In the reaction represented by the equation 2 Al2O3 --> 4 Al 3 O2, what is the correct mole ratio of aluminum to oxygen
Answers
For every 2 moles of Al₂O₃, we get 4 moles of Al and 3 moles of O₂. Therefore, the mole ratio of aluminum to oxygen is: Aluminum (Al): Oxygen (O) = 4:3
In the reaction represented by the equation:
2 Al₂O₃ → 4 Al + 3 O₂
The correct mole ratio of aluminum (Al) to oxygen (O) can be determined by comparing the coefficients in front of each compound. The coefficients represent the relative number of moles of each substance involved in the reaction.
From the equation, we can see that for every 2 moles of Al₂O₃, we get 4 moles of Al and 3 moles of O₂. Therefore, the mole ratio of aluminum to oxygen is:
Aluminum (Al): Oxygen (O) = 4:3
Learn more about mole ratio, here:
https://brainly.com/question/14425689
#SPJ4
Related Questions
A balanced chemical equation always obeys law of conservation of ___.
Answers
A balanced chemical equation always obeys law of conservation of mass.
A balanced chemical equation generally obeys to law of conservation of mass. As per this regulation the mass of items should be equivalent to the mass of reactants. A response is supposed to be adjusted when the complete mass of particles on the two sides of the substance response is equal.According to the law of conservation of mass, the mass of the items in a synthetic response should rise to the mass of the reactants.
The law of conservation of mass is helpful for various computations and can be utilized to tackle for obscure masses, such how much gas consumed or delivered during a reaction.They should submit to the Law of conservation of Mass that expresses that matter can't be made or obliterated, it is saved. The mass of the reactants should rise to the mass of the products.Thus, the mass of substances created in a synthetic response is consistently equivalent to the mass of responding substances. Consequently, you really want to have a similar number of each kind of component on each side of a synthetic condition. This is the entire reason for adjusting a synthetic condition.
To know more about balanced chemical equation,visit here:
https://brainly.com/question/28294176
#SPJ4
The diagram shows electrolysis of molten KCl. When the switch is closed: (a) Positive ions move to the anode and gain electrons (b) Positive ions move to the anode and lose clectrons (c) Positive ions move to the cathode and gain electrons (d) Positive ions move to the cathode and lose electrons
Answers
When the switch is closed during the electrolysis of molten KCl, the correct statement is: (c) Positive ions move to the cathode and gain electrons.
During electrolysis, the cathode attracts positively charged ions (cations) from the electrolyte solution. These cations, in this case, positive potassium ions (K+), migrate towards the cathode. At the cathode, they gain electrons, which allows them to be reduced and form neutral potassium atoms. This process is facilitated by the external power source connected to the electrodes.
Conversely, the anode attracts negatively charged ions (anions) from the electrolyte solution. In the case of molten KCl, the primary anions are chloride ions (Cl-). At the anode, chloride ions lose electrons through oxidation, forming chlorine gas (Cl2).
Therefore, positive ions (K+) move to the cathode (negative electrode) and gain electrons, while negative ions (Cl-) move to the anode (positive electrode) and lose electrons.
Learn more about electrolysis at: https://brainly.com/question/12994141
#SPJ11
What is the process of splitting into two cells called?
Answers
how many kilograms of chalcopyrite must be mined to obtain 465 g of pure cu ?how many kilograms of chalcopyrite must be mined to obtain 465 g of pure cu ?
Answers
Chalcopyrite must be mined to obtain 465 g of pure cu will be 1342.92g.
The chemical formula for Chalcopyrite will be CuFeS2 having a brassy to golden yellow color. It contains 34.5%Cu, 35.0% S and 30.5% Fe. Mineral is the principal source of a copper metal, as well as one of the major source of sulfur. Chalcopyrite will corresponds having a molecular mass of 183.54.
