solvent is the first stage in the dissolving process. The molecules of these two substances begin to interact, causing the solute molecules to disperse and become encircled by solvent molecules.
Which of the following three compounds dissolves in water?In water, salt, sugar, and coffee all dissolve. They disintegrate quickly. They typically dissolve more rapidly and completely in hot or warm water. Even very hot water won't be able to dissolve sand or pepper.
To know more about temperature visit:
https://brainly.com/question/14633960
#SPJ1
the process used to mine salts by filling shallow ponds with sea water is
Answers
The process used to mine salts by filling shallow ponds with seawater is known as solar salt production.
The process of mining salts by filling shallow ponds with seawater is called solar salt production. It is a method commonly used to extract salt from seawater on a large scale. The process takes advantage of the natural evaporation of water under the sun, leaving behind concentrated salt crystals that can be collected and processed.
The process begins by selecting suitable coastal areas or salt flats, typically in arid or semi-arid regions with access to the sea. These areas are usually flat and have low rainfall, facilitating the evaporation process. Shallow ponds or basins, also known as salt pans or evaporation ponds, are constructed to contain the seawater.
Seawater is then pumped into these ponds or is allowed to flow in naturally during high tide. The ponds are designed to maximize the exposure of seawater to sunlight and heat. The sun's energy drives the evaporation process, causing the water to gradually evaporate, leaving behind concentrated brine solutions.
Over time, as the water continues to evaporate, the salt concentration in the remaining brine increases. The concentrated brine, also known as bittern, becomes supersaturated with dissolved salts, including sodium chloride and other minerals. As the saturation point is reached, salt crystals begin to precipitate and form salt beds at the bottom of the ponds.
Learn more about evaporation here:
https://brainly.com/question/33571997
#SPJ11
Based on your knowledge of the periodic table, identify the element Y.

Answers
Answer: argon
Explanation:
using the last clue “avg atomic mass of 3.33x greater than carbon-12” we can deduce the atomic mass of the element is 12x3.33=40(2sf) hence its argon. we can further confirm from third clue that it is a noble gas hence unreactive
what is nacl compound name?
Answers
Answer:
Below
Explanation:
Sodium chloride also known as table salt or just 'salt'
Which of the following MOST directly influences a measurable outcome in an experiment? a. An independent variable b. A variable hypothesis c. A dependent variable d. Previous information
Answers
Answer:
A). An independent variable
Explanation:
The Independent variable is characterized itself as the variable that is controlled or manipulated by the experimenter to observe its direct impact on the dependent variable. It is a variable that is not dependent and can stand alone. For example, if a student aims to observe the impact of temperature on solubility of a substance(sugar/salt) in a liquid, the temperature will be the independent variable as any change in the temperature will directly affect the solubility power of the liquid. Thus, option A is the correct answer.
Answer:
D.
Explanation:
. what products would you expect from oxidation of the following compounds with cro3 in aqueous acid? with pyridinium chlorochromate (pcc, c5h6ncro3cl) in dichloromethane? (a) 1-hexanol (b) 2-hexanol (c) hexanal
Answers
The oxidation of a) 1-hexanol b) 2-hexano c) hexanal with CrO3 in aqueous acid gives 1-hexanoic acid, 2-hexanone and 1-hexanoic acid respectively. The oxidation with PCC gives 1-hexanaldehyde, 2-hexanone and no reaction respectively.
What is PCC?
PCC is also called as pyridinium chlorochromate which is a yellow-orange salt. this is primarily used for the oxidation of alcohols which gives carbonyl compounds as products. It is one of the highly effective oxidizing agents for alcohols.
The oxidation of alcohols with CrO3 gives carbonyl compounds like aldehydes and ketones. With primary alcohol and aldehydes it gives carboxylic acid, whereas with secondary alcohol it gives ketones as products. PCC with primary alcohols give aldehyde, with secondary alcohols give ketone and with aldehyde there is no reaction.
Therefore, CrO3 with 1-hexanol and hexanal gives 1-hexanoic acid, with 2-hexanol gives 2-hexanone. PCC with 1-hexanol gives 1-hexanaldehyde, 2-hexanol gives 2-hexanone and with hexanal there is no reaction.
To learn more about PCC click on the given link https://brainly.com/question/28385165
#SPJ4
imagine you have collected data on the temperature and dissolved oxygen in a eutrophic lake over five years. you want to see if dissolved oxygen varies with temperature in this lake. what statistical test would you do ? explain why you chose this test
Answers
We can make use of the correlation approach, T test, and linear regression techniques in this situation.
The two variables in this example of a eutrophic lake are temperature and dissolved oxygen, and both of these variables change over time. Therefore, a correlation test must be performed to see whether a relationship between these two variables exists. Here, the correlation coefficient value will be given to us, allowing us to calculate the linear relationship between these two variables.
Additionally, we can use the T test to determine whether or not the correlation between these two variables is strong enough to reject the null hypothesis.
Finally, we can also utilize the linear regression method to get a more accurate relationship between those two variables.
Learn more about Hypothesis here:
https://brainly.com/question/12687557
#SPJ4
If the AGº for ATP hydrolysis is -30 kJ/mol and the AG" for phosphoenolpyruvate hydrolysis is -62 kJ/mol, what is the AGº for the phosphorylation of ADP by phosphoenolpyruvate? -92 kJ/mol +31 kJ/mol +92 kJ/mol -62 kJ/mol -32 kJ/mol
Answers
The AGº for the phosphorylation of ADP by phosphoenolpyruvate is equal to 32 kJ/mol.
The AGº for the phosphorylation of ADP by phosphoenolpyruvate can be calculated using the equation:
AGº = AGº (ATP hydrolysis) - AGº (phosphoenolpyruvate hydrolysis)
Given that AGº (ATP hydrolysis) = -30 kJ/mol and AGº (phosphoenolpyruvate hydrolysis) = -62 kJ/mol, then:
AGº = -30 kJ/mol - (-62 kJ/mol) = 32 kJ/mol
NADH: An increase in the concentration of NADH can inhibit PDH, as it competes with pyruvate for binding to the active site of the enzyme.
Acetyl-CoA: An increase in the concentration of acetyl-CoA can also inhibit PDH, as it acts as an allosteric inhibitor
Therefore, the AGº for the phosphorylation of ADP by phosphoenolpyruvate is equal to 32 kJ/mol.
Learn more about concentration here
https://brainly.com/question/10725862
#SPJ4
A scientist has found a protein that is involved in an important chemical
reaction. She sets up two tests: one with the protein and one without the protein.
What should she measure to determine whether the protein is an
enzyme?
The amount of protein consumed
The rate of the chemical reaction
The amount of products bound to the active site
The temperature change of the reaction
D
Answers
To determine whether the protein is an enzyme, the scientist should primarily measure the rate of the chemical reaction. Option(b)
Enzymes are specialized proteins that act as catalysts, facilitating chemical reactions by lowering the activation energy required for the reaction to occur. By comparing the rate of the chemical reaction with and without the protein, the scientist can determine if the presence of the protein enhances the reaction rate. If the reaction proceeds at a significantly higher rate in the presence of the protein, it suggests that the protein is acting as an enzyme, catalyzing the reaction. Enzymes typically accelerate reaction rates by providing an alternative reaction pathway with a lower activation energy, allowing the reaction to occur more readily. While measuring the amount of protein consumed may provide information about the protein's involvement in the reaction, it does not directly determine whether the protein is an enzyme. Similarly, measuring the amount of products bound to the active site or the temperature change of the reaction can provide additional insights, but they alone are not sufficient to confirm enzymatic activity. The most reliable indicator to ascertain whether the protein is an enzyme is to compare the rate of the chemical reaction in the presence and absence of the protein. Option(b)
For such more questions on enzyme
https://brainly.com/question/18743961
#SPJ11
EXTRA POINTS.
WILL MARK BRAINIEST.
How many grams of AgCl are produced from 167 grams of AgNO3?
Answers
Answer:
1+2=5
Explanation:
1+2=5
The table shows the relative atomic masses of some common elements. Use this information to work out the relative formula mass of magnesium sulfate. The formula for magnesium sulfate is MgSO4.