The distribution of a mass for this compound will be
Iron Fe 55.845 (1) 30.430%
Copper Cu 63.546 (1) 34.626%
Sulfur S 32.065 (2) 34.944%
Cu represents 34.626% of the total mass of a compound
To mine 425g of pure copper, requires a quantity of the Chalcopyrite which is equal to
\frac{465g}{0.34626} =1342.92g }{}
To knw more about chalcopyrite here
https://brainly.com/question/8534303
#SPJ4
[H+] for a solution is 1 x 10^-7 M. This solution is _____________.
A. acidic
B. basic
C. neutral
Answers
Answer:
the answer is C neutral
Explanation:
if the ph is lower then 7 its acidic
if its higher then 7 its basic
and a ph of 7 is neutral
Use the particle theory to explain why 10 mL
of liquid cannot
fill a 20 mL container.
Answers
Answer:
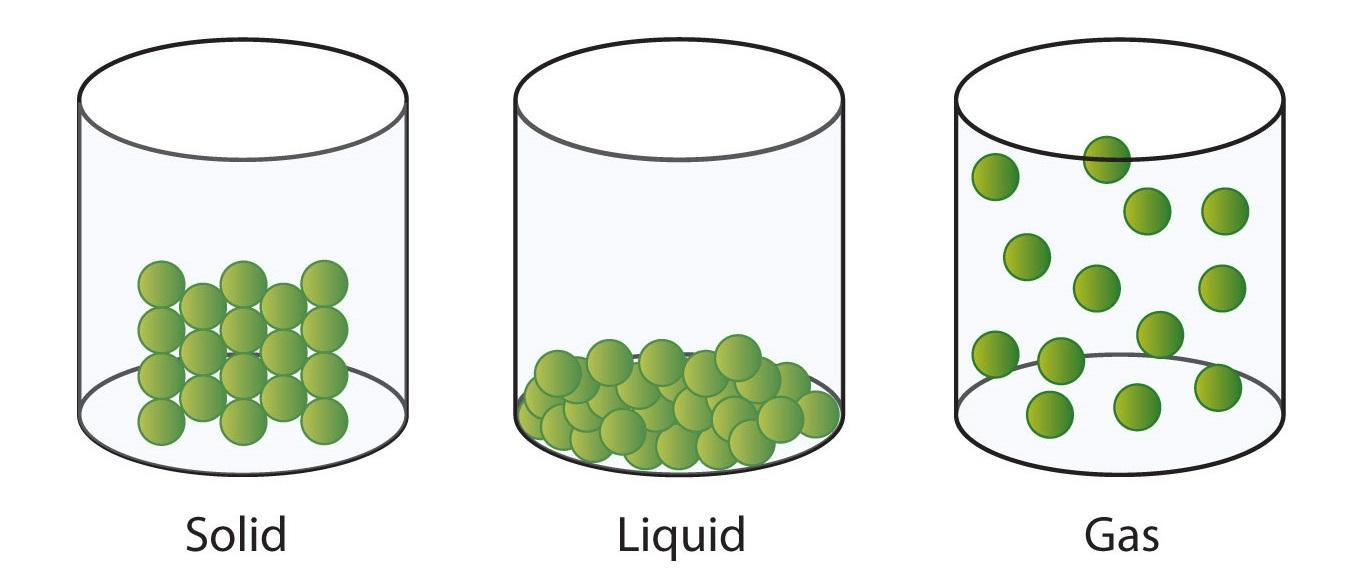
The particle theory is the belief that everything in our solar system and beyond is made of very small matter called atoms. The 10 mL is a matter in our solar system and even though we cannot see, there are millions- if not billions of smaller particles that make up the liquid. If you view the picture below, you can see that the particles in a liquid are close to one another, particles in a gas are far apart, and a particle in a solid is tightly pushed together. This gives them their distinctive shape. Since this is a liquid, this means that the particles are close together, but not very close. The particles glide over one another. If you want to have more space between the particles and expand the size of the liquid you can boil the water, however boiling the water turns it into gas and causing the liquid to evaporate. Freezing the liquid would cause the particles to be closer packed together, making a solid. The amount of liquid you have can not change. There is still 10 mL even when the liquid is frozen or when the liquid boils into vapor in the air. Therefore using particle theory, we can know that a shape can only expand or shrink when changing states of matter.
I hope this helped & Good Luck <3 !!
You're a dentist and want your patients to have the cleanest smiles possible. You're not sure which toothpaste to recommend and decide to design an
experiment to determine which toothpaste gives the brightest and whitest smile.
1) What variables would you need to control in your
experiment?
2) Which variable would you change to determine which toothpaste is best?
3) What is the measurable outcome for this experiment?
d) What would the procedure be for your experiment? Please help me to answer this questions:(
Answers
Answer:
Why do you need good toothpaste?
Explanation:
Answer:
Do this yourself smh this isnt what the wesite is for
Explanation:
why is edta used to determine the hardness of water
Answers
EDTA (ethylenediaminetetraacetic acid) is commonly used to determine the hardness of water due to its ability to form complexes with metal ions, particularly calcium and magnesium ions.
Water hardness refers to the concentration of calcium and magnesium ions present in water. These ions can cause scaling, reduce the effectiveness of soaps, and have other negative effects. EDTA acts as a chelating agent, meaning it can bind to metal ions and form stable complexes.
In the process of determining water hardness, a known amount of EDTA solution is added to a water sample. The EDTA molecules form complexes with the calcium and magnesium ions present in the water.
The endpoint of the titration is reached when all the metal ions are complexed by the EDTA, resulting in a color change or an indicator reaching a specific endpoint.
To know more about hardness of water refer:
https://brainly.com/question/28178305
#SPJ11
How do mass and type of material affect thermal energy transfer?
Answers
Answer:
Temperature, mass, and the type of material are factors that affect the thermal energy of an object.
Material with the higher specific heat will have more thermal energy than material with lower specific heat if they both have the same mass and temperature.
The thermal energy within the thing increases if the temperature remains constant but the object's mass rises.
If two materials have the same mass and temperature, the material with the greater specific heat will have more thermal energy than the material with the lower specific heat.
How does thermal energy transfer depend on the kind of material?
The speed at which thermal energy moves from one end of a substance to another determines thermal conductivity. Insulators transport thermal energy slowly while conductors transfer thermal energy quickly.
The thermal energy within the thing increases if the temperature remains constant but the object's mass rises.
Conduction, convection, or radiation are all ways that thermal energy can be moved from one location to another.
To learn more about energy refer to:
https://brainly.com/question/26520543
#SPJ2
The number 6 that he put in from of the O2, CO2, and H2O is called a:
superscript
coefficient
subscript
Answers
The number 6 that he put in from of the \(O_{2}\) , \(CO_{2}\) , and \(H_{2} O\) is called a coefficient .
So , second option is correct one .
In chemistry, coefficient is a number used in chemical equations, simply as a prefix of chemical formula to define the number of molecules reacting and creating in a reaction. This is similar to how in mathematics, coefficient is the number used in front of a variable.
When dealing with concentration terms in a chemical equation, coefficients are crucial to the equation's stability. In order to ensure that the law of conservation of mass is being obeyed, we add coefficients to the equation to make it balanced.
To learn more about coefficient here ,
https://brainly.com/question/26322576
#SPJ1
When volume of gas in a closed system decreases, the pressure of the gas in the system______.
Answers
When the volume of a gas in a closed system decreases, the pressure of the gas in the system increases.
This relationship is described by Boyle's Law, which states that the pressure and volume of a gas are inversely proportional at a constant temperature.
Boyle's Law can be mathematically represented as P₁V₁ = P₂V₂, where P₁ and V₁ are the initial pressure and volume, and P₂ and V₂ are the final pressure and volume, respectively.