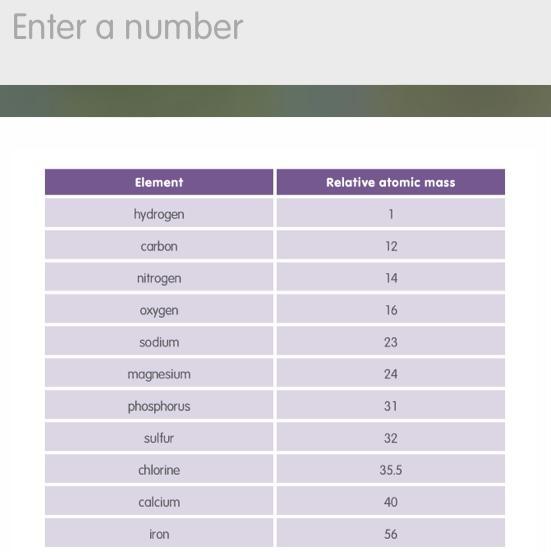
Answers
The relative atomic mass of Mg is 24.3, the relative atomic mass of S is 32.1, and the relative atomic mass of O is 16.0.
What is relative atomic mass?The relative atomic mass is a unit of measurement used in chemistry to express the mass of an atom of an element.It is defined as the ratio of the mass of an atom of an element to one twelfth of the mass of an atom of carbon-12.It is often used to compare the masses of atoms of different elements.The relative atomic mass is usually represented by the symbol "A" or "Ar" and is measured in atomic mass units (amu).It is important to note that the relative atomic mass is different from the atomic mass, which is the actual mass of an atom in grams.To calculate the relative formula mass of magnesium sulfate, we add up the relative atomic masses of each element in the formula:Mg = 24.3S = 32.14 x O = 4 x 16 = 64.0So, the relative formula mass of magnesium sulfate is 24.3 + 32.1 + 64.0 = 120.4.To learn more about relative atomic mass refer:
brainly.com/question/28882057
#SPJ1
84 g of a metal at an initial temperature of 72°C releases 553 J of heat. Calculate the final temperature of the metal if its specific heat capacity is 0.13 J/g•K.
Answers
84 g of a metal at an initial temperature of 72°C releases 553 J of heat, then the final temperature of the metal is 21.36°C if its specific heat capacity is 0.13 J/g•K.
To calculate the final temperature of the metal given its initial temperature of 72°C, the amount of energy released (553 J), and its specific heat capacity (0.13 J/g•K), we can use the following equation:
Q = mc∆T,
where Q is the amount of heat energy released,
m is the mass of the metal (84 g),
c is the specific heat capacity (0.13 J/g•K), and
∆T is the change in temperature. Solving for ∆T gives us:
∆T = Q/mc
∆T = \(\frac{553J}{84g\times0.13\frac{J}{gK}}\) = 50.641
Therefore, the final temperature of the metal is 72°C - 50.641 K = 21.36°C.
To learn more about heat capacity refer to: brainly.com/question/29965746
#SPJ11
When a 8 gram slice of bread is burned under a beaker of 4500 grams of water, it heats up the water by 1.3 degrees Celsius. The specific heat of water is 1 cal/g degree C. How many calories does this slice of bread contain
Answers
Answer:
\(5850\ \text{cal}\)
Explanation:
m = Mass of water = 4500 g
c = Specific heat of water = \(1\ \text{cal/g}^{\circ}\text{C}\)
\(\Delta T\) = Change in temperature of water = \(1.3^{\circ}\text{C}\)
Heat is given by
\(q=mc\Delta T\\\Rightarrow q=4500\times 1\times 1.3\\\Rightarrow q=5850\ \text{cal}\)
A slice of the bread contains \(5850\ \text{cal}\).
Justin and Abby need to carry out an experiment where they separate salt and water. They know they need water, a bunsen burner and flint striker, an evaporating dish, and salt. What other pieces of equipment might they need for this experiment? Choose 5.
Answers
Answer:
Beaker.Stirring rodGauzeFilter FunnelConical FlaskTripodSuppose an ideal gas undergoes isobaric (constant pressure) compression. Write an expression about the entropy of the environment.
Answers
The expression for the change in entropy of the environment (ΔS_env) during isobaric compression of an ideal gas can be given by ΔS_env = -ΔH / T, where ΔH is the enthalpy change of the gas and T is the temperature of the environment.
Entropy is a measure of the randomness or disorder of a system. In the case of an ideal gas undergoing isobaric compression, the pressure of the gas remains constant while it is being compressed. This means that the work done on the gas is being absorbed by the environment, which is usually assumed to be at a constant temperature.