When the volume decreases, the same amount of gas is now confined to a smaller space. As a result, the gas molecules collide more frequently with the walls of the container, exerting a greater force per unit area. This increase in force per unit area is what we refer to as an increase in pressure.
To understand this concept intuitively, imagine squeezing a balloon. As you apply pressure and decrease the volume of the balloon, you will feel the resistance of the air inside. The increased pressure arises because the same number of gas molecules are now occupying a smaller space, leading to more frequent and intense collisions with the balloon's surface.
Therefore, when the volume of gas in a closed system decreases, the pressure of the gas in the system increases due to the more confined space for the gas molecules.
For more such question on volume visit;
https://brainly.com/question/29796637
#SPJ8
what is the formula for chlorine
Answers
which statment best explains why liquids and gases can flow but solids cannot
Answers
Solids have a dignified structure. The particles are stuck together causing them to not flow. Liquids and gases have particles that move around so they can take the shape of their container.
How would our life be affected if plants could not do photosynthesis?
Answers
Answer:
If photosynthesis does not occur in plants then plants cannot synthesize the food. ... The plants will not produce oxygen and then no animal life will be able to survive due to the absence of oxygen. We will not get oxygen, food, and life on this planet will be extinct.
There are __ ___________ that make up everything we know and love.
Answers
Answer:
There are 92 elements that make up everything we know and love.
Explanation:
a 23.6 ml solution of 0.150 m ch3cooh (aq) is titrated with 0.25 m naoh. how many ml of naoh are needed to reach the half-equivalence point for this titration? express your answer in units of milliliters (ml) using at least three significant figures.
Answers
The amount naoh in ml required to reach the half-equivalence point for this titration is calculated to be 7.08 ml.
The half-equivalence point of a titration is reached when half of the acid has reacted with the base. At this point, the moles of acid and base are equal, and we can use this information to calculate the volume of base needed to reach the half-equivalence point.
First, we can calculate the number of moles of acetic acid present in the solution:
moles of CH3COOH = concentration x volume
moles of CH3COOH = 0.150 mol/L x 0.0236 L
moles of CH3COOH = 0.00354 mol
At the half-equivalence point, half of the acetic acid will have reacted, so the number of moles of acetic acid remaining will be:
moles of CH3COOH remaining = 0.00354 mol / 2
moles of CH3COOH remaining = 0.00177 mol
Since the reaction between acetic acid and sodium hydroxide is 1:1, we will need an equal number of moles of sodium hydroxide to react with the remaining acetic acid:
moles of NaOH needed = 0.00177 mol
Finally, we can use the concentration of sodium hydroxide to calculate the volume needed to provide this many moles:
volume of NaOH = moles of NaOH / concentration of NaOH
volume of NaOH = 0.00177 mol / 0.25 mol/L
volume of NaOH = 0.00708 L
volume of NaOH = 7.08 mL
Therefore, the volume of NaOH needed to reach the half-equivalence point is 7.08 mL.
Learn more about Titration and Moles :
https://brainly.com/question/31229711
#SPJ4
how many atoms in 10.0 g of Ar?
Answers
Answer:
1.51 x 10^23 atoms of Ar
Explanation:
Start out with what you're given.
10.0 g of Ar/1 * 1 mol/39.948 g Ar * 6.022*10^23 atoms/1 mol
(It's easier if you write it out.)
You always want to start out w/ what you're given and continue your unit conversions from there. Since you started out with grams of Argon, you want grams of Argon on the bottom to cancel it out. Once you found the unit conversion: 1 mol/39.948 g Ar (molar mass of Ar), now it's time to convert to atoms. Since 1 mol is up on top, put 1 mol on the bottom. Considering you're looking for atoms, use Avagradro's number: 6.022*10^23.
Once simplified, you should get 1.51 x 10^23 atoms of Ar.
***I hope this helps! I also suggest using a mole map, if you're interested.***
Any individual offspring produced through a sexual reproduction is always,
Answers
Answer:
Any individual offspring produced through asexual reproduction is always: genetically identical to its parent. made up of cells with a cell wall. different from all of its siblings
What is the trend in the ionic charges of the elements in groups 1, 2 and 13 of the periodic table (I will be giving brainliest to whoever gives the best answer and explains it)
Answers
Group 1: 1+
Group 2: 2+
Group 13: 3+
Explanation:The charges of the ions are dependent on the number of valence electrons.
Valence Electrons
Group 1 has 1 valence electron, group 2 has 2, and group 13 has 3. It is important to remember that elements will attempt to have a full valence shell. They may either attempt to lose all of their valence electrons or fill the energy level with 8 electrons.
Since all of these groups have less than 4 valence electrons, they will attempt to lose their valence electrons.
Determining Charges
When atoms lose electrons, they gain a positive charge. Since all of these elements will lose electrons, all of the ions will be positive, also known as a cation.
The positive charge of an element is equal to the number of electrons the atom loses. This means that group 1 will have a 1+ charge because it loses 1 electron. Continue this pattern to determine that group 2 will have a 2+ charge and group 13 will have a 3+ charge.
To what final volume in L should 380mL of a 0.47M solution be diluted if you want a final concentration of 0.094M?
Answers
ANSWER
The final volume of the solution is 1900mL
EXPLANATION
Given information
The initial volume is 380mL
The initial concentration is 0.47M
The final concentration is 0.094M
To find the final volume of the solution, follow the steps below
Step 1: Write the dilution formula
\(\begin{gathered} \text{ C1V1 = C2V2} \\ \text{ Where} \\ \text{ C1 = initial concentration} \\ V1\text{ = Final volume} \\ \text{ C2 = initial concentration} \\ \text{ V2 = Final concentration} \end{gathered}\)Step 1: Substitute the given data into the formula in step 1
Let the final volume be V2
\(\begin{gathered} \text{ 0.47 }\times\text{ 380 = 0.094}\times V2 \\ 178.6\text{ = 0.094}\times\text{ V2} \\ \text{ Divide both sides by 0.094} \\ \text{ }\frac{178.6}{0.094}\text{ = }\frac{0.094\times V2}{0.094} \\ \text{ V2 = 1900mL} \end{gathered}\)Hence, the final volume of the solution is 1900mL
Arrange the following sets of elements according to increasing first ionization energy and according to increasing metallic character.
S, Sr, Se, O, Ba, Ca
Answers
Answer:
Explanation:
Given elements:
S, Sr, Se, O, Ba, Ca
We are to sort the elements:
1. based on their increasing first ionization energy
2. based on their increasing metallic character
First ionization energy is the energy needed to remove the most loosely bonded electron of an atom in the gas phase in the ground state.
Ionization energy decreases down the group and increases across the period;
So;
Ba < Sr < Ca < Se < S < O
Metallic character is the electropositivity of an element. The agility to lose electrons is a measure of an atom's electropostivity;
Down a group metallic character increases and across a period it reduces.
O < S < Se < Ca < Sr < Ba
relate the following structures to your body organs_ walls: _houses_: bricks_:_ a room_: rooms
Answers
Answer:
what is this I am not getting which type of this question you have taken from where
HELP PLEASE DUE SOON!!!
calculate the following the correct amount of significant figures 1.25+3.69277+125.993
Answers
Answer: 129.93577
Explanation:
There-
Question
2 Points
Describe the path of a light ray that hits a mirror at its vertex.
A. It is reflected back through the focus.
B. It is absorbed by the mirror.
C. It is reflected out at exactly the same angle at which it came in,
according to the Law of Reflection.