According to the second law of thermodynamics, the change in entropy of a system is related to the heat transfer (ΔQ) and the temperature (T) of the surroundings. In this case, as the gas is being compressed, heat is being transferred to the environment, causing an enthalpy change (ΔH) in the gas. The negative sign in the expression for ΔS_env indicates that the entropy of the environment decreases during isobaric compression.
The expression ΔS_env = -ΔH / T shows that the change in entropy of the environment is proportional to the enthalpy change of the gas and inversely proportional to the temperature of the environment. This means that as the enthalpy change of the gas increases, the entropy change of the environment decreases, and vice versa.
Additionally, as the temperature of the environment increases, the entropy change of the environment decreases, indicating that heat transfer to a higher temperature environment results in a smaller change in entropy.
To know more about second law of thermodynamics refer here:
https://brainly.com/question/7206767#
#SPJ11
Atoms of two different elements must have different electrical charges. numbers of neutrons. atomic numbers. energy levels.
Answers
Answer:
atomic numbers
Explanation:
Atoms are the unit of molecules and compounds or matter. They have their atomic numbers and mass. Atoms of different elements must have different atomic numbers. Thus, option C is correct.
What is an atomic number?An atomic number is the total number of protons in a nucleus that is an essential factor in determining the place and position of the atom in the periodic chart.
Though the nucleus is made of neutrons and protons, the atomic number is only defined by the number of protons alone. Hence, the number of protons is equivalent to the atomic number.
The number of the protons and neutrons together makes the atomic mass of the element that is expressed at the lower of the symbol, whereas the atomic number is written at the upper of the symbol.
Therefore, the atomic number is different in each atom.
Learn more about atomic numbers here:
https://brainly.com/question/13464692
#SPJ6
select the substituents will direct the incoming group in the ortho/para- position during electrophilic aromatic substitution? question 13 options: -och3 -ch3 -cf3 -cl -cooch3
Answers
The substituents -OCH3, -CH3, and -COOCH3 will direct the incoming group in the ortho/para- positions during electrophilic aromatic substitution.
During electrophilic aromatic substitution, the presence of certain substituents on an aromatic ring can influence the position at which the incoming group attaches. Substituents that possess electron-donating effects or are capable of stabilizing positive charge on the ring can direct the incoming group to the ortho or para positions.
In this case, the substituents -OCH3 (methoxy), -CH3 (methyl), and -COOCH3 (methoxy carbonyl) have electron-donating effects. These substituents increase the electron density on the aromatic ring, making it more nucleophilic and susceptible to attack by electrophiles.
The presence of these electron-donating groups increases the likelihood of the incoming group attaching to the ortho or para positions relative to the substituent. The -CF3 (trifluoromethyl) and -Cl (chloro) substituents, on the other hand, have electron-withdrawing effects, which decrease the electron density on the ring and direct the incoming group to the meta position.
Therefore, in the context of ortho/para- directing groups during electrophilic aromatic substitution, the substituents -OCH3, -CH3, and -COOCH3 will direct the incoming group to the ortho or para positions.
Learn more about electrophilic aromatic substitution here:
https://brainly.com/question/30761476
#SPJ11
Which substance is a mixture? Table salt, gasoline, aluminum, or carbon dioxide.
Answers
Answer:
gasoline
Explanation:
mention one real life significance of the covalent bond.
Answers
Answer:
it helps in respiration
Help me i don’t know what to put here pls thank you!

Answers
How many moles is 6.88 x 10^-2 g silver chloride ?
Answers
Answer:hey
Explanation:
a mixture of ch4 (g) and c2h6 (g) has a total pressure of 0.53 atm. just enough o2 was added to the mixture to bring about it's complete combustion to co2 (g) and h2o (g). the total pressure of the two product gases is found to be 2.2 atm. assuming constant volume and temperature, find the mole fraction of ch4 in the original mixture.
Answers
the mole fraction of \(CH_4\) in the original mixture is 0.73 = 73%.
How do we calculate?Total pressure of the mixture = 0.53 atm
Total pressure of the product gases = 2.2 atm
The combustion equation for \(CH_4\) is given as :
\(CH_4\)(g) + \(2O_2\)(g) -> \(CO_2\)(g) + \(2H_2O\)(g)
We then apply Dalton's law of partial pressures, and write
Pressure of \(CO_2\) + Pressure of \(H_2O\) = Pressure of product
x + Pressure of \(H_2O\) = Pressure of product
x + Pressure of \(H_2O\) = 2.2 atm
the mole fraction and the partial pressure relationship is :
2x = Pressure of \(H_2O\)
x + 2x = 2.2 atm
3x = 2.2 atm
x = 2.2 atm / 3
x = 0.73
Learn more about mole fraction at;
https://brainly.com/question/29065092
#SPJ4
The table below shows the height of a ball x seconds after being kicked.