D. It is reflected back parallel to the principal axis.
SUBMI
Hurry
Answers
Answer:D
Explanation:
Answer:
D
Explanation:
Calculate the settling velocity of a particle with 10 µm diameter and a specific gravity of 1.05 in 15 ˚C water (1.140 x 10^ -3 N-s / m^s and the density of water is 999.1 kg / m^3).
Answers
For a 10 µm diameter particle with a specific gravity of 1.05 in 15 ˚C water, the settling velocity can be determined using Stoke's law.
The settling velocity of a particle can be calculated using Stoke's Law, which is given by the equation:
\(V = (2/9) * (g * (ρp - ρf) * d^2) / η\)
Where:
V is the settling velocity,
g is the acceleration due to gravity (approximately 9.81\(m/s^2\)),
ρp is the density of the particle,
ρf is the density of the fluid,
d is the diameter of the particle, and
η is the dynamic viscosity of the fluid.
Given that the diameter of the particle is 10 µm (or \(10 x 10^-6 m\)) and the specific gravity is 1.05, we can calculate the density of the particle using the equation:
ρp = ρf * (specific gravity)
Substituting the values, we have ρp = (999.1 \(kg/m^3\)) * 1.05 = 1049.545 \(kg/m^3\).
Using the known values of ρp, ρf (density of water), d, and the dynamic viscosity of water at 15 ˚C (\(1.140 * 10^ -3 N-s/m^2\)), we can substitute these values into the Stoke's Law equation to calculate the settling velocity of the particle.
Learn more about velocity here:
https://brainly.com/question/17005285
#SPJ11
Name main factors that affect on rates of chemical reaction
write in your own words, please. Don't copy-paste from the internet, please.
Answers
Answer:
There are four main factors that can affect the reaction rate of a chemical reaction:
Reactant concentration. Increasing the concentration of one or more reactants will often increase the rate of reaction. ...
Physical state of the reactants and surface area. ...
Temperature. ...
Presence of a catalyst.
Solar systems are larger than galaxies. Also, galaxies are larger than the universe.
True
False
Answers
ATCG combine different patterns to form different ____
Answers
Answer:
Chemicals
Explanation:
Your answer would be chemicals because of you have different inputs you would also have a different output depending.
I hope it helps! Have a great day!
Anygays-
Answer:
Chemicals
Explanation:
Different ATCG makes different chemicals.
I hope it helps! Have a great day!
Muffin °-°
A gas syringe contains 42.3 milliliters of a gas at 371 K. Determine the volume that the gas will occupy if the temperature is decreased to 254 K.
Answers
Answer:
28.82 mL
Explanation:
This is about gases laws, in this case about Charles Gay Lussac.
At constant pressure the volume is proportional to the T°.
V₁ / T₁ = V₂ /T₂
42.1 mL / 371K = V₂ /254K
(42.1 mL / 371K) . 254K
V₂ = 28.82 mL
In conclussion:
As T° was decreased, the volume also decreased too.
Is Ni(NO3)3 ionic or covalent and why
Answers
The Ni ( NO₃ )₃ is an anhydrous covalent compound. Ni ( NO₃ )₃ is prepared by the reaction of red fuming nitric acid and nickel nitrate hemihydrate.
What is covalent compound ?An electron exchange that results in the formation of electron pairs between atoms is known as a covalent bond. Bonding pairs or sharing pairs are the names given to these electron pairs. Covalent bonding is the stable equilibrium of the attractive and repulsive forces between atoms when they share electrons.
At room temperature, covalent compounds can be found in all three of their physical states and often have low boiling and melting temperatures. Because covalent compounds lack charged particles with the ability to carry electrons, they do not conduct electricity.
Two atoms sharing a pair of electrons make a covalent connection. Because the electron pair is drawn to both nuclei, the atoms are kept together.
Thus, The Ni ( NO₃ )₃ is an anhydrous covalent compound.
To learn more about covalent compound, follow the link;
https://brainly.com/question/21505413
#SPJ2