A 2-column table with 5 rows. The first column is labeled time (seconds) with entries 0, 0.5, 1, 1.5, 2, 2.5, 3. The second column is labeled height (feet) with entries 0, 35, 65, 85, 95, 100, 95.
What values, rounded to the nearest whole number, complete the quadratic regression equation that models the data?
f(x) =
x2 +
x + 0
Based on the regression equation and rounded to the nearest whole number, what is the estimated height after 0.25 seconds?
feet
Answers
Answer:
-16
81
19
Explanation:
The quadratic regression equation is \(y = -16x^2 +81x\), and the estimated height in 0.25 seconds is 19 feet
What are regression equations?Regression equations are used to determine the relationship between sets of data
The dataset is given as:
Time (seconds) Height (feet)
0 0
0.5 35
1 65
1.5 85
2 95
2.5 100
3 95
To determine the quadratic regression equation, we make use of a graphing calculator.
From the graphing calculator, we have the following calculation summary:
a = -16.429
b = 81.071
c = -0.357
A quadratic regression equation, is represented as:
\(y = ax^2 + bx + c\)
So, we have:
\(y = -16.429x^2 +81.071x -0.357\)
Approximate
\(y = -16x^2 +81x\)
The estimated height in 0.25 seconds is:
\(y = -16* 0.25^2 +81 * 0.25\)
\(y = 19.25\\\)
Approximate
\(y = 19\)
Hence, the estimated height in 0.25 seconds is 19 feet
Read more about quadratic regression at:
https://brainly.com/question/25794160
A scientist conducts experiments to test what temperature causes the fastest growth rate in sunflower plantsafter multiple trials the results show that sunflower plants grow the fastest at 75 FIs this experement an example of replication or repetition?
Answers
Answer:
Repetition
Explanation:
In the world of science, experiments are regularly conducted to test out hypotheses. These experiments are however, not conducted by scientist once to derive a result. They are rather conducted multiple times (repeated) to ascertain the accuracy i.e not as a result of random occurrence. The experiments that undergo multiple trials are said to undergo REPETITION.
This is the case of the scientist in this question, who is conducting an experiment to test what temperature causes the fastest growth rate in sunflower plants. He conducted the experiment multiple times, making it an example of REPETITION.
N.B: Replication is when the experiment is re-conducted by another scientist to see if same result is derived.
When iron oxide (Fe2O3) forms what type of bonding happens?
Answers
Answer:
It is represented by Fe2O3. The oxidation state of iron oxide is +3 and +2. The bond formed between iron and oxygen is due to the difference in electronegativity between the two atoms. Since iron is metal and oxygen is non-metal therefore the bonding between oxygen and iron is ionic.
Explanation:
the name of binary ionic cpd composed of thelithium cation and the chlorine anion is called
Answers
The name of binary ionic cpd composed of the lithium cation and the chlorine anion is called Lithium chloride (LiCl). In Chemistry, the lithium chloride is an ionic compound consisting of lithium and chlorine.
It has a white or colourless solid structure in the form of a crystal. The lithium chloride formula is LiCl. It is a compound that is highly soluble in water and its solutions conduct electricity. Lithium chloride is used as a desiccant, a flux for welding and soldering, and a starting material in the synthesis of various other lithium compounds.
It is also used as an important reagent in organic synthesis. It is also useful in the production of ceramics and in the manufacture of lithium-ion batteries. In conclusion, the name of the binary ionic cpd consisting of the lithium cation and chlorine anion is Lithium chloride (LiCl).
To know more about cation visit;-
https://brainly.com/question/28710898
#SPJ11
Can someone please describe the nature and variety of instruments used to detect radiation?
Answers
Answer:
The Answer is below :)
Explanation:
A variety of handheld and laboratoryinstruments is available for detectingand measuring radiation. ... Geiger Counter, with Geiger-Mueller (GM) Tube or Probe—A GM tube is a gas-filled device that, when a high voltage is applied, creates an electrical pulse when radiation interacts with the wall or gas in the tube